Abstract
Context: Cinnamomum osmophloeum Kaneh. (Lauraceae) is one of the indigenous tree species in Taiwan. This tree species has been of interest to researchers because the chemical constituents of its essential oil are similar to those of Cinnamomum cassia Presl. bark oil, known as cinnamon oil, which is commonly used in foods and beverages.
Objective: The anti-inflammatory activities of the leaf essential oils and their major compounds from seven provenances of C. osmophloeum are investigated here for the first time.
Materials and methods: Chemical compositions of hydrodistilled essential oils obtained from C. osmophloeum leaves were analyzed by gas chromatography-mass spectrometry (GC-MS), and the effects of essential oils on nitric oxide (NO) production in lipopolysaccharide (LPS)-activated RAW 264.7 macrophages were investigated.
Results: The leaf essential oils of cinnamaldehyde type and mixed type strongly inhibited NO production, with IC50 values ranging from 9.7-15.5 μg/mL. Furthermore, trans-cinnamaldehyde is responsible for the inhibitory activity of cinnamaldehyde type, and T-cadinol and α-cadinol are responsible for the inhibitory activity of mixed type.
Discussion and conclusion: These findings demonstrate that the leaf essential oils and their constituents of C. osmophloeum have excellent anti-inflammatory activities and thus have great potential as a source for natural health products.
Introduction
Nitric oxide (NO) is an endogenous free radical species that is synthesized from l-arginine by nitric oxide synthase (NOS) in various animal cells and tissues. Small amounts of NO are important regulators of physical homeostasis, whereas larger amounts of NO have been closely correlated with the pathophysiology of a variety of diseases and inflammation. After exposure to inducers, such as lipopolysaccharide (LPS) from Gram-negative bacteria, inducible NOS (iNOS) can be induced in various cells, such as macrophages, Kupffer cells, smooth muscle cells, and hepatocytes, thereby triggering cytotoxicity, tissue damage, inflammation, sepsis, and stroke (CitationMarletta, 1993; CitationJiang et al., 2006). Thus, measuring of NO production may be a method for assessing the anti-inflammatory effects of plant extracts.
Cinnamomum osmophloeum Kaneh. (Lauraceae) is a tree indigenous to natural hardwood forests of Taiwan at elevations between 400-1500 m. This tree species has been of interest to researchers because the chemical constituents of its essential oil are similar to those of Cinnamomum cassia Presl. bark oil, known as cinnamon oil, which is commonly used in foods and beverages (CitationOoi et al., 2006). Recent phytochemical analyses and biological screenings of C. osmophloeum have focused on the leaf essential oil constituents, which have shown excellent antibacterial, antitermite, antimite, antimildew, antimosquito larvae, and antifungal effects (CitationChang et al., 2001; CitationChang & Cheng, 2002; CitationChen & Chang, 2002; CitationChen et al., 2002; CitationCheng et al., 2004, Citation2006). Furthermore, CitationChao et al. (2005) reported that essential oil of C. osmophloeum leaves at a dose of 60 μg/ mL also exhibited effective inhibitory effects on IL-1β and IL-6 production in LPS-stimulated macrophages. CitationTung et al. (2008) also found that C. osmophloeum twigs strongly suppress NO synthase in LPS-stimulated macrophages. However, to the best of our knowledge, there is no prior study on the effects of provenances for leaf essential oils of C. osmophloeum. In this study, the leaf essential oils of C. osmophloeum from seven provenances were analyzed by gas chromatography-mass spectrometry (GC-MS), and the effects of essential oils and their constituents on nitric oxide production in lipopolysaccharide-activated RAW 264.7 macrophages were investigated.
Materials and methods
Chemicals
Lipopolysaccharide (LPS), Greiss reagent and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma (St. Louis, MO). Linalool, d-(+)-camphor, 3-phenylpropionaldehyde, trans-cinnamaldehyde, l-bornyl acetate, cinnamyl acetate, (-)-aromadendrene, caryophyllene oxide, curcumin, and methyl sulfoxide (DMSO) were all purchased from Acros (Geel, Belgium). Dulbecco’s modified Eagle medium (DMEM) was purchased from Gibco (Grand Island, NY, USA). α-Cadinol and T-cadinol were isolated from leaf essential oil of C. osmophloeum.
Plant material
The leaves of seven Cinnamomum osmophloeum provenances (COA–COG) were collected at the end of October 2005 from the Taiwan Sugar Company Research Center located in Nantou County in central Taiwan. The species were confirmed by Yen-Ray Hsui of the Taiwan Forestry Research Institute and voucher specimens (CO0109, CO4407, CO1709, CO0403, CO0902, CO0502, and CO0609) were deposited at the laboratory of wood chemistry (School of Forestry and Resource Conservation, National Taiwan University).
Essential oil distillation
Leaves of C. osmophloeum, in triplicate, were subjected to hydrodistillation for 6 h in a Clevenger-type apparatus (CitationTung et al., 2008). The yellow essential oils with characteristic odor and hot water extracts were obtained and stored in airtight containers prior to analysis.
Analysis of essential oil
Essential oils from leaves were analyzed by a Trace GC Ultra (Thermo, Austin, TX) gas chromatograph coupled with a Polaris Q (Thermo, Austin, TX) ion-trap mass spectrometer, equipped with a 30 m × 0.25 mm × 0.25 µm DB-5MS (Agilent J & W Scientific, USA). The mass spectrometer was operated in the electron-impact mode, with an ionization energy of 70 eV. The sector mass analyzer was set to scan from 50 to 400 amu. The GC oven temperature was programmed to start from 60°C, hold 1 min, rise to 220°C at 4°C/min, hold for 2 min, and rise to 250°C at 20°C/min, hold for 3 min. The injector temperature was 250°C; and the flow rate of carrier gas, helium, was at a 1 mL/min; 1:10 split ratio. Diluted samples (1 μL, 1/10000, v/v, in ethyl acetate) were injected manually in the split mode. The Kovats indices were calculated for all volatile constituents using a homologous series of n-alkanes C9–C19 on DB-5MS column. The major constituents of C. osmophloeum leaf oil were identified by co-injection with standards (wherever possible), confirmed with Kovats indices (CitationAdams, 2007) using the Wiley (V. 7.0) and National Institute of Standards and Technology (NIST) V. 2.0 GC-MS library. The relative concentration of each compound in essential oil was quantified based on the peak area integrated by the analysis program.
Anti-inflammatory activity
Cell line and cell culture
RAW 264.7 cells, a murine macrophage cell line, were obtained from the Culture Collection and Research Center (CCRC), Hsinchu, Taiwan. Cells were cultured at 37°C in a 5% CO2 incubator and maintained in disks with DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin.
Anti-inflammatory activity assay
To investigate the anti-inflammatory activity of the leaf essential oil from different C. osmophloeum provenances, NO production in LPS-stimulated RAW 264.7 cells were examined. For NO determination, RAW 246.7 cells were seeded in 96-well plates at a density of 2 × 105 cells/well and grown for 4 h for adherence. The cells were treated with test samples for 1 h and then incubated for 24 h in fresh DMEM with or without 1 μg/mL LPS. The nitrite concentration in the culture medium was measured as an indicator of NO production according to the Griess reaction (CitationWu et al., 2008). Briefly, 100 μL cell culture supernatant was reacted with 100 μL Griess reagent (1:1 mixture of 0.1% N-(1-naphthyl) ethylene-diamine dihydrochloride in water and 1% sulfanilamide in 5% phosphoric acid) in a 96-well plate, and absorbance at 540 nm was recorded using an ELISA reader.
Cell viability
The cell viability assay was determined on the basis of MTT assay, as described above with a slight modification. After culturing, supernatants were collected for NO measurement, 100 μL tetrazolium salt solution (1 mL MTT/10 mL DMEM) was added to each well, and then incubated for 1 h at 37°C in a 5% CO2 incubator. The medium was then aspirated, and the insoluble formazan product was dissolved in 100 μL of DMSO. The extent of MTT reduction was quantified by measuring the absorbance at 570 nm.
Cluster analysis and principal components analysis
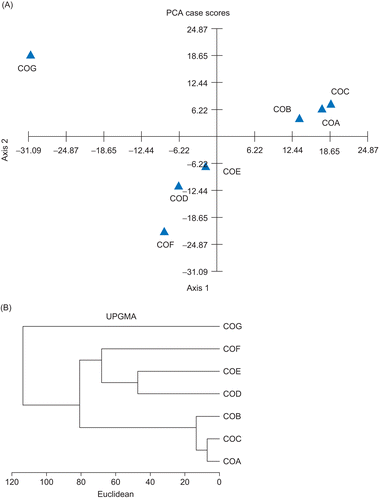
The cluster analysis and principal components analysis (PCA) were performed using multivariate statistical package (MVSP) software to identify relatively homogeneous groups of seven provenances (COA-COG) of C. osmophloeum based on the percentage composition of their essential oil samples. Euclidean distance was selected as a measure of similarity, and the unweighted pair-group method with arithmetic average (UPGMA) was used for cluster definition.
Statistical analysis
All results are expressed as mean ± SD (n = 3). The significance of difference was calculated by SAS Scheffe’s test, and values <0.05 were considered to be significant.
Results and discussion
Yields and constituents of essential oil
A total of 42 compounds of COA, COB, COC, COD, COE, COF, and COG were identified in the leaf essential oils, representing 99.3, 99, 99.4, 76.1, 85.7, 98.4, and 99%, respectively of the essential oils. Major compositions of seven essential oils are reported in . The leaf essential oils of COA, COB, and COC were found to contain mainly trans-cinnamaldehyde (72.9, 62.6, and 77.2%, respectively). The major constituent of leaf essential oil from COF was d-(+)-camphor (56.6%). The major constituent of leaf essential oil from COG was linalool (96.3%). The leaf essential oil from COD was found to be rich in l-bornyl acetate (22%), α-cadinol (11.2%), T-cadinol (10.9%), caryophyllene oxide (5.6%), and (-)-aromadendrene (5.5%). The leaf essential oil of COE was found to be rich in cinnamyl acetate (40.8%), trans-cinnamaldehyde (9.1%), caryophyllene oxide (6.2%), T-cadinol (5.5%), and α-cadinol (5.4%). Principal component analysis (PCA) and cluster analysis were performed to detect the degrees of similarity of the compositions of the leaf essential oils analyzed (). Different groups can be identified in the loading plots of PC1 and PC2. The leaf essential oils obtained from different geographical provenances were classified into four chemotypes: cinnamaldehyde type (COA, COB, and COC), camphor type (COF), linalool type (COG), and mixed type (COD and COE).
Table 1. Composition of essential oils from C. osmophloeum leaves.
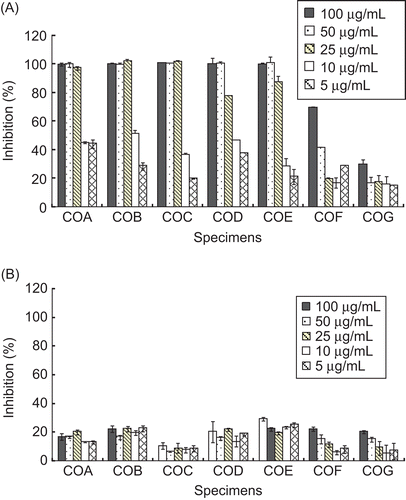
Effects of essential oils and hot water extracts from different provenances on NO production in LPS-stimulated RAW 264.7 cells
As for the inhibitory effects of the leaf essential oils and hot water extracts from four chemotypes of C. osmophloeum on NO production, data in and show that the leaf essential oils had better inhibitory effect than the hot water extracts. The leaf essential oils of cinnamaldehyde type (COA, COB, and COC) and mixed type (COD and COE) showed excellent inhibitory effects. The IC50 values of cinnamaldehyde type (COA, COB, and COC) and mixed type (COD and COE) were 11.7, 9.7, 13.1, 11.6, and 15.5 μg/mL, respectively. However, the IC50 values of camphor type and linalool type were 65.8 and >100 μg/mL, respectively. In addition, MTT assay revealed that concentrations up to 100 μg/mL produced no significant cytotoxic effects on cells treated with the leaf essential oils. These results indicate that of the 4 chemotypes tested, the leaf essential oils of cinnamaldehyde type and mixed type inhibit NO production most strongly. Cinnamaldehyde type (COA, COB, and COC) contained more trans-cinnamaldehyde and also strongly inhibited NO production, consistent with our previous study showing that trans-cinnamaldehyde has excellent inhibitory activity on NO production (CitationTung et al., 2008). Furthermore, CitationLee et al. (2002) showed that trans-cinnamaldehyde strongly suppresses NO synthase.
Table 2. IC50 values (μg/mL) of essential oils and hot water extracts from C. osmophloeum leaves on nitric oxide production of LPS-stimulated RAW 264.7 macrophage cells.
Effects of the constituents on NO production in LPS-stimulated RAW 264.7 cells
To understand the relationship between the constituents of C. osmophloeum leaf essential oils and inhibition of NO production in RAW 264.7 macrophages, ten constituents, namely linalool, d-(+)-camphor, 3-phenylpropionaldehyde, trans-cinnamaldehyde, l-bornyl acetate, cinnamyl acetate, (-)-aromadendrene, caryophyllene oxide, T-cadinol, and α-cadinol were tested. Curcumin, well known for its anti-inflammatory activity, was used in parallel as a positive control. As shown in and , trans-cinnamaldehyde, (-)-aromadendrene, caryophyllene oxide, T-cadinol, and α-cadinol exhibited strong NO production inhibitory effects. The IC50 values of these five compounds were 15.7, 16.5, 43.2, 20.8, and 14.0 μg/ mL (118.6, 80.5, 195.9, 93.6, and 63.2 μM), respectively. Nevertheless, linalool, d-(+)-camphor, 3-phenylpropionaldehyde, l-bornyl acetate, and cinnamyl acetate were found to be the least active (IC50 value > 200 μM). In addition, MTT assay revealed no significant cytotoxic effects on cells treated with the ten constituents at the dosage of 100 μM ().
Table 3. IC50 values (μM) of compounds from C. osmophloeum leaves on nitric oxide production of LPS-stimulated RAW 264.7 macrophage cells.
Figure 3. Effects of compounds from C. osmophloeum leaves and curcumin on nitric oxide production of LPS-stimulated RAW 264.7 macrophage cells. (A) represents the concentration-dependent inhibition of nitric oxide production; and (B) indicates the cytotoxicity of pure compound on RAW 264.7 cells in the presence of LPS, measured by the MTT assay. Results are mean ± SD (n = 3).
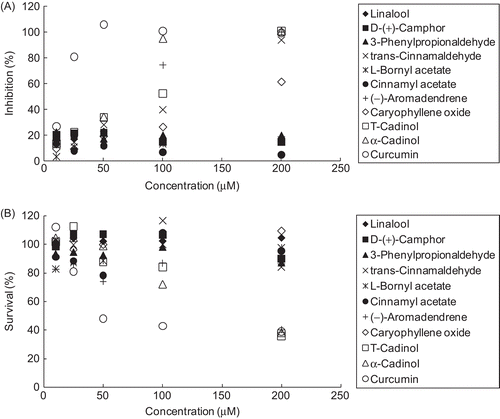
Although the three major compounds, 3-phenylpropionaldehyde, trans-cinnamaldehyde, and cinnamyl acetate, all exist in the leaf essential oils of cinnamaldehyde type (COA, COB, and COC), trans-cinnamaldehyde clearly is responsible for the excellent inhibition of NO production. Similar results in previous studies showed that in the major compounds of C. osmophloeum leaf essential oils trans-cinnamaldehyde performed best against bacteria, termites, mites, mildew, pathogens, mosquitoes, and fungi (CitationChang et al., 2001; CitationChang & Cheng, 2002; CitationChen et al., 2002; CitationChen & Chang, 2002; CitationCheng et al., 2004, Citation2006). Furthermore, of the major compounds of COD, (-)-aromadendrene, caryophyllene oxide, T-cadinol, and α-cadinol are responsible for the excellent inhibitory activity on NO production. In addition, trans-cinnamaldehyde, caryophyllene oxide, T-cadinol, and α-cadinol are responsible for the inhibitory activity on NO production of COE. Therefore, it is reasonable to conclude that T-cadinol and α-cadinol of mixed type (COD and COE) are mainly responsible for the inhibitory activity on NO production. However, the IC50 values of camphor type (COF) and linalool type (COG) were 65.8 and > 100 μg/mL, and the major constituents of leaf essential oils from COF and from COG were camphor (56.6%) and linalool (96.3%), respectively. Nevertheless, D-(+)-camphor and linalool were found to be the least active (IC50 value > 200 μM).
Conclusions
According to GC-MS and cluster analyses the leaf essential oils from seven provenances and their relative contents were classified into four chemotypes: cinnamaldehyde type, linalool type, camphor type, and mixed type. It was also found that the major constituents of C. osmophloeum leaf essential oils such as trans-cinnamaldehyde, (-)-aromadendrene, caryophyllene oxide, T-cadinol, and α-cadinol had excellent anti-inflammatory activities in suppressing nitric oxide production by LPS-stimulated macrophages. However, the leaf essential oils of cinnamaldehyde type and mixed type had excellent inhibitory activity on NO production, with IC50 values ranging from 9.7 to 15.5 μg/ mL. The essential oils of C. osmophloeum leaves and their active compounds are worthy of further exploration, due to their excellent performance found in this study.
Declaration of interest
The authors declare no conflict of interest.
References
- Adams RP (2007): Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Carol Stream, Illinois, USA: Allured Publishing Corporation.
- Chang ST, Chen PF, Chang SC (2001): Antibacterial activity of leaf essential oils and components from Cinnamomum osmophloeum. J Ethnopharmacol 77: 123–127.
- Chang ST, Cheng SS (2002): Antitermitic activity of leaf essential oils and components from Cinnamomum osmophloeum. J Agric Food Chem 50: 1389–1392.
- Chao LK, Hua KF, Hsu HY, Cheng SS, Liu JY, Chang ST (2005): Study on the anti-inflammatory activity of essential oil from leaves of Cinnamomum osmophloeum. J Agric Food Chem 53: 7274–7278.
- Chen PF, Chang ST (2002): Application of essential oils from wood on the manufacture of environment-friendly antimicrobial paper products. Quart J Chin For 35: 69–74.
- Chen PF, Chang ST, Wu HH (2002): Antimite activity of essential oils and their components from Cinnamomum osmophloeum. Quart J Chin For 35: 397–404.
- Cheng SS, Liu JY, Hsui YR, Chang ST (2006): Chemical polymorphism and antifungal activity of essential oils from leaves of different provenances of indigenous cinnamon (Cinnamomum osmophloeum). Bioresource Technol 97: 306–312.
- Cheng SS, Liu JY, Tsai KH, Chen WJ, Chang ST (2004): Chemical composition and mosquito larvicidal activity of essential oils from leaves of different Cinnamomum osmophloeum provenances. J Agric Food Chem 52: 4395–4400.
- Jiang JS, Shih CM, Wang SH, Chen TT, Lin CN, Ko WC (2006): Mechanisms of suppression of nitric oxide production by 3-O-methylquercetin in RAW 264.7 cells. J Ethnopharmacol 103: 281–287.
- Lee HS, Kim BS, Kim MK (2002): Suppression effect of Cinnamomum cassia bark-derived component on nitric oxide synthase. J Agric Food Chem 50: 7700–7703.
- Marletta MA (1993): Nitric oxide synthase structure and mechanism. J Biol Chem 268: 12231–12234.
- Ooi LS, Li Y, Kam SL, Wang H, Wong EY, Ooi VE (2006): Antimicrobial activities of cinnamon oil and cinnamaldehyde from the Chinese medicinal herb Cinnamomum cassia. Blume Am J Chin Med 34: 511–522.
- Tung YT, Chua MT, Wang SY, Chang ST (2008): Anti-inflammatory activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresource Technol 99: 3908–3913.
- Wu JH, Tung YT, Chien SC, Wang SY, Kuo YH, Shyur LF, Chang ST (2008): Effect of phytocompounds from the heartwood of Acacia confusa on inflammatory mediator production. J Agric Food Chem 56: 1567–1573.