Abstract
Context: Woodfordia fruticosa Kurz. (Lythraceae), a non-rasayana immunomodulatory Indian medicinal plant, used traditionally as an anthelmintic, in dysentery, leprosy, blood diseases, leucorrhea, and menorrhagia.
Objective: To investigate the effect of ethanol extract of W. fruticosa flowers on non-specific immune responses in mice.
Materials and methods: In vitro immunomodulatory activity of the extract was examined on murine peritoneal macrophage phagocytosis (nitroblue tetrazolium (NBT) dye reduction, lysosomal enzyme activity, nitric oxide and myeloperoxidase) and on proliferation of bone marrow cells by sulforhodamine B (SRB) assay, while the in vivo potential on macrophages and bone marrow cells was evaluated by using carbon clearance test and cyclophosphamide-induced myelosuppression, respectively.
Results: Significant increase in the release of myeloperoxidase, nitric oxide lysosomal enzyme and superoxide from macrophages along with significant increase in phagocytic index in carbon clearance test indicate stimulatory activity of the extract on macrophages. The extract also demonstrated 60% increase in bone marrow cell proliferation and offer protection towards cyclophosphamide-induced myelosuppression which represents the stimulation of bone marrow activity.
Discussion: Significant increase in mediators released from macrophages and phagocytic index in carbon clearance test suggests the release of cytokines from macrophages and stimulation of reticulo-endothelial system. Proliferation of bone marrow cells indicates the plausible release of colony stimulating factors, which further stimulates the immune system through generation of immune cells.
Conclusion: The result described here indicates the immunostimulatory activity of ethanol extract of W. fruticosa flowers by stimulating non-specific immune responses, macrophages and bone marrow cells.
Introduction
Woodfordia fruticosa Kurz. (Lythraceae), a non-rasayana immunomodulatory Indian medicinal plant, is a plant of tropical and subtropical regions with a long history of medicinal use. A wide range of bioactive compounds including tannins (especially those of macrocyclic hydrolysable class), flavonoids, anthraquinone glycosides, and polyphenols have been isolated from this species in recent times. Extracts and metabolites of this plant, particularly those from flowers and leaves, possess some useful medicinal properties (CitationPratap et al., 2007).
Although different parts of the plant have long been recognized in folklore medicine, there is a heavy demand for the flowers, both in domestic and international markets specialized in the preparation of herbal medicines (CitationOudhia, 2003). According to the Indian systems of medicine, flowers of this plant have pungent, acrid, cooling, toxic, alexiteric properties and are used as a uterine sedative and as an anthelmintic. These flowers are also useful in thirst, dysentery, leprosy, blood diseases, leucorrhea, menorrhagia, and toothache. Further, it is considered as “kapha” (mucilage-type body secretion) and “pitta” (energy-dependent metabolic activity) suppressant in the Ayurvedic concepts of medicine (CitationSharma, 1956). The flowers are used in the preparation of Ayurvedic fermented drugs called “aristhas” and “asavas” (CitationAtal et al., 1982). Aristhas are believed to be general health tonics in nature, having overall health stimulating properties via ameliorating and/or delaying one or other systemic disorders. Of the 18 aristhas mentioned in the Indian Ministry of Health & Family Welfare’s monograph (CitationCCRIMH, 1978), 17 have been found to contain Woodfordia fruticosa. Tribal people in the Chhattisgarh district of central India will use fresh flowers to stop bleeding in emergency cuts, but they prefer to employ dried flower powder to heal wounds more efficiently.
A study conducted by CitationKroes et al. (1993) also reported an immunomodulatory activity of the Ayurvedic drug nimba aristha, which contains Woodfordia fruticosa flowers. Substantial increase in the inhibition of both human complement activity and chemoluminescence generated by zymogen-stimulated human polymorphonuclear leukocytes was observed. It was demonstrated that such increased biological activity was attributed to an immunoactive constituents released from the flowers of Woodfordia fruticosa (CitationKroes et al., 1992). In addition to this, a popular crude drug (called sidowaya or sidawayah) of Indonesia and Malaysia mainly contains dried flowers of Woodfordia fruticosa (CitationBurkill, 1966). It has been used as an astringent to treat dysentery and sprue, and also for the treatment of bowel complaints, rheumatism, dysuria and hematuria in many southeast Asian countries. Further, it is also one of the ingredients of a preparation used to increase fertility in women (CitationBurkill, 1966; CitationDey, 1984).
In light of the above facts, as a part of our continuous research, we have made an attempt to investigate the immunomodulatory potential of the ethanol extract of Woodfordia fruticosa flowers in mice.
Materials and methods
Plant material and extraction
The fresh flowers of Woodfordia fruticosa were collected in the month of January from Kasara, Maharashtra state; India. Flowers of Woodfordia fruticosa were identified and authenticated by Ganesh Iyer, Botanist, Ramnarayan Ruia College, Mumbai, India. They were shade-dried, freed from their stalks and pulverized with the help of an electric grinder to get a free-flowing coarse powder. The coarse powder of the dried flowers was defatted with petroleum ether and then exhaustively extracted using ethanol in a Soxhlet extractor. The obtained ethanol extract was concentrated in a rotary evaporator and dried at 40°C in an oven. The yield of ethanol extract was found to be 20% (w/w of dried flower powder). The freshly prepared solution of ethanol extract was utilized for the further treatment of animals.
Experimental animals
Albino mice (20-22 g) of either sex were used for immunomodulatory activity. The animals were housed under good hygienic conditions in the departmental animal house. They were housed under standard conditions of temperature (22° ± 2°C), 12 h light and dark cycle, fed with standard pellet diet (Amrut, Pune) and had access to water ad libitum. All the experiments were performed in accordance with the Institutional Animal Ethics Committee (IAEC) constituted as per directions of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), under the Ministry of Animal Welfare Division, Government of India, New Delhi. The provided animal house registration number of our institute is 87/1999/CPCSEA.
Chemicals
Ovalbumin, Freund’s complete adjuvant and TMB/H2O2 were procured from Bangalore Genei (Karnataka). Streptomycin, penicillin and HEPES buffer were purchased from Himedia, Mumbai, India. Fetal bovine serum (FBS) and phytohemagglutinin (PHA) were procured from Sigma Aldrich (St. Louis, MO). All other chemicals were procured from standard local sources.
Isolation of peritoneal macrophages and culture conditions
Peritoneal macrophages were obtained from mice that had been injected intraperitoneally 3 days prior with 2 mL 3% thioglycolate broth (Himedia, Mumbai). Three days later the peritoneal exudates were collected in RPMI-1640 medium containing 10% FBS, 20 μM 2-mercaptoethanol, 100 U/mL penicillin, 100 μg/mL streptomycin and 25 mM HEPES buffer. The peritoneal exudate cells (PEC) were centrifuged at 1000 rpm, 25°C for 20 min and erythrocytes were lysed by hypotonic lysis. The mixture was again centrifuged and the cell pellets were washed twice and resuspended in complete RPMI-1640 medium. The cell numbers were determined by a hemocytometer and cell viability was tested by Trypan blue dye exclusion technique. The collected cells were then adjusted to required cell counts per mL, and seeded into a 96-well plate with RPMI-1640 medium. The cells were then cultured at 37°C for 2 h under a humidified atmosphere of 95% air and 5% CO2. The growth medium was replaced to a sample dissolved in the medium containing 0.1% DMSO, and it was then maintained for 24 h under the same condition.
Nitric oxide assay
Nitric oxide (NO) production was determined by assaying culture supernatants for nitrite using Griess reagent by the method of CitationKeller et al. (1990). PEC at 5 × 106cells/mL was incubated with different concentrations of the extract and PHA 100 μg/mL for 24 h at 37°C in 5% CO2 atmosphere. Cell-free supernatant (75 μL) was mixed with 75 μL of Griess reagent (sulfanilamide 1%, phosphoric acid 5%, and naphthylethylenediamine 0.1%) and incubated at room temperature for 10 min. Cells incubated with PHA (100 μg/mL) were used as a positive control. After incubation, the absorbance of the wells was determined by using an ELISA reader (Biotek Mumbai, Maharashtra, India) equipped with a 540 nm filter.
Superoxide assay: Nitroblue tetrazolium dye reduction
The reduction of nitroblue tetrazolium (NBT) to insoluble blue formazan was used as a probe for superoxide generation (O2−) (CitationAuclair & Voisin, 1985). A 0.3% NBT solution (50 μL) in the medium was distributed in the wells. Following incubation for 2 h, the supernatants were removed and the macrophages were fixed by the addition of 200 μL of absolute methanol, washed twice with 70% methanol and then dried. The formazan deposits were solubilized in 120 μL 2 M KOH and 140 μL DMSO. Following homogenization of the contents in the wells, the extinction was read at 630 nm.
Measurement of cellular lysosomal enzyme activity
The determination of lysosomal enzyme activity was carried out using the cultured macrophage monolayers in a 96-well plate (1.5 × 105 cells/well). The cultured macrophages were solubilized by the addition of 25 μL of 0.1% triton X-100. Following incubation at room temperature for 15 min, 100 μL of 10 mM p-nitrophenyl phosphate as a substrate for acid phosphatase, and thereafter 0.1 M citrate buffer (50 μL, pH 5) was added respectively. After incubating for 1 h at 37°C, 0.2 M borate buffer (150 μL, pH 9.8) was added to the mixture to end the reaction. Finally, the absorbance was measured at 405 nm (CitationSuzuki et al., 1990).
Measurement of myeloperoxidase activity
The cells (5 × 106 cells/mL) were washed three times with fresh medium, and the mixture (100 μL) of O-phenylene diamine (0.4 mg/mL) and 0.002% H2O2 in phosphate-citrate buffer (pH 5) was added. The reaction was stopped after 10 min using 0.1 N H2SO4, and the optical density was measured at 490 nm (CitationPinegin et al., 1995).
Preparation of mouse bone marrow cells
In order to perform in vitro assays, mice were sacrificed by spinal dislocation under light ether anesthesia. The femur bone was isolated aseptically and bone marrow cell suspension was prepared by means of flushing (CitationManosroi et al., 2003). The cell suspension was centrifuged and the cell pellets were washed twice and resuspended in complete RPMI-1640 medium. The cell numbers were determined by a hemocytometer and cell viability was tested by Trypan blue dye exclusion technique. The final volume was adjusted to concentration of 1.5 × 106 cells/mL. The cells were cultured at 37°C for 3 h under a humidified atmosphere of 95% air and 5% CO2. The growth medium was replaced to a sample dissolved in the medium containing 0.1% DMSO, and then it was maintained for 48 h under the same conditions.
Cell proliferation assay
After 48 h, cells were fixed by adding ice-cold 50% trichloroacetic acid (TCA) and incubating for 1 h at 4°C. The plates were washed with distilled water, air-dried and stained with SRB solution (0.4% w/v in 1% acetic acid) for 30 min at room temperature. Unbound SRB was removed by washing thoroughly with 1% acetic acid and the plates were air-dried. The bound SRB stain was solubilized with 100 μL of 10mM Tris buffer, and the optical density was read at 540 nm by using UV visible spectrophotometer (CitationAjaya Kumar et al., 2004).
Acute toxicity study
Acute oral toxicity study with ethanol extract of W. fruticosa was carried out in albino mice in accordance with the OECD guidelines no. 425 and the extract was found to be safe up to 2000 mg/kg.
Phagocytic activity
Phagocytic index was determined as per the method reported by CitationGonda et al. (1990). Mice were divided into three groups of six animals each. Group I, the control group, received 0.2% sodium carboxymethyl cellulose solution only as vehicle; while animals of groups II and III were given treatment of test extract (250 and 500 mg/kg p.o. suspended in 0.2% sodium carboxymethyl cellulose) daily for 20 days, respectively. Carbon ink suspension was injected via tail vein into each mouse 48 h after the 20 days treatment. Blood samples were drawn from the orbital vein at 0 and 15 min. Blood (25 μL) was mixed with 0.1% sodium carbonate (2 mL) and subjected for determination of optical densities at 660 nm. The phagocytic index “K” was calculated by using following equation:
where OD1 and OD2 are the optical densities at times t1 and t2, respectively.
Cyclophosphamide-induced myelosuppression
Cyclophosphamide-induuced myelosuppression was studied according to the method described by CitationManjarekar et al. (2000). Animals were divided into four groups of six animals each. The negative control group and normal group received sodium carboxymethylcellulose solution only as vehicle daily for 16 days while animals in treatment groups were given the test extract (250 and 500 mg/kg p.o.) suspended in sodium carboxymethylcellulose daily for 16 days. On days 17, 18, 19 all the animals except those in the normal group were injected with cyclophosphamide (30 mg/kg i.p.) 1 h after administration of the extracts. Blood samples were collected by retro-orbital plexus under ether anesthesia on day 20 and total white blood cell (WBC) count was determined.
Statistical analysis
Results in the present study are expressed as mean ± standard deviation (SD). Statistical analysis was performed using a one-way analysis of variance (ANOVA) followed by Dunnett’s test for multiple comparisons. P < 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad InStat 3.06 software.
Results
Nitrite and superoxide production
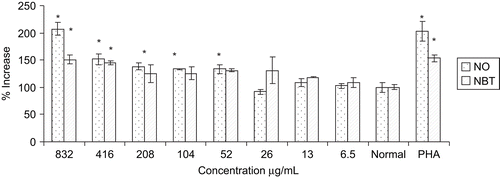
Increase in the nitrite and superoxide production has a significant effect on the function of macrophages which subsequently results in increase in the cytotoxic activity of the macrophages. Effects of different concentrations of the extract on the superoxide generation in terms of the reduction of NBT dye and nitrite release in culture supernatant were demonstrated in . The extract showed significant increase in the NO production dose-dependently from peritoneal macrophage at 208, 416 and 832 μg/mL concentration with 38, 51, and 107%, respectively as compared to control. The extract also increased superoxide production significantly at 416 and 832 μg/mL with 44 and 51%, respectively, compared to control. The extract at 832 μg/mL showed similar effects on NO and superoxide generation to that of positive standard PHA 100 μg/mL.
Myeloperoxidase enzyme activity
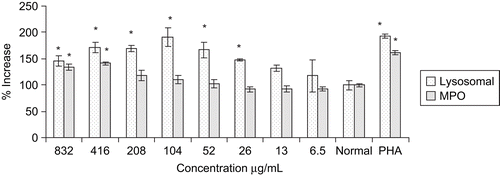
Myeloperoxidase produces hypochlorous acid (HOCl) and hydrogen peroxide to exhibit cytotoxic effect. In the present study, the extract showed maximum increase in the enzyme activity (41%) at 416 μg/mL. ().
Cellular lysosomal enzyme activity
The extract significantly increased the release of cellular lysosomal enzyme from peritoneal macrophages. The significant release of enzyme started at 26 μg/mL and reached maximum at 104 μg/mL. At a concentration 104 μg/mL, the extract exhibited 91% increase in cellular lysosomal activity as compared to control (), which was similar to that of PHA, a potent activator of macrophages.
Bone marrow proliferation assay
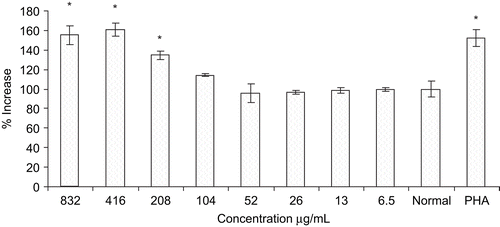
Effects of plant extract on bone marrow proliferation are shown in . The extract showed significant increased in the proliferation of bone marrow cells at the concentrations of 208, 416, and 832 μg/ml with 35, 60, and 55%, respectively, which was similar to 54% of PHA at 100 μg/mL. The extract demonstrated cell proliferation stimulation in a dose- response manner up to 416 μg/mL.
Carbon clearance test
Ethanol extract of W. fruticosa flowers possess macrophage stimulatory activity as evidenced by increased phagocytic index in the carbon clearance test. The phagocytic activity of the reticulo-endothelial system is generally measured by the rate of removal of carbon particles from the blood stream. The phagocytic index for the control group was found to be 0.064, whereas the extract increased it to 0.087 and 0.108 at a doses of 250 and 500 mg/kg, respectively ().
Table 1. Effect of ethanol extract of Woodfordia fruticosa flowers on phagocytic index and WBC count in cyclophosphamide induced myelosuppression.
Cyclophosphamide-induced myelosuppression
Administration of cyclophosphamide significantly lowered the levels of total WBC (5180) as compared to normal control (9180) in blood. Ethanol extract demonstrated a significant protection in cyclophosphamide-induced myelosuppression at a dose of 500 mg/kg as evidenced by increasing levels of total WBC count ().
Discussion
Modulation of the immune response through stimulation or suppression may help in maintaining a disease-free state. Agents that activate host defense mechanisms in the presence of an impaired immune responsiveness can provide supportive therapy to conventional chemotherapy (CitationWagner & Proksch, 1985). The results obtained in the present study indicate that an extract possesses immunostimulating property which is attributed to stimulation of macrophages and bone marrow cells in vitro and in vivo.
Macrophages on activation produce a large number of cytotoxic molecules, i.e., superoxide (O2−), H2O2 and HOCl via release of various mediators like NO, superoxide and myeloperoxidase. In our study, we found that the extract induced NO in mouse peritoneal macrophages, with highest release at 832 μg/mL, which is comparable to that of positive standard PHA. It has been reported that NO is synthesized by NO synthase (NOS) (CitationXie et al., 1992) and mediates diverse functions, including vasodilation, neurotransmission and inflammation (CitationLowenstein & Synder, 1992). Further, NO has been shown to be the principal effector molecule produced by macrophages for cytotoxic activity and can be used as a quantitative index of macrophage activation (CitationDing et al., 1988). In the present study, macrophages incubated with an extract at concentrations of 6.5-832 μg/mL for 24 h, showed a significant and dose-dependent activation of macrophages by modulating the secretion of mediators responsible for the observed cytotoxicity.
The extract also showed potent cellular lysosomal enzyme activity on murine peritoneal macrophages which is accountable for lysis of antigen and assists in presenting the antigen to immune cells. Besides, the increase in carbon clearance index reflects the enhancement of phagocytic function of mononuclear macrophages and non-specific immunity which act through the release of cellular lysosomal enzyme and other mediators. Phagocytosis by macrophages is important against the smaller parasites and its effectiveness is markedly enhanced by opsonization of parasite with antibodies and complement C3b leading to more rapid clearance of parasite from blood (CitationWalport, 1993). These results led to the assumption that the extract has the ability to stimulate the macrophages for the lysis of foreign materials as a result of engulfment by phagocytosis.
Proliferation of bone marrow cells in vitro indicate the in vivo potential of the extract and showed protective effect against cyclophosphamide-induced myelosuppression. Increase in number of WBC counts in cyclophosphamide-induced myelosuppression indicates the stimulatory activity of the extract on bone marrow proliferation. Stimulation of bone marrow signifies the plausible release of colony stimulating factors and subsequently enhancement of the bone marrow cellularity (CitationOredipe et al., 1991; CitationJiang et al., 1994). In addition to this, CitationUjihara et al. (2001) reported that release of granulocytes macrophages colony stimulating factor ensures the survival of macrophages and other mediators, which subsequently increase the number of macrophages and neutrophils in blood and thus enhance phagocytic activity. Further studies are needed towards a better understanding of the mode of actions and the responsible underlying biological principles.
Conclusion
The present investigation suggests that ethanol extract of W. fruticosa flowers stimulates non-specific immune responses by stimulating macrophages and bone marrow cells. It can therefore be concluded that ethanol extract of Woodfordia fruticosa flowers is a potential immunostimulant against cytotoxic drugs and can be used as a complimentary therapeutic agent.
Acknowledgements
The authors thank Ganesh Iyer, Botanist at Ramnarayan Ruia College, Mumbai - 400019 for authentication of the fresh flowers of Woodfordia fruticosa.
Declaration of interest
The authors are very thankful to the University Grant Commission for their generous financial support.
References
- Ajaya Kumar R, Sridevi K, Vijaya Kumar N, Nanduri S, Rajagopal S (2004): Anticancer and immunostimulatory compounds from Andrographis paniculata. J Ethnopharmacol 92: 291–295.
- Atal CK, Bhatia AK, Singh RP (1982): Role of Woodfordia fruticosa Kurz (Dhataki) in the preparation of Asavas and Aristhas. J Res Ayu and Siddha III: 193–199.
- Auclair C, Voisin E (1985): Nitroblue tetrazolium reduction, in: Greewald RA, ed., Handbook of Methods for Oxygen Radical Research Boca Raton, FL, CRC Press, pp. 123–132.
- Burkill IH (1966): A Dictionary of Economic Products of the Malay Peninsula Kuala Lumpur, Ministry of Agriculture and Co-operatives, p. 2305.
- CCRIMH (Central Council for Research in Indian Medicine and Homeopathy) (1978): Hand Book of Domestic Medicine and Common Ayurvedic Remedies New Delhi, Ministry of Health and Family Welfare, p. 334.
- Dey KL (1984): The Indigenous Drugs of India Dehradun, International Book Distributors, p. 311.
- Ding AH, Nathan CF, Stuehr DJ (1988): Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages: Comparison of activating cytokines and evidence for independent production. J Immunol 141: 2407–2412.
- Gonda R, Tomoda M, Shimizu N, Kanari M (1990): Characterization of an acidic polysaccharide from the seeds of Malva verticillata stimulating the phagocytic activity of cells of the RES. Planta Med 56: 73–76.
- Jiang S, Levine JD, Fu Y, Deng B, London R, Groopman JE, Avraham H (1994): Cytokine production by primary bone marrow megakaryocytes. Blood 84: 4151–4156.
- Keller R, Keisi R, Wechsler A, Leisi TP, Van Der Meide PH (1990): Mechanisms of macrophage-mediated tumor cell killing: A comparative analysis of the roles of reactive nitrogen intermediates and tumor necrosis factor. Int J Cancer 46: 682–686.
- Kroes BH, Van-den Berg AJJ, Abeysekera AM, de Silva KTD, Labadie RP (1992): Investigation on Nimba arishta, an immunomodulating Ayurvedic drug obtained by fermentation. J Eur Ayu Soc 2: 123–133.
- Kroes BH, Van-den Berg AJJ, Abeysekera AM, de Silva KTD, Labadie RP (1993): Fermentation in traditional medicine: The impact of Woodfordia fruticosa flowers on the immunomodulatory activity, and the alcohol and sugar contents of Nimba Aristha. J Ethnopharmacol 40: 117–125.
- Lowenstein CJ, Snyder SH (1992): Nitric oxide, a novel biologic messenger. Cell 70: 705–707.
- Manjarekar PN, Jolly CI, Narayanan S (2000): Comparative studies of the immunomodulatory activity of Tinospora cordifolia and Tinospora sinensis. Fitoterapia 71: 254–257.
- Manosroi A, Saraphanchotiwitthaya A, Manosroi J (2003): Immunomodulatory activities of Clausena excavata Burm. f. wood extracts. J Ethnopharmacol 89: 155–160.
- Oredipe OA, White SL, Grzegorzewski K, Gause BL, Cha JK, Miles VA, Olden K (1991): Protective effects of swainsonine on murine survival and bone marrow proliferation during cytotoxic chemotherapy. J Natl Cancer Inst 83: 1149–1156.
- Oudhia P (2003): Interactions with the herb collectors of Gandai region, Chhattisgarh, India having rich traditional medicinal knowledge about useful herb Dhawai (Woodfordia fruticosa). Research note available at http://www.botanical.com/site/column_poudhia/149_gandai.html (accessed on March 20, 2009).
- Pinegin BV, Butakov AA, Shelcina TL (1995): Complex of methods for determination of functional activity of phagocyting cells, in: Khaitov RM, Pinegin BV, Istamov HI. Eds, Ecological Immunology Moscow, VNIRO, pp. 146–154.
- Pratap KD, Suchandra G, Annalakshmi C, Nilendu P, Sukdeb B, Niranjan PS, Basudeb A. (2007). Woodfordia fruticosa: Traditional uses and recent findings. J Ethnopharmacol 110: 189–199.
- Sharma PV (1956): Dravyagun Vigyan Varanasi, India, The Chowkhamba Bharati Academy, pp. 472.
- Suzuki I, Tanaka H, Kinoshita A, Oikawa S, Osawa M, Yadomae T (1990): Effect of orally administered β-glucan on macrophage function in mice. Int J Immunopharmacol 12: 675–684.
- Ujihara M, Nomura K, Yamada O, Shibata N, Kobayashi M, Takano K (2001): Granulocyte-macrophage colony-stimulating factor ensures macrophage survival and generation of the superoxide anion: A study using a monocytic-differentiated HL60 subline. Free Radic Biol Med 31: 1396–1404.
- Wagner H, Proksch A (1985): Immunostimulatory drugs of fungi and higher plants, in: Wagner H, Hikino H, Farnsworth NR, eds, Economic and Medicinal Plant Research, Vol. I. London, Academic Press, pp. 113–153.
- Walport M (1993): Complement, in: Roitt I, Brostoff J, Male D, eds, Immunology third edition. London, Mosby-Europe, pp. 1–16.
- Xie OW, Cho HJ, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Ding A, Troso T, Nathan C (1992): Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science 256: 225–228.