Abstract
Context: The roots of Plumbago zeylanica Linn. (Plumbaginaceae) are reputed to have a wide spectrum of therapeutic properties in the Ayurvedic system of medicine. They are useful in curing many ailments such as skin diseases, diarrhea, plague and leprosy.
Objective: The study was aimed at isolating, separating and evaluating the antimicrobial properties of compounds such as neoisoshinanolone and 1-epineo-isoshinanolone from the roots of P. zeylanica.
Materials and methods: The crude petroleum ether extract of roots of P. zeylanica was subjected to repeated chromatographic techniques to separate compounds 2 and 3 along with plumbagin. Structure elucidation was carried out using nuclear magnetic resonance (NMR), infra red (IR) and mass spectroscopy. The serial dilution method was used to test antimicrobial activities and their minimum inhibitory concentration (MIC) expressed in µg/mL.
Results: 1-Epineo-isoshinanolone is more active with a MIC of 12.5-25 µg/mL whereas neoisoshinanolone has recorded a MIC of 50-100 µg/mL. The activities are compared with plumbagin (0.78-3.13 µg/mL) and standards streptomycin for bacteria and nystatin for fungi.
Discussion: Earlier researchers have established the presence of plumbagin in the roots of P. zeylanica and its antimicrobial activities. The structure elucidation of two more biologically active biogenetic precursors along with their activities in the root extracts has been established for the first time in the present study.
Conclusion: The root extract of P. zeylanica possesses good antimicrobial activity, which suggests its therapeutic use in the Ayurvedic system of medicine to cure skin diseases.
Introduction
Higher plants have been the source of medicinal agents since early times and today they continue to play a dominant role in the primary healthcare of about 80% of the world’s population (CitationBaker et al., 1995). Research into the chemical and biological properties of natural products over the past two centuries has not only yielded drugs for the treatment of many human ailments, but has provided the stimulus for the development of modern synthetic organic chemicals and the emergence of medicinal chemistry as a major route for the discovery of novel and more effective therapeutic agents. Sophisticated plant-based traditional medicine systems have been in existence for thousands of years in countries such as China (CitationChang & But, 1986) and India (CitationKapoor, 1990).
The roots of P. zeylanica Linn. (Plumbaginaceae) are reputed to have a wide spectrum of therapeutic properties in the Ayurvedic system of medicine. The roots of this plant are used in the treatment of skin diseases, diarrhea, dyspepsia, piles, anasarca, plague, leprosy, urinary tract infections, scabies and ulcers. In addition to the above uses, chewing of Plumbago root is said to be effective for relieving toothache (CitationChopra et al., 1956) and is a strong abortifacient (CitationSivarajan & Balachandran, 1994).
In our pursuit to isolate novel antimicrobial compounds, we have earlier worked on several plants (CitationAnnapurna et al., 1983, Citation1989, Citation1999, Citation2003, Citation2004; CitationAnnapurna & Iyengar, 2000; CitationLalitha & Annapurna, 2008; CitationMarthanda et al., 2005, Citation2006). The present investigation describes the isolation, purification and characterization of plumbagin (1) and the two biogenetic isomers and evaluated their antimicrobial properties. For the first time the diastereoisomers neoisoshinanolone (2) and 1-epineo-isoshinanolone (3) from P. zeylanica roots were separated and characterized by spectral data.
Methods
Plant material and extraction procedure
P. zeylanica (Plumbaginaceae) also called as leadwort and chitrakmool is a perennial shrub found wild in West Bengal and also in peninsular India (CitationKrishnamurthi, 1969). The roots were collected from Vageswarapuram, West Godavari District, Andhra Pradesh, India, in March 2003 and authenticated by Prof. Prabhakar Malve, Department of Botany, Osmania University, Hyderabad, India and a voucher specimen No. 00403 OUAH has been deposited there. Air-dried root powder of P. zeylanica was extracted four times at room temperature (35° ± 2°C) for every 24 h for 4 days with distilled petroleum ether bp (60-80°C). The pooled extracts were concentrated in a rotary vaporizer under reduced pressure < 40°C.
Isolation and Characterization of plumbagin (1)
Finely powdered air-dried roots (1 kg) were extracted with petroleum ether bp (60-80°C) at room temperature (35° ± 2°C). The brown-colored concentrated extract 2.57 g was subjected to column chromatography using silica gel finer than 200 mesh with 0.08 mm particle size. The column (60 cm high and 3 cm diameter) was prepared using benzene-petroleum ether (60°-80°C) (3:1) and eluted using benzene-petroleum ether (3:1) and finally with ethyl acetate. The eluates were pooled based on TLC to give eight fractions. The residue from fraction 4 gave orange-colored crystalline needles (melting point 78°C) and it has been identified as plumbagin by comparison with an authentic sample (CitationSankaram et al., 1976).
Isolation and separation of neoisoshinanolone (2) and 1-epineo-isoshinanolone (3)
The dark residue from the polar fraction 8 which was eluted with ethyl acetate was further purified by repetitive column chromatography (silica gel Acme finer than 200 mesh, 0.08 mm particle size; column prepared using chloroform-ethyl acetate (20:1); eluent: chloroform-ethyl acetate (20:1)) resulting in two fractions (A) and (B). Fraction A, white flakes identified as β-sitosterol on comparison with authentic sample (CitationSinha, 1959).
Fraction B further purified by column chromatography [silica gel Acme finer than 200 mesh, 0.08 mm particle size; column prepared using petroleum ether (60-80°C); eluents: petroleum ether (60-80°C), benzene, chloroform and ethyl acetate] followed by preparative TLC (Silica gel C, eluent: di-isopropyl ether) resulting in two compounds neoisoshinanolone (2) and 1-epineo-isoshinanolone (3).
Microorganisms
Ten bacterial strains and two fungal strains were obtained from various collection centers such as American Type Culture Collection (ATCC), National Collection of Industrial Microorganisms (NCIM) Pune, India and Microbial Type Culture Collection (MTCC) Chandigarh, India. They were Gram-positive (Gr+) Arthrobacter citreus (ATCC 11624), Bacillus cereus (ATCC 11778), Bacillus pumilus (ATCC 14884), Clostridium acetobutylicum (NCIM 2337), Staphylococcus aureus (ATCC 9144), Staphylococcus epidermidis (NCIM 2493), and Gram-negative (Gr-) Escherichia coli (ATCC 10536), Klebsiella aerogenes (ATCC 15380), Sarcina lutea (NCIM 2103) and Pseudomonas aeruginosa (ATCC 25619). Two fungal strains, Candida albicans (MTCC 227) and Saccharomyces cerevisiae (MTCC 170), were also used.
Antimicrobial assay
All the media and standard antibiotics used were from Hi Media, Mumbai. Bacterial strains were grown on nutrient agar and suspended in Mueller Hinton broth and fungal strains in Sabouraud broth. Two-fold serial dilutions were employed to determine MIC values (CitationJones et al., 1985). The sample (1 mg) dissolved in 1 mL of 10% acetone/water (1000 µg/mL) was transferred aseptically to 9 mL of broth (first tube, 100 µg/mL) and subsequently 5 mL from the first tube was added to 5 mL of second tube and so on to get serial dilutions of concentrations ranging from 100-1 µg/mL and below. To the above tubes, 30 µL of suitably diluted inoculum grown at 35° ± 2°C for 20 h (approximately 107 CFU/mL) was added and the culture tubes, along with controls and standard antibiotic streptomycin for bacteria and nystatin for fungi, were incubated at 37°C for 24 h. Each experiment was replicated three times and the results are presented in µg/mL along with standards. MIC values were recorded as the lowest concentration resulting in complete inhibition of bacterial growth.
Results
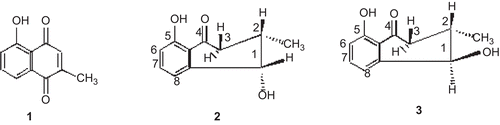
shows the structures of two newly separated compounds along with known plumbagin and their structures were elucidated. The yields of the pure compounds 2 and 3 were calculated as percentage based on the crude petroleum extract. Neoisoshinanolone (2) (yield 0.11%) low melting solid; [α]D; +24.86° (c.1.49, CHCl3); M+,192, C11H12O3; UV max (MeOH): 259 and 333 nm; IR bands (KBr): 3430, 2960, 2920, 1640, 1580, 1455, 1410, 1340, 1240, 1215, 1165, 1019, 1030, 980, 940, 900, 870, 810, 800 and 740 cm−1; 1H NMR (300 MHz, CDCl3); δ 12.41 (s, 5-OH), 7.48 (t, H-7), 6.93 (dd, H-6 and H-8), 4.74 (d, J = 2.5Hz, H-1), 2.56 (dd, J = 17.6 and 4.3Hz (He-3), CH2-3), 2.86 (dd, J = 17.6 and 10.9Hz (Ha-3), CH2-3), 2.43 (m, H-2), 1.18 (d, J = 6Hz, CH3-2).
1-Epineo-isoshinanolone (3) (yield 0.008%); Mp 80°C; M+, 192, C11H12O3, UV max (MeOH): 259 and 333 nm; IR bands (KBr): 3430, 2960, 2920, 1640, 1580, 1455, 1340, 1240, 1165, 1030, 940, 900, 810, and 740 cm−1; 1H NMR (100 MHz, CDCl3) δ; 12.04 (s, 5-OH), 7.34 (t, H-7), 6.86 (dd, H-6), 7.14 (dd, H-8), 4.46 (d, J = 8Hz, H-1), 2.38 (dd, J = 17.5 and 11Hz Ha-3, CH2-3), 2.88 (dd, J = 17.5 and 4.3Hz He-3, CH2-3), 1.86 (m, H-2), 1.17 (d, J = 6Hz, CH3-2).
shows the antimicrobial activity of plumbagin and the two biogenetic precursors neoisoshinanolone (2) and 1-epineo isoshinanolone (3). The most sensitive bacteria to plumbagin were S. epidermidis and S. lutea at 0.78 µg/mL, while the other bacteria recorded MIC of 1.56-3.13 µg/mL. Fungal strains such as C. albicans and S. cerevisiae had MIC of 1.56 and 3.13 µg/mL, respectively, which is comparable with standard nystatin. Of the two isomers tested, 1-epineo isoshinanolone (3) was more active when compared to neoisoshinanolone (2).
Table 1. Antimicrobial activity of Plumbago zeylanica root extracts.
In general, compound 3 had shown activity towards Gram positive bacteria at 12.5-25 µg/mL, while Gram negative had recorded 50-100 µg/mL. The activities were less pronounced when compared to standard antibiotic, streptomycin, which was in the range of 1.56-3.13 µg/ mL of recorded MIC against all tested bacteria. The most sensitive Gram positive bacteria towards compound 3 are B. cereus and S. aureus and Gram negative E. coli with MIC of 12.5 µg/mL, whereas Gram positive bacteria such as A. citreus, B. pumilus, C. acetobutylicum, and S. epidermidis have MIC of 25 µg/mL. The other Gram negative bacteria such as P. aeruginosa and S. lutea have recorded MIC at 25 µg/mL, while K. aerogenes showed MIC of 50 µg/mL.
Compound 2, i.e., neoisoshinanolone, in general showed activity in the range of 50-100 µg/mL against all the tested organisms. The most sensitive bacteria to compound 2 with 50 µg/mL were Gram positive A. citreus, C. acetobutylicum, K. aerogenes, and S. aureus, while the rest of the other bacterial species such as B. cereus, B. pumilus, E. coli, S. epidermidis P. aeruginosa, and S. lutea showed MIC of 100 µg/mL. Compound 3 is more active against both the fungal species such as C. albicans and S. cerevisiae at 25 µg/mL, whereas compound 2 showed MIC of 50 µg/ mL.
Discussion
Plants with antimicrobial properties have received much attention because of current problems associated with resistance. One approach that has been used for the discovery of antimicrobial agents from higher plants is based on the evaluation of medicinal and aromatic plant extracts. Currently this approach to the problem has the advantage that human clinical experience of the use of these agents is well established.
In the present investigation we have studied the antimicrobial activities of petroleum ether extracts of roots of P. zeylanica along with plumbagin. Though plumbagin is a known biologically active compound (CitationKrishnaswamy & Purushothaman, 1980), the two newly purified and separated compounds 2 and 3 were reported for the first time from this plant root extract. Earlier workers have reported the existence of epiisoshinanolone from P. scandens as a mixture (CitationBhattacharyya & Carvalho, 1986). The structures of the above two diastereoisomers have been determined from a consideration of its 1H NMR, 2D-NMR (NOESY, H...H) and CD spectral evidence. We have evaluated the antimicrobial activities of the above two compounds and compared their activities with known antibiotic streptomycin for bacteria and nystatin for fungi.
Several naphthoquinones, binaphthoquinones, flavonoids and β-sitosterol have been isolated from this plant by earlier workers (CitationGunaherath et al., 1983) and different biological activities such as antifeedant (CitationKubo et al., 1980) and antifertility (CitationAzad et al., 1982) were also investigated. It was found to possess antitubercular activity also (CitationRanganatha Rao & Seshadri, 1955; CitationMossa et al., 2004).
Many pathogenic microbes can be controlled with the antibiotics presently available. However, the need for new antibiotics has increased due to current problems of resistance associated with continuous use of antibiotics rendering pathogen resistance. Thus, new and effective antimicrobial agents from non-microbial sources also are needed. Compounds isolated from plants have the potential to fill this need, as their structures are different from those of microbial sources and hence their mode of action may very likely differ.
Plumbagin (1), which is a known biologically active compound (CitationSivarajan & Balachandran, 1994), inhibited both Gram positive and Gram negative bacteria with MIC values in the range of 0.78-3.13 µg/mL. The antifungal activity of plumbagin has been attributed to the presence of vacant quinonoid position of the molecule (CitationSankaram et al., 1976).
In the present study, compound 2 was more active than its isomer compound 3 against all the bacteria tested. The results obtained in this study demonstrated that the isolated two new diastereoisomers from the roots of P. zeylanica had shown broad-spectrum antimicrobial activity in vitro.
Compound 3 was more active against both the fungal species at 25 µg/mL, whereas compound 2 had a MIC of 50 µg/mL. Though the antimicrobial activity is less than that of the known compound, plumbagin, the reduced activity might be due to the reduced quinone to hydroxy group in both the structures (2 and 3). The ethnomedical use and Ayurvedic preparations made from this plant root extracts are justified to alleviate skin infections caused by bacteria and fungi.
Conclusions
From the present investigation it can be concluded that 1-epineo-isoshinanolone (3) was found to be more active than its diastereoisomer neoisoshinanolone (2) against all the tested bacteria and fungi. Earlier these compounds were reported together as a mixture from P. scandens. We have separated the isomers from P. zeylanica for the first time in the pure form and evaluated their antimicrobial activities along with plumbagin (1).
Acknowledgements
The authors are grateful to J. S. Yadav, Director IICT, for encouragement and cooperation and Prof. Prabhakar Malve, Taxanomist, Osmania University, Hyderabad for authentication of the plant material.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Annapurna Y, Mitra S, Iysengar DS, Nagabushan Rao S, Bhalerao UT (1983): Antimicrobial activity of leaf extracts of Polyalthia longifolia. Phytopath Z 106: 183–185.
- Annapurna J, Iyengar DS, Bhalerao UT (1989): Antimicrobial activity of leaf extracts of Enterolobium saman. Indian Drugs 26: 272–274.
- Annapurna J, Bhalerao UT, Iyengar DS (1999): Antimicrobial activity of Saraca asoca leaves. Fitoterapia 70: 80–82.
- Annapurna J, Iyengar DS (2000): Antimicrobial activity of Millingtonia hortensis leaf extract. Pharm Biol 38: 157–160.
- Annapurna J, Amarnath P, Kumar A, Ramakrishna SV, Raghavan KV (2003): Antimicrobial activity of Ixora coccinea leaf extract. Fitoterapia 74: 291–293.
- Annapurna J, Chowdary IP, Lalitha G, Ramakrishna SV, Iyengar DS (2004): Antimicrobial activity of Euphorbia nivulia leaf extract. Pharm Biol 42:91–93.
- Azad Chowdhury AK, Sushanta KC, Azad Khan AK (1982): Antifertility activity of Plumbago zeylanica Linn. root. Indian J Med Res 76: 99–101.
- Baker JT, Borris RP, Carte B, Cordell GA, Soejarto DD, Cragg GM, Gupta MP, Iwu MM, Madulid DR, Tyler VE (1995): Natural product drug discovery and development. J Nat Prod 58: 1325–1357.
- Bhattacharyya J, Carvalho VRD (1986): Epi-isoshinanolone from Plumbago scandens. Phytochemistry 25: 764–765.
- Chang HM, But PPH (1986): Pharmacology and Applications of Chinese Materia Medica. Singapore, World Scientific.
- Chopra RN, Nayar SL, Chopra IC (1956): Glossary of Indian Medicinal Plants, New Delhi, Council of Scientific and Industrial Research, pp.114–124.
- Gunaherath GMKB, Gunatilaka AAL, Sultanbawa MUS, Balasubramanian S (1983) 1,2(3)-Tetrahydro-3,3′-biplumbagin: A naphthalenone and other constituents from Plumbago zeylanica. Phytochemistry 22: 1245–1247.
- Jones RN, Barry A, Gaven TL, Washington JA (1985): Susceptibility tests: Microdilution and macrodilution broth procedures, in:Lenette EH, Ballows A, Havsle WJ, Shadomy HJ, eds., Manual of Clinical Microbiology, Washington DC, American Society for Microbiology, pp. 972–977.
- Kapoor LD (1990): CRC Handbook of Ayurvedic Medicinal Plants. Boca Raton FL, CRC Press, pp. 208–217.
- Krishnamurthi A (1969). The Wealth of India, Vol. 8. New Delhi, CSIR, pp. 163–165.
- Krishnaswamy M, Purushothaman KK (1980): Plumbagin: A study of its anticancer, antibacterial and antifungal properties. Indian J Exp Biol 18: 876- 877.
- Kubo I, Taniguchi M, Chapya A, Tsujimoto K (1980): An insect antifeedant and antimicrobial agent from Plumbago capensis. Planta Med 40:185–187.
- Lalitha Kumari, Annapurna J (2008): Ethno botany of Indian plant species used against snakebite–A review. In: Singh VK, Govil JN, Sharma RK, eds., Recent Progress in Medicinal Plants. Phytopharmacology and Therapeutic Values I. Studium Press, LLC, USA, pp. 237–292.
- Marthanda Murthy M, Subramanyam M, Hima Bindu M, Annapurna J (2005): Antimicrobial activity of clerodane diterpenoids from Polyalthia longifolia seeds. Fitoterapia 76: 336–339.
- Marthanda Murthy M, Subramanyam M, Giridhar KV, Annapurna J (2006): Antimicrobial activity of bharangin and bharangin monoacetate from Premna herbacea roots. J Ethnopharmcol 104: 290–292.
- Mossa JS, El-Feraly FS, Muhammad I (2004): Antimicrobial constituents from Juniperus procera, Ferula communis and Plumbago zeylanica and their in vitro synergistic activity with isonicotinic acid hydrazide. Phytother Res 18: 934–937.
- Ranganatha Rao K, Seshadri TR (1955): The tuberculostatic activity of some naturally occurring quinones. J Sci Ind Res 14: 189–191.
- Sankaram AVB, Srinivasa Rao A, Sidhu GS (1976) Chitranone- a new binaphthaquinone from Plumbago zeylanica. Phytochemistry 15, 237–238.
- Sinha A (1959): Sterol from seeds of Pongamia glabra. Indian J Appl Chem 22: 86–88.
- Sivarajan VV, Balachandran I (1994): Ayurvedic Drugs and their Plant Sources, New Delhi, Oxford & IBH Publishing, pp. 119–122.