Abstract
Context: Solidago chilensis Meyen (Asteraceae) is widely used in South America in traditional medicine as an anti-inflammatory and diuretic, and to treat gastrointestinal disorders. However, no scientific evidence exists in literature to corroborate the therapeutic use of the plant. Despite its traditional uses, no reports are available on the safety of this utilization or on the relationship between the pharmacological activities and its phytochemical compounds.
Objective: This study investigates for the first time the acute toxicity and the gastroprotective effect of the aqueous extract from inflorescences of S. chilensis.
Materials and methods: The gastroprotective activity was evaluated in mice subjected to ethanol-induced gastric ulcer model at 125, 250, 400, 800, 1200, and 2000 mg/kg doses. Acute toxicity study was performed at one dose of 2000 mg/kg. At the end of the exposure behavioral and functional parameters and motor activity were assessed in all animals.
Results: Results demonstrated that the extract exhibited a significant antiulcer activity when given at 125-2000 mg/kg (P <0.05), but did not show acute toxicity in mice treated with 2000 mg/kg p.o.
Discussion and conclusion: This study demonstrated that the oral administration of S. chilensis aqueous extract prevents the formation of gastric lesions caused by an aggressive factor as ethanol but does not produce toxicity by acute exposure in mice. These promising results support a better pharmacological study of S. chilensis as a potential antiulcerogenic species for studies targeted towards the development of antiulcerogenic agents.
Introduction
Peptic ulcer is one of the most important diseases of the digestive system and is a medical-social problem of global economic importance due to its high incidence, broad geographical distribution, morbidity and drug consumption. It is estimated that nearly 20% of the population may suffer from peptic ulcer throughout their life, its causes being factors such as stress, diet, smoking, alcohol and certain types of drugs (CitationEverhart et al., 1998; CitationLevenstein, 2000).
Treatment of symptomatologies related to gastric ulcers or gastritis with medicinal plants are quite common in traditional medicine worldwide; however, current treatments to combat ulcers cause side effects and actions contrary to what is required. Because of this, it has increased the search and evaluation of new agents against gastrointestinal diseases, mainly from plants, in the treatment of gastrointestinal disorders.
CitationBucciarelli and Skliar (2007) determined that infusions of several medicinal species growing in Argentina prevent gastric ulcers induced by ethanol in mice, a representative model of peptic ulcer disease in humans (CitationSilen, 1988).
Solidago chilensis Meyen (Asteraceae) is a native species from South America, where is popularly known as “vara dorada” and is employed in folk medicine as an anti-inflammatory and diuretic, and to treat gastrointestinal disorders (CitationGoleniowski et al., 2006). Despite its wide utilization, the pharmacological, chemical and toxicological investigations are rather scarce.
Plants of the genus Solidago, such as S. canadensis and S. virgaurea, contain terpenoids, saponins, phenolic acids, phenolic glycosides, and high amounts of flavonoids, mainly quercetin, kampferol, and rutin (CitationBatyuk & Kovaleva, 1985; CitationLorenzi & Matos, 2002). Other authors reported the presence of sesquiterpenes, labdane diterpenes, saponins, chlorogenic acid and caffeic acid in Solidago species (CitationBader et al., 1995; CitationApati et al., 2002; CitationBradette-Hébert et al., 2008; CitationSteliopoulos et al., 2008).
Besides their antiinflammatory effect, flavonoids have been demonstrated to have important gastroprotective activities. Some natural flavonoids have been shown to increase the mucosal content of prostaglandins and mucus in gastric mucosa, thus exhibiting cytoprotective effects. Some of them prevent gastric mucosal lesions produced by several methods, and protect it against different necrotic agents (CitationRobert et al., 1979a, Citation1979b; CitationAlarcón de la Lastra et al., 1994; CitationIzzo et al., 1994; CitationMartín et al., 1994; CitationMotilva et al., 1994).
Considering these data, Solidago chilensis was selected with the aim of evaluating its gastroprotective effect at different doses in mice. Due to the possible therapeutical use of this plant, we also evaluated the acute toxicity in mice by means of a functional observational battery (FOB) and by assessing the motor activity in an open field. Finally, the histopathological examination was realized on several tissues.
Materials and methods
Plant material
Fresh inflorescences of Solidago chilensis Meyen (Asteraceae) were collected in Bahía Blanca, province of Buenos Aires, Argentina, in January 2008. A voucher specimen (AB 9), collected by Alejandro Bucciarelli, was identified and authenticated by Carlos B. Villamil, Director of Departamento de Biología, Bioquímica y Farmacia Herbarium (BBB).
Preparation of extracts
Plant material, oven dried at 40°C, was extracted using hot water at 90°C under infusion for 10 min (plant:solvent, 1:10 w/v). The extract was collected, filtrated and concentrated to dryness under reduced pressure (yield: 14%).
Standard screening tests were utilized for detecting the major constituents: alkaloids (Dragendorff reaction), anthraquinones (Borntraeger reaction), flavonoids (Shinoda test), phenolic compounds (Ferric chloride reaction), sesquiterpene lactones (Baljet reaction), sterols/triterpenes (Liebermann-Burchard reaction).
Animals
Female CF-1 albino mice of ten weeks were used for toxicity and gastroprotective studies. Animals were maintained under constant temperature conditions (22° ± 1°C) in a 12 h light/dark cycle (lights on at 7:00 h), provided with food and water ad libitum.
The experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health, USA.
Gastroprotective effect study
The dried extract and the antiulcer reference drug (omeprazole) were separately suspended in vehicle (0.05% Tween 80 and 0.1% carboxymethylcellulose in water, 1:1 w/w).
Mice were divided into eight groups (n = 6) and fasted for 24 h with free access to water prior to oral administration of 0.3 mL vehicle, omeprazole (10 mg/ kg) or aqueous extract (125, 250, 400, 800, 1200 and 2000 mg/kg). Forty five min after each treatment, all animals received 7.5 mL/kg absolute ethanol to induce the formation of gastric lesions. Animals were sacrificed 45 min after administration of the necrotizing agent. The stomachs were dissected out, inflated with 2 mL of saline solution and fixed in 10% formaldehyde solution for 2 h. Stomachs were opened along the greater curvature and the mucosa examined under stereoscope to score the ulcers (CitationMartín et al., 1998) and to determine the ulcer index (UI). The percentage of ulcer inhibition (Inhibition %) was determined by the following formula:
Acute toxicity study
The plant extract was suspended in water and incorporated into the diet and fed to mice over 24 h at one dose of 2000 mg/kg. For this, the amount of food consumed and body weight of each mouse were determined previously. Eight mice were used in control and exposed groups. The treated animals received standard food mixed with plant extract while control mice only received the same standard diet without the plant extract. The elected dose is the maximum dose indicated for this assay of toxicity (CitationOECD, 2001). The limit test was performed because no severe toxic effects were expected by ingestion of this plant extract. At the end of the exposure, behavioral and functional parameters and motor activity were assessed in all animals. Subsequently, the animals were maintained with standard diet without the plant extract for 14 days and the same parameters were reevaluated to determine reversibility, persistence, or delayed occurrence of toxic effects. Finally, all animals were euthanized by cervical dislocation, and necropsy observations and histopathological examinations were realized on several tissues: brain, liver, kidney, spleen, stomach and intestine. These tissues were weighed immediately after dissection, embedded in paraffin and 5 μm thick sections were stained with hematoxylin and eosin.
Functional observational battery
The FOB is a series of observational and manipulative tests designed to assess the neurological integrity of the test subject (CitationMoser et al., 1988), whereas motor activity is an apical measure of neurobehavioral function (CitationMacPhail, 1987).
The FOB included a thorough description of the animals’ appearance, behavior, and functional integrity (CitationMoser & Ross, 1996; CitationUSEPA, 1998). This was assessed through observations in the home cage, while animals were moving freely in an open field, and through manipulative tests. Procedural details and scoring criteria for the FOB protocol were based on the FOB developed for rats (CitationMcDaniel & Moser, 1993).
Briefly, measurements were first carried out in the home cage. The observer recorded each animal’s posture, activity and palpebral closure. The presence or absence of clonic and tonic movements was noted and, if present, described. The presence or absence of spontaneous vocalizations and biting was also noted. The observer then removed the animal, rating the ease of removal and handling. Lacrimation or salivation was rated. The presence or absence of piloerection and limb pressure grade was also noted. Fur appearance, respiration, cardiovascular signs, limb and abdominal tone, and other abnormal clinical signs were also recorded.
The animal was next placed in an open field arena having a piece of clean absorbent paper on the surface and allowed to freely explore for 3 min. During that time, the observer ranked the mouse’s arousal, activity level and rearing as well as any abnormal gait and mobility. Gait, stereotypy, pelvic elevation, tail position and vocalizations were also described. At the end of the 3 min, the number of fecal boluses and urine pools, and presence or absence of diarrhea on the absorbent paper was recorded. Next, sensorial responses were ranked according to a variety of stimuli (click stimulus using a metal clicker, approach and touch rump with a blunt object, pinch of the tail using forceps, and touch of the corner of the eye and the inside of the ear with a fine object). Also, motor reflexes were evaluated (flexor and extensor thrust reflexes). Degree of righting reflex was rated next. In the wire maneuver, the animal was suspended from horizontal wire by forelimbs and released; the ability to bend or hang was rated. In landing foot splay, the tarsal joint pad of each hindfoot was marked with ink and the animal was then dropped from a height of 15 cm onto a recording sheet. This procedure was repeated twice. The distance (cm) from center-to-center of the ink marks was measured and the average of the two splay values was used for statistical analysis.
Motor activity
An open field of 50 × 50 × 60 cm whose floor was divided into 12 × 12 cm squares by black lines was used. The number of squares entered with all four paws, rearings, groomings, and fecal boluses were scored for 15 min. After each animal was removed, the open field was carefully cleaned with a damp cloth.
Statistical analysis
The effects of the different treatments in the gastroprotective study were evaluated by one-way analysis of variance (ANOVA) followed by Tukey’s test, with the level of significance at P <0.05.
The dietary intake and body weight of mice subjected to the acute toxicity study were tested using an independent Student’s t-test. Behavioral test measures in FOB were continuous (providing interval data), ranked (based on a defined scale), descriptive or binary (presence or absence of a sign). Continuous data were tested using an independent Student’s t-test. The ranked data were analyzed using the Mann-Whitney U-test. For descriptive and binary data, each experimental group was compared to the control group using a Chi-square test. The open field data were analyzed by independent Student’s t-test. Resulting P values <0.05 were considered significant.
Results
Phytochemical screening
Preliminary phytochemical screening performed on the plant extract revealed the presence of flavonoids and phenolic compounds.
Gastroprotective activity study
Mice that were only given vehicle (control) showed marked mucosal damage, including hyperemia, submucosal edema and severe congestion of vessels (). There was a complete incidence in the formation of lesions in this group. The severity of ulcers is reflected in the ulcer index, which was significantly higher in the control group as compared with the treated groups (). The pretreatment with omeprazole or aqueous extracts inhibited the formation of gastric lesions in varying degrees (P <0.05). Doses of 125, 250 and 400 mg/kg did not prevent completely any stomach from ulceration but elicited significant gastroprotection (61.4%, 75.1% and 78.4%, respectively). Ulcerative lesions were not observed in two of the six animals treated with S. chilensis extract at 800, 1200 and 2000 mg/kg doses, exhibiting a gastroprotective activity of 81.3, 86.8 and 91.5%, respectively. Although the antiulcer reference drug omeprazole did not prevent completely any stomach from gastric ulceration, it achieved 76.5% inhibition.
Figure 1. Gastroprotective activity of Solidago chilensis aqueous extract. (A) Representative stomach of control group. (B) Representative stomach of group treated with 1200 mg/kg of extract. Arrows indicate some of the ulcers produced by ethanol. Bars = 5 mm.
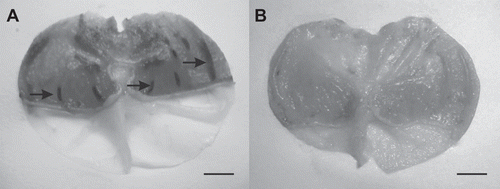
Table 1. Effect of Solidago chilensis aqueous extract (AE) against gastric lesions induced by ethanol in mice.
Acute toxicity study
The mice exposed to aqueous extract exhibited a similar dietary intake to control groups. No significant differences were observed between the groups when we analyzed the body weight (data not shown).
The acute exposure to Solidago chilensis did not produce alterations in all parameters evaluated in the home cage or during the manipulative tests. In both days (Day 1 and Day 14), no significant differences were observed between control and experimental group in the different parameters analyzed during home cage, hand-held and open field observations.
Motor activity evaluations in the square open field indicated that the acute exposure modified neither the number of squares crossed nor rearings on days 1 and 14 after the exposure. No significant differences were observed between control and experimental group in the number of squares crossed after the exposure or 14 days after it. The same was observed when we evaluated the rearings in the square open field.
When we analyzed the emotionality parameters as the number of groomings and fecal boluses, no measures demonstrated any significant differences between control and experimental group in both evaluated days.
The histopathological examinations of brain, liver, kidney, spleen, stomach, and intestine in all animals did not show any changes as consequence of the exposure.
Discussion
There are several factors that may induce ulcers in human beings, such as stress, chronic use of anti-inflammatory drugs and continuous alcohol ingestion, among others. Although in most cases the etiology of ulcer is unknown, it is generally accepted that it is the result of an imbalance between aggressive factors and maintenance of the mucosal integrity through an endogenous defense mechanism. The candidate for an effective drug against peptic ulcer should basically act either by reducing the aggressive factors on gastric mucosa or by increasing mucosal resistance against them.
Ethanol-induced gastric ulcer method has been widely used for the experimental evaluation of antiulcer activity. Disturbances in gastric secretion, damage in gastric mucosa, alterations in permeability, gastric mucus depletion and free radical production are reported to be the pathogenic effects of ethanol. Several experimental studies have demonstrated that oxygen-generated free radicals and lipid peroxidation play an important role in the pathogenesis of acute gastric lesions induced by this substance (CitationSalim, 1990).
Solidago chilensis was shown to be effective in reducing the gastric lesions in mice caused by an aggressive factor such as ethanol. The effects observed in this model of gastric ulceration support a possible mechanism of cytoprotection mediated by prostaglandins (CitationRobert et al., 1979a, Citation1979b).
Although none of the stomachs was completely prevented from lesions when animals received 125, 250 and 400 mg/kg of the aqueous extract, these doses displayed significant gastroprotection (61.4, 75.1 and 78.4%, respectively) as compared with the control. Groups exposed to 800, 1200 and 2000 mg/kg extract showed higher gastroprotection, achieving 91.5% inhibition at 2000 mg/kg dose. The latter may be the maximum achievable for this aqueous extract, since previous studies with higher doses did not increase the antiulcer activity (CitationBucciarelli et al., 2006).
Reactions performed on the plant extract revealed the presence of flavonoids. These compounds possess broad biological effects which include antiulcerogenic activity, which suggest that there could be, at least in part, active compounds responsible for the gastroprotective effect exhibited by the extract in this experimental model, although the action of other constituents present in the plant cannot be discarded. Several mechanisms have been proposed to explain their activity, such as an increase in prostaglandins mucosal content, decrease of histamine secretion, elimination of free radicals, increase in vascular perfusion and reduction of leukocyte adhesion (CitationBorrelli & Izzo, 2000). Some of them act as inhibitors of gastrointestinal motility, which is closely related to the ability of the extracts to remain more time in contact with the stomach wall, prolonging the cytoprotective effect. Thus, they prevent gastric mucosal lesions produced by different methods and protect the mucosa against various necrotic agents.
With regard to the acute toxicity of the extract, the mice exposed did not show alterations in all parameters evaluated in the FOB nor in motor activity at the end of exposure and 14 days later. This fact indicates that the evaluated extract does not produce neurotoxicity due to it does not affect the functionality of the nervous system at neuromuscular, sensory and autonomic level. The histopathological examinations also demonstrated the absence of abnormalities in all tissues studied. Considering the results in the toxicity study it was demonstrated that the oral administration of the aqueous extract of S. chilensis does not produce toxicity by acute exposure in mice. The lack of toxicity indicates that the possible therapeutic use of the extract may be safe, future research such as potential chronic toxicity associated with this extract will need to be evaluated through long-term bioassays in order to ensure its safety.
Conclusion
As this is the first report on the gastroprotection and toxicology of the inflorescences of the plant, a further investigation is being conducted to isolate and identify the potential antiulcerogenic compounds responsible for the reported gastroprotective activity in order to elucidate their mode of action. These promising results support a better pharmacological study of S. chilensis as a potential antiulcerogenic plant for studies targeted towards the development of new antiulcerogenic agents.
Acknowledgements
We thank Biochemist Ariel Gandini and Biochemist María Eugenia Fermento for their assistance in the histopathological studies.
Declaration of interest
This research was supported by a grant from Secretaría General de Ciencia y Tecnología, Universidad Nacional del Sur (PGI 24/B129).
References
- Alarcón de la Lastra C, Martín MJ, Motilva V (1994): Antiulcer and gastroprotective effect of quercetin. A gross and hystologic study. Pharmacology 48: 56-63.
- Apati P, Szentmihályi K, Balázs A, Baumann D, Hamburger M, Cristo TSz, Szőke E, Kéry A (2002): HPLC analysis of the flavonoids in pharmaceutical preparations from Canadian goldenrod (Solidago canadensis). Chromatographia 56: S65–68.
- Bader G, Wray V, Hiller K (1995): The main saponins from the aerial parts and the roots of Solidago virgaurea subsp. virgaurea. Planta Med 61: 158–161.
- Batyuk VS, Kovaleva SN (1985): Flavonoids of Solidago canadensis and Solidago virgaurea. Khimiia Prirodnykh Soedinenii 21: 566–567.
- Borrelli F, Izzo AA (2000): The plant kingdom as a source of antiulcer remedies. Phytother Res 14: 581–591.
- Bradette-Hébert ME, Legault J, Lavoie S, Pichette A (2008): A new labdane diterpene from the flowers of Solidago canadensis. Chem Pharm Bull 56: 82–84.
- Bucciarelli A, Mancini MM, Skliar MI (2006) Studies of gastroprotective activity of medicinal plants from southwestern Buenos Aires, in: Vaquero MC, Cazzaniga N, eds. Natural environment, countryside and city: Strategy use and conservation in southwestern Buenos Aires. Bahía Blanca, ediUNS, 155–160.
- Bucciarelli A, Skliar MI (2007): Medicinal plants from Argentina with gastroprotective activity. Ars Pharm 48: 361–369.
- Everhart JE, Byrd-Holt D, Sonnenberg A (1998): Incidence and risk factors for self-reported septic ulcer disease in the United States. Am J Epidemiol 147: 529–536.
- Goleniowski ME, Bongiovanni GA, Palacio L, Nuñez CO, Cantero JJ (2006): Medicinal plants from the “Sierra de Comechingones”, Argentina. J Ethnopharmacol 107: 324–341.
- Izzo AA, Di Carlo G, Mascolo N, Capasso F, Autore G (1994): Antiulcer effect of flavonoids. Role of endogenous PAF. Phytother Res 8: 179–181.
- Levenstein S (2000): The very model of a modern etiology: A biopsychosocial view of peptic ulcer. Psychosom Med 62: 176–185.
- Lorenzi H, Matos FJA (2002): Plantas Medicinais no Brasil: Nativas e Exóticas Cultivadas. São Paulo, Brasil, Instituto Plantarum de Estudos da Flora Ltda.
- MacPhail RC (1987): Observational batteries and motor activity. Zentralblatt fur Bakteriologie, Mikrobiologie und Hygiene, Series B 185: 21–27.
- McDaniel KL, Moser VC (1993): Utility of a neurobehavioral screening battery for differentiating the effects of two pyrethroids, permethrin and cypermethrin. Neurotoxicol Teratol 15: 71–83.
- Martín MJ, La Casa C, Alarcón de la Lastra C, Cabeza J, Villegas I, Motilva V (1998): Anti-oxidant mechanisms involved in gastroprotective effects of quercetin. Z Naturforsch 53c: 82–88.
- Martín MJ, Marhuenda E, Pérez-Guerrero C, Franco JM (1994): Antiulcer effect of naringin on gastric lesions induced by ethanol in rats. Pharmacology 49: 144–150.
- Moser VC, McCormick JP, Creason JP, MacPhail RC (1988): Comparison of chlordimeform and carbaryl using a functional observational battery. Fund Applied Toxicol 11: 189–206.
- Moser V, Ross J (1996): Training Video and Reference Manual for a Functional Observational Battery. United States Environmental Protection Agency and Industrial Health Council (USEPA/AIHC), Washington D.C., USA.
- Motilva V, Alarcón de la Lastra C, Martín MJ (1994): Ulcer-protecting effects of naringenin on gastric lesions induced by ethanol in rats: Role of endogenous prostaglandins. J Pharm Pharmacol 46: 91–94.
- OECD (2001): Acute Oral Toxicity - Acute Toxic Class Method. OECD Test Guideline for Testing of Chemicals, Section 4, Health Effects. OECD Guideline No. 423, Adopted December 17. Organization for Economic Cooperation and Development, Paris, France.
- Robert A, Nezamis JE, Lancaster C, Hanchar AJ (1979a): Cytoprotection by prostaglandins in rats: Prevention of gastric necrosis produced by alcohol, HCl, NaOH, hypertonic NaCl and thermal injury. Gastroenterology 77: 433–443.
- Robert A, Nezamis AE, Lancaster C, Hanchar AJ (1979b): Cytoprotection by prostaglandins. Gastroenterology 77: 761–767.
- Salim AS (1990): Removing oxygen-derived free radicals stimulates healing of ethanol-induced erosive gastritis in the rat. Digestion 47: 24–28.
- Silen W (1988): Experimental models of gastric ulceration and injury. Am J Physiol Gastrointest Liver Physiol 255: G395–G402.
- Steliopoulos P, Wüst M, Adam KP, Mosandl A (2008): Biosynthesis of the sesquiterpene germacrene D in Solidago canadensis: 13C and 2H labeling studies. Phytochemistry 60: 13–20.
- USEPA (1998) Neurotoxicity Screening Battery. EPA 712-C-98-238. Health Effects Test Guidelines. OPPTS 870.6200. United States Environmental Protection Agency, Washington D.C., USA.