Abstract
Context/objective: Herbal preparations derived from various species and parts of Echinacea (Asteraceae) have been advocated for various medical applications, as a result of the many antimicrobial and immunomodulatory activities attributed to them.
Materials and methods: In order to investigate their effects on parasites, four preparations of Echinacea, with distinct chemical compositions, were evaluated for growth inhibition of three species of trypanosomatids: Leishmania donovani, Leishmania major, and Trypanosoma brucei. In addition one Echinacea preparation was tested for anti-inflammatory activity in cell culture models designed to measure pro-inflammatory cytokines induced by L. donovani.
Results and discussion: All Echinacea preparations inhibited growth of the organisms, though with different relative potencies, and in some cases morphological changes were observed. However, there was no obvious correlation with the composition of the marker compounds, alkylamides, caffeic acid derivatives, and polysaccharides. L. donovani stimulated the production of the pro-inflammatory cytokines IL-6 and IL-8 in human bronchial epithelial cells and in human skin fibroblasts, but in both cases the standardized ethanol extract of E. purpurea (L.) Moench (Echinaforce®) abolished the stimulation, indicating anti-inflammatory activity of this extract.
Conclusions: Thus various Echinacea extracts can inhibit the proliferation of these parasites and at least one can reverse the pro-inflammatory activity of Leishmania donovani.
Introduction
Echinacea (Asteraceae) is one of the most popular herbal medicines for the treatment of cold and flu symptoms, and other respiratory disorders. In laboratory and animal studies extracts of several Echinacea species, particularly E. purpurea (L.) Moench. and E. angustifolia (D.C.), have been shown to possess a wide variety of bioactivities, including immune modulation, anti-inflammatory, antiviral, and antibacterial properties (CitationBarnes et al., 2005; CitationVimalanathan et al., 2005; CitationSharma et al., 2008 CitationVohra et al., 2009). However, similar studies on the effects of Echinacea on parasites have not been reported.
Leishmaniasis and trypanosomiasis are diseases caused by protozoans belonging to the Trypanosomatidae family: Leishmania and Trypanosoma. There are four forms of Leishmaniasis depending on the species of Leishmania responsible: cutaneous, diffuse cutaneous, mucocutaneous, and visceral Leishmaniasis (WHO, Citation2008a). Likewise there are two forms of trypanosomiasis depending on the species of Trypanosoma responsible: African trypanosomiasis (sleeping sickness) and American trypanosomiasis (Chagas disease).
Leishmania are mainly transmitted by the bite of an infected female phlebotomine sandfly (WHO, Citation2008a). In the sandfly, Leishmania are in the promastigote stage (flagellated protozoans), which later transform into amastigotes (non-flagellated protozoans) after they are engulfed by macrophages.
Similar to Leishmania, Trypanosoma brucei gambiense and Trypanosoma brucei rhodensiense (parasites responsible for African trypanosomiasis), or Trypanosoma cruzi (parasite responsible for American trypanosomiasis) are transmitted to humans by the bite of an infected tsetse fly (Glossina genus) or an infected assassin bug (subfamily Triatominae) respectively (WHO, Citation2008b). Procyclic (non-infective) trypanosomes multiply in the insect vector whereby they transform into metacyclic (infective) trypanosomes that are then injected into humans after a blood meal.
Both parasites cause widespread disease, with hundreds of thousands of new cases each year. Although drugs are available for the treatment of different stages of the diseases, they are frequently associated with severe side effects (CitationHay et al., 2008; WHO Citation2008a, Citation2008b). However, some recent studies have examined antiparasitic properties of several plant extracts, with promising results (CitationAtawodi et al., 2003; CitationBraga et al., 2007; CitationGamboa-Leon et al., 2007; CitationDesrivot et al., 2007; CitationHay et al., 2008). But similar studies with Echinacea have not been reported.
We therefore decided to investigate possible antiparasitic properties of several standardized commercial preparations of Echinacea. In addition, since we know that Echinacea can act as an anti-inflammatory agent in cells exposed to various viruses and bacteria (CitationSharma et al., 2006, Citation2008, Citation2009), we tested for possible anti-inflammatory activity (anticytokine activity) in cells exposed to the parasites.
Materials and methods
Echinacea extracts
Commercial Echinacea preparations from the aerial and/or root portions of Echinacea purpurea and Echinacea angustifolia (EA, EP-2, and EP-3) were obtained from J.T. Arnason (University of Ottawa), who also performed the HPLC analyses (). Complete details on the origin and chemical analyses of these preparations were given in CitationVohra et al. (2009). EA, EP-1, and EP-2 were coded respectively as brands D1-60, IL-1 and Il-12 (CitationVohra et al., 2009). EP-1 was a standardized commercial extract of E. purpurea (Echinaforce®, A. Vogel/Bioforce, Roggwil, Switzerland). It was also analyzed by HPLC. The samples were stored at 4°C in tubes wrapped with foil (to prevent light exposure) during the experimental study. These extracts were chosen based on their representation of various types of commercial Echinacea extracts and different compositions of marker compounds (CitationVohra et al., 2009). EP-1 and EA were ethanol extracts containing alkylamides (PID 8/9) and little or no polysaccharide. On the other hand, EP-2 and EP-3 were aqueous extracts rich in polysaccharides but lacking alkylamides. Caffeic acid derivatives were present in accordance with expected compositions (CitationBauer, 1998; CitationBinns et al., 2002). shows the origins of the extracts and the composition of these standard markers.
Table 1. Composition of Echinacea extracts.
Parasite cultures
L. donovani promastigotes were cultured at 26°C in M199 medium (Sigma, M4530, St. Louis MO) supplemented with 10% fetal bovine serum (FBS) (Sigma), 25 mM HEPES (Stem Cell Technologies), penicillin and streptomycin (100μg/mL) (Stem Cell Technologies Vancouver, BC, Canada), 25 mM adenosine, hemin (6 μg/mL) and folic acid. Exponentially growing cells were maintained at 26°C in non-vented culture flasks (Corning Inc. New York, USA). Procyclic T. brucei, and the growth medium, were obtained from Terry Pearson (University of Victoria, BC). They were cultured at 26°C.
Proliferation assay
Exponentially growing Leishmania promastigotes (day 3 growth) were resuspended at 2 × 106 cells/mL after hemocytometer counting with Trypan blue (Sigma). The cell suspension (1 mL) was pipetted into each well of a 24-well plastic tissue culture plate (Corning). Echinacea extracts were then pipetted directly into the wells and immediately mixed with the pipette tip to prevent possible local concentration effects. Possible secondary effects of ethanol on the organisms were also assessed by adding equivalent amounts of 65% or 48% ethanol solutions (95% ethanol diluted accordingly in sterile distilled water) in accordance with the EP-1 and EA extracts, which contained 65% and 48% ethanol, respectively. The plates were then sealed around the edges with parafilm to limit airflow and incubated at 26°C for 24, 48, or 72 h, after which cell proliferation was assayed using hemocytometer counting with Trypan blue. Growth controls were assessed by culturing Leishmania in the absence of Echinacea or ethanol. The effect of Echinacea or ethanol on cell proliferation was then expressed as the percent of the control cultures for each time point.
Trypanocidal activity of Echinacea was assayed in a similar manner described above; procyclic T. brucei were resuspended at 2 × 106 cells/mL after hemocytomer counting with Trypan blue (Sigma). This cell suspension (0.5 mL) was pipetted into 1.5 mL sterile microfuge tubes. Microfuge tubes were used because T. brucei do not grow well in the 24-well tissue culture plates used for L. donovani. Means and standard deviation (SDM) values were calculated for all replicate samples.
Anti-inflammatory activity
Details of the test system were described previously (CitationSharma et al., 2008, Citation2009). BEAS-2B human epithelial cells, originally obtained from ATCC (American Type Culture Collection, Rockville, MD), were grown in Dulbecco MEM (DMEM) in 10% fetal bovine serum. For the experiments, cells were sub-cultured and grown to confluency in 6-well trays, after which the medium was changed to DMEM without serum. Under these conditions the cultures remained viable for at least 5 days. Human skin fibroblasts (courtesy of Aziz Ghahary) in their sixth passage were also cultivated in DMEM with 10% serum. No antibiotics or antimycotic agents were used. Rhinovirus type 1A (RV1A from ATCC) was propagated and assayed, by plaque assay, in H-1 cells (CitationSharma et al., 2008). The stock virus had a titer of 1 × 108 pfu/mL.
Cells were infected with 58-64 × 106 /mL of L. donovani (approximately 50 organisms per epithelial cell), or with 1 pfu/cell of RV1A as positive control, for 1 h, followed by Echinacea or medium for 48 h. Cell free supernatants were then removed for cytokine assay by ELISA which was carried out according to the instructions supplied by the companies (either R&D Systems, Minneapolis, MN, for IL-8, or e-Bioscience, San Diego CA, for IL-6). Absorbance readings at 540 nm were converted to pg/mL cytokine by means of standard curves.
Results
The growth and viability of each organism were measured in the presence and absence of each of the test Echinacea extracts, or the appropriate solvent controls, and also the ability of selected extracts to inhibit Leishmania donovani induced pro-inflammatory cytokine secretion. The origins and phytochemical characteristics of the extracts are summarized in .
Inhibition of growth of Leishmania promastigotes
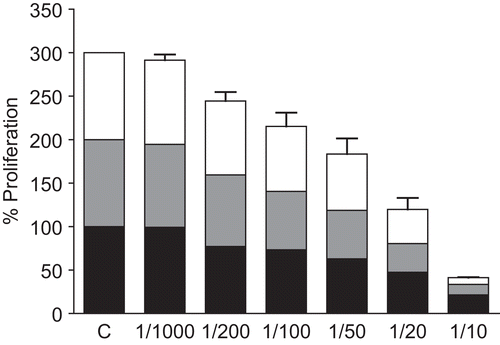
shows the inhibitory effect of increasing concentrations of aqueous Echinacea extract EP-3 on the growth of L. donovani. At 1:1000 dilution the cell number was indistinguishable from the untreated control; but increasing concentrations of the extract resulted in proportionately greater inhibition in growth at all three time points examined. At 1:10 dilution (10 mg/mL) the cell numbers were only 21%, 12%, and 8% of controls at 24, 48, and 72 h, respectively (). Furthermore, at this concentration the treated cells appeared rounded with minimal movement. Similar results were obtained for the other aqueous Echinacea extract EP-2 (data not shown).
Figure 1. Effect of aqueous Echinacea extract EP-3 on the extracellular proliferation of L. donovani. Exponentially growing L. donovani were incubated for 24, 48 and 72 h with the indicated dilutions of Echinacea extract EP-3. Control Leishmania were grown under identical conditions without Echinacea. At each time point the numbers of motile surviving parasites were counted by Trypan blue exclusion. The data shown are representative of three independent experiments: lower black bars, 24 h; grey bars, 48 h; upper white bars, 72 h.
In comparison, L. major appeared to be somewhat more resistant to EP-3, in spite of their equivalent concentrations (). In this case the final cell counts at 24, 48, and 72 h were 56%, 54%, and 35% of controls, respectively. In addition the treated cells appeared to have normal morphology though slower movements.
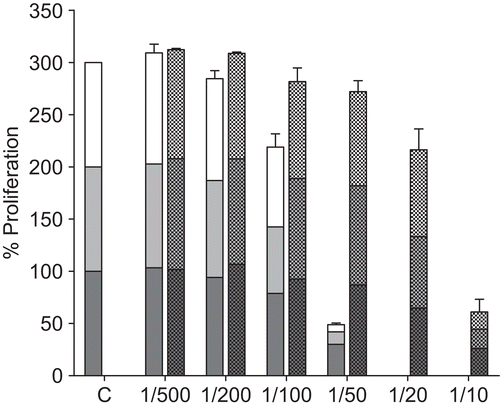
The ethanol extracts also showed inhibition of L. donovani and L. major proliferation. shows the results for L. donovani and extract EA. Dilutions of 1:500 and 1:200 showed little effect, but inhibition was evident in concentrations above 1:100 (0.9 mg/mL). The ethanol solvent was also inhibitory at 1:20 or greater concentrations (>5% v/v). At 1:50 the cell numbers were unaffected by ethanol, but Echinacea-treated cell numbers were reduced to 30%, 12%, and 7% of controls at 24, 48, and 72 h, respectively (). Corresponding values for L. major were 59%, 26%, and 10%. Morphologically, the cells treated with 1:50 dilution (1.8 mg/mL) were rounded and slower moving compared with control cells. The other ethanol extract, EP-1, showed similar responses (data not shown).
Figure 2. Effect of ethanol Echinacea extract EA on the extracellular proliferation of L. donovani. Exponentially growing L. donovani were incubated for 24, 48 and 72 h with the indicated dilutions of EA (left hand bars; lower black bars, 24 h; middle grey bars, 48 h; upper white bars, 72 h). Controls consisted of dilutions of ethanol corresponding to those in the diluted extracts (right hand bars; lower bars, 24 h; middle bars, 48 h; upper bars, 72 h). At each time point the numbers of motile surviving parasites was counted by Trypan blue exclusion. The data shown are representative of three independent experiments.
Inhibition of growth of trypanosomes
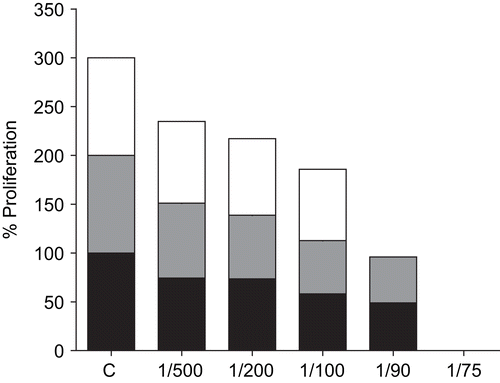
shows the inhibitory effect of extract EP-3 on proliferation of T. brucei. Progressive inhibition was evident with increasing concentrations of extract, and at 1:50 or greater (>2 mg/mL) the effect was completely trypanocidal. Under these conditions the cells were non-motile and curled into balls. Extract EP-2 showed similar results (data not shown).
Figure 3. Effect of aqueous Echinacea extract EP-3 on the extracellular proliferation of T. brucei. Exponentially growing T. brucei were incubated for 24, 48 and 72 h with the indicated dilutions of Echinacea extract EP-3. Control trypanosomes were grown under identical conditions without Echinacea. At each time point the numbers of motile surviving parasites was counted by Trypan blue exclusion. The data shown are representative of three independent experiments: lower black bars, 24 h; grey bars, 48 h; upper white bars, 72 h.
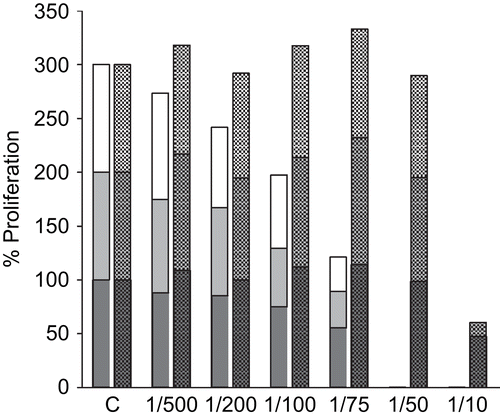
shows the effects of the ethanol extract EA. The higher concentrations were again trypanocidal, although the ethanol solvent itself also contributed to the inhibitory effect at the highest concentration tested (1:10). The other ethanol extract EP-1 gave similar results (data not shown).
Figure 4. Effect of ethanol Echinacea extract EA on the extracellular proliferation of T. brucei. Exponentially growing T. brucei were incubated for 24, 48 and 72 h with the indicated dilutions of Echinacea extract EA (left hand bars; lower black bars, 24 h; middle grey bars, 48 h; upper white bars, 72 h). Controls consisted of dilutions of ethanol corresponding to the diluted extracts (right hand bars; lower bars, 24 h; middle bars, 48 h; upper bars, 72 h). At the end of the experiment, the number of motile surviving parasites was counted by Trypan blue exclusion. The data shown are representative of three independent experiments.
Inhibition of pro-inflammatory activity
In order to investigate the possibility of Leishmania induction of pro-inflammatory cytokines and the anti-inflammatory effect of Echinacea, we used the model system characterized in our previous reports (CitationSharma et al., 2006, Citation2009), in which RV1A and other viruses were shown to induce substantial levels of secretion of IL-6 and IL-8 (CXCL8) at 48 h post infection.
IL-6 and IL-8 secretion were measured by ELISA tests in control uninfected cells, cells plus Echinacea, and L. donovani or RV1A infected cells with and without Echinacea. L. donovani induced the secretion of both IL-6 and IL-8, in BEAS-2B bronchial epithelial cells and in human skin fibroblasts, and Echinacea was very effective in inhibiting these responses. In many cases the Leishmania + Echinacea values were indistinguishable from control uninfected cells (). There were no evident L. donovani-induced cytopathic effects within 48 hours post infection. In one experiment the supernatants were also evaluated by means of the Quantibody® cytokine array system (CitationSharma et al., 2009). Leishmania showed stimulation of IL-6 and IL-8, in agreement with the ELISA results, and again EP-1 abolished this stimulation (data not shown).
Table 2. Effect of Echinacea extract EP-1 on induced secretion of pro-inflammatory cytokines.
Discussion
All four Echinacea preparations exhibited dose-dependent antileishmanial and trypanocidal activities after 24, 48 and 72 h incubation. However, the relative potency of Echinacea varied depending on the preparations used. Of the Echinacea preparations tested on L. donovani, the 48% ethanol extract EP-1 (E. angustifolia) was the most effective. In contrast, the aqueous extract EP-3 (E. purpurea) proved to be the most potent against T. brucei.
It was anticipated that different Echinacea preparations might have different effects on the protozoans tested. Research has shown that the efficacy of Echinacea preparations depends on their chemical composition, wherein different concentrations of components either alone or together can result in different and even opposing immunomodulating effects (CitationMatthias et al., 2008; CitationVimalanathan et al., 2009). Therefore, a next step to this study would be to further analyze the chemical compositions of the Echinacea extracts to determine which compounds in the preparations are responsible for the antileishmanial and/or trypanocidal activities. It should be noted that concentrations of ethanol alone showed some negative effect on the proliferation of L. donovani and T. brucei. Nevertheless, incubation with the ethanol Echinacea extracts resulted in enhanced inhibition of proliferation. It is possible that these compounds work synergistically, in which case differing relative amounts of the compounds in Echinacea preparations may result in an extract with high antileishmanial and/or trypanocidal activities.
Despite being classified as trypanosome protozoans, the different results observed between L. donovani and T. brucei suggest that Echinacea acts on or involves structures that are different between Leishmania and Trypanosoma. Research has shown that plant extracts can have different trypanocidal effects depending on the species tested (CitationAtawodi et al., 2003). Further tests of the Echinacea preparations with other species of Leishmania or Trypanosoma may show whether the effects of Echinacea are also species-dependent.
The mode of action of Echinacea on these parasites is not known at present. However, morphological observations were made and these show that Echinacea slowed/eliminated their motility and caused rounding of both L. donovani and T. brucei at high concentrations. Observing the cellular structures of Echinacea-treated parasites by electron microscopy could provide valuable insights to its mode of action.
It is questionable whether Echinacea negatively affects proliferation by killing the parasites (which would then question whether it stimulates apoptosis or necrosis) or through growth inhibition. For example, 1/10 dilution of EP-3 treated L. donovani promastigotes showed that upon re-suspension in M199 media lacking Echinacea (after 5 days incubation with EP-3), the cells were able to recover over a period of four days (data not shown). More of these tests need to be carried out with the other Echinacea extracts and with T. brucei.
L. donovani also showed pro-inflammatory activity by stimulating the secretion of IL-6 and IL-8 (CXCL8) in two different human cell lines, a bronchial epithelial line and a skin fibroblast line. Similar stimulation of these cytokines was shown in these cell lines by a variety of other viral and bacterial pathogens (CitationSharma et al., 2008, Citation2009). In both cell types, the selected Echinacea preparation (EP-1) inhibited these Leishmania-induced responses, as it did for the viral inducers.
Thus certain Echinacea preparations are capable of controlling growth of these parasites, and in at least one case can inhibit the inflammatory activity induced by them.
Declaration of interest
No funding was received for this study. All authors declare no conflict of interest
References
- Atawodi SE, Bulus T, Ibrahim S, Ameh DA, Nok AJ, Mamman M, Galadima M (2003): In vitro trypanocidal effect of methanolic extract of some Nigerian savannah plants. Afr J Biotechnol 2: 317–321.
- Barnes J, Anderson LA, Gibbons S, Phillipson JD (2005): Echinacea species (Echinacea angustifolia (DC.) Hell. Echinacea pallida (Nutt.) Nutt. Echinacea purpurea (L.) Moench.: A review of their chemistry, pharmacology and clinical properties. J Pharm Pharmacol 57: 929–954.
- Barrett B (2003): Medicinal properties of Echinacea: A critical review. Phytomedicine 10: 66–86.
- Bauer R (1998): Echinacea: Biological effects and active principals. Phytomedicines of Europe: Chemistry and biological activity, in: Lawson LD, Bauer R, eds, ACS symposium series 691. Washington DC, American Chemical Society, pp. 140–157.
- Binns SE, Livesey JF, Arnason JT, Baum BR (2002): Phytochemical variation in Echinacea from roots and flower heads of wild and cultivated populations. J Agric Food Chem 50: 3673–3687.
- Braga FG, Bouzada MLM, Fabri RL, Matos MO, Moreira FO, Scio E, Coimbra ES (2007): Antileishmanial and antifungal activity of plants used in traditional medicine in Brazil. J Ethnopharmacol 111: 396–402.
- Desrivot J, Waikedre J, Cabalion P, Herrenknecht C, Bories C, Hocquemiller R, Fournet A (2007): Antiparasitic activity of some New Caledonian medicinal plants. J Ethnopharmacol 112: 7–12.
- Gamboa-Leon MR, Aranda-Gonzalex I, Mut-Martin M, Garcia MR, Dumonteil E (2007): In vivo and in vitro control of Leishmania mexicana due to garlic-induced NO production. Scand J Immunol 66: 508–514.
- Hay A-E, Merza J, Landreau A, Litaudon M, Pagniez F, Pape PL, Richomme P (2008): Antileishmanial polyphenols from Garcinia vieillardii. Fitoterapia 79: 42–46.
- Matthias A, Banbury L, Bone KM, Leach DN, Lehmann RP (2008): Echinacea alkylamides modulate induced immune responses in T-cells. Fitoterapia 79: 53–58.
- Sharma M, Arnason JT, Burt A, Hudson JB (2006): Echinacea extracts modulate the pattern of chemokine and cytokine secretion in rhinovirus-infected and uninfected epithelial cells. Phytother Res 20: 147–152.
- Sharma M, Schoop R, Hudson JB (2008): Echinacea as an antiinflammatory agent: The influence of physiologically relevant parameters. Phytotherapy Res 23: 863–867.
- Sharma M, Anderson S, Schoop R, Hudson JB (2009): Induction of multiple pro-inflammatory cytokines by respiratory viruses and reversal by standardized Echinacea, a potent antiviral herbal extract. Antiviral Res 83: 165–170.
- Vimalanathan S, Kang L, Treyvaud Amiguet V, Livesey J, Arnason JT, Hudson J (2005): Echinacea purpurea aerial parts contain multiple antiviral compounds. Pharm Biol 43: 740–745.
- Vimalanathan S, Arnason JT, Hudson JB (2009): Anti-inflammatory activities of Echinacea extracts do not correlate with traditional marker components. Pharm Biol 47: 430–435.
- Vohra S, Adams D, Hudson JB, Moore JA, Vimalanathan S, Sharma M, Burt AJ, Lamont E, Lacaze N, Arnason JT, Lee TDG (2009): Selection of natural health products for clinical trials: A preclinical template. Can J Physiol Pharmacol 87: 371–378.
- WHO (World Health Organization) (2008a): Leishmaniasis. Available At http://www.who.int/leishmaniasis/disease_epidemiology/en/index.html (accessed April 28, 2008).
- WHO (World Health Organization) (2008b) African trypanosomiasis (sleeping sickness). Available at http://www.who.int/mediacentre/factsheets/fs259/en/ (accessed April 28, 2008).