Abstract
Context: Anacyclus pyrethrum DC (Compositae) roots, commonly known as Pellitory root and locally as akarkara, are widely recognized in the Indian traditional systems of medicine, Ayurveda, as a ‘rasayana’, i.e. a plant with immunomodulatory properties.
Objective: Evaluation of A. pyrethrum extract for its effect on normal and chemically suppressed immune systems in vivo.
Materials: Petroleum ether extract (PEE) of roots was tested at 50 and 100 mg/kg dose. The effect of both doses on total and differential leukocyte count, cyclophosphamide-induced immunosuppression, survival rate against Candida albicans infection, delayed type hypersensitivity reaction, percentage neutrophil adhesion, and phagocytic activity were tested.
Results: The PEE-treated rats were able to overcome cyclophosphamide-induced myelo-suppression as evidenced by the normalization of blood parameters. Survival rate of albino rats was improved in Candida albicans-infected animals by treatment with the extract (p <0.05). An increase in delayed type hypersensitivity response (DTH), percentage neutrophil adhesion, and in vivo phagocytosis by carbon clearance method was observed after treatment. Extract administration also increased the HA titer value and IgG antibodies.
Discussion: Immunostimulant activity increased two-fold upon doubling the dosage of extract administered. While a significant (p <0.05) improvement was observed in the humoral component, a highly significant (p <0.01) effect was observed in the cellular components of the immunity evaluated. The results thus provide a basis for the use of A. pyrethrum as an adaptogen and immunomodulator in the Ayurvedic system of medicine.
Introduction
Ayurveda, an alternative system of medicine in India, uses a number of plants either individually or in combination for treatment of a variety of diseases. Natural complementary medical therapy like Ayurveda has always aimed for long-term stimulation of natural resistance and improvement of defective immunological conditions without causing any untoward or harmful effects to the biological homeostasis (CitationHajto et al., 2005). Traditional systems of medicine such as Ayurveda may play a pivotal role in modern healthcare, particularly in the treatment or amelioration of diseases where satisfactory treatments are not available. The rasayana class of medicinal plants in Ayurveda, are reported to have a triphasic activity, i.e. the ability to improve health and longevity, enhance memory, intelligence, youthfulness, and improve complexion. Rasayanas are reputed to possess immunomodulatory activity (CitationAtal et al., 1986; CitationPatwardhan et al., 2005; CitationRamnath et al., 2008).
Currently, there is a need to evaluate the potential of Ayurvedic remedies for therapy and as adjuvants to counteract the adverse effects of modern therapy and imbalance of the immune system upon long-term treatment with allopathic drugs (CitationDahanukar & Thatte, 1997). The word rasayana is composed of two words Rasa signifying vital elixir of life and ayana signifying the home of: rasayana in total signifying the vitality and immunity enhancing properties. Rasayana therapy prevents diseases and counteracts the aging process by means of optimization or homeostasis (CitationPuri, 2003; CitationThakur et al., 2007). Many plants have been extensively used as rasayana drugs in Ayurveda for the management of neurodegenerative diseases, as tonics and rejuvenators, immunomodulators, aphrodisiacs, and nutritional supplements (CitationThakur & Dixit, 2008).
Anacyclus pyrethrum DC (Compositae) roots, commonly known as akarkara (pellitory root), have been widely acclaimed in the Ayurvedic system of medicine for their rasayana properties (CitationMinistry of Health and Family Welfare, 2004). Akarkara has been described in ancient Indian literature, i.e. Charak Samhita as ‘vajikaran rasayana’ which signifies a special category of immunomodulators (CitationPuri, 2003). It is widely used in folk remedies for stimulating salivary glands. It is also useful as a remedy for toothache, paralysis of the tongue and throat muscles, and in neuralgic problems of the teeth (CitationBentley & Trimen, 2004). Pellitorine, an alkylamide, is one of the constituents amongst various phytochemicals reported from the roots of A. pyrethrum; it is a colorless crystalline acidamide, and is also known as pyrethrine. Pellitorine is an alkylamide consisting of undecadienic acid or nonadiene carboxylic acid that possesses an intensely pungent taste and produces a sialogogue effect (CitationMukerji, 1953). Other chemical constituents reported in the plant include N-isobutyldienediynamide (CitationCrombie, 1954), and polysaccharides (CitationBendjeddou et al., 2003). Since alkylamide has been acclaimed for potent immunomodulatory activity, the petroleum ether extract of A. pyrethrum was evaluated for its in vivo immunomodulatory activity.
Materials and methods
Plant material
Dried whole roots of A. pyrethrum (Akarkara) were purchased in August 2007 from local market of Sagar, India. The sample, was authenticated by A.M. Mujumdar, at the Agharkar Research Institute, Pune, India (voucher number Auth.07-86).
Extraction
Dried A. pyrethrum roots were crushed to moderately coarse powder (60-180 mesh) using a mechanical grinder. Root powder (500 g) was fed into a Soxhlet extractor and extracted with petroleum ether (60–80°C) for 72 h. After ensuring complete extraction, the extract was collected, filtered, and dried under vacuum using a rotary evaporator (Heidolph, Schwabach, Germany) the yield was found to be 1.8% w/w. Extract was stored in an air tight vial till further use. For oral administration the extract was triturated with Arachis (peanut) oil and administered as a suspension orally. Cyclophosphamide was a gift sample from Khandelwal Laboratories (Mumbai, India). Candida albicans was purchased from IMTECH (Chandigarh, India). All other chemicals and reagents used were of analytical grade and procured from Qualigens (Mumbai, Maharashtra, India).
Animals
The Institutional Animal Ethics Committee of Dr H.S. Gour University, Sagar, India, approved the protocol for animal experimentation. The guidelines of the Committee for the Purpose of Supervision and Control of Experiments on Animals (CPCSEA, India) were strictly followed.
Wistar albino rats of either sex weighing 120–150 g were fed on a standard diet and water ad libitum. The animals were housed at room temperature (24° ± 2°C) under a normal light/dark cycle of 12:12 h. Animals were allowed to acclimatize to the cage environment for one week prior to experimentation. Sheep red blood cells (SRBCs) were collected in Alsever’s solution, washed three times with large volumes of pyrogen-free sterile normal saline, and the cells were adjusted to a concentration of 5 × 109 cells per mL for immunization and challenge.
Statistical analysis
Data is expressed as mean ± SEM and was analyzed for significance of variance by one-way ANOVA followed by Dunnett’s test using InSTAT v 2.01.
Chromatographic analysis
High performance thin layer chromatography (HPTLC)and gas chromatography-mass spectometry (GC-MS) analysis of the petroleum ether extract was performed in order to characterize the extract. For HPTLC analysis pre washed silica gel GF254 plates (Merck, Darmstadt, Germany) were used. The mobile phase used was toluene:ethyl acetate (97:3 v/v). The sample (5 µL) was applied on a thin layer chromatography (TLC) plate using a Linomat V applicator (Camag, Muttenz, Switzerland). The plate was scanned densitometrically at 220 nm using a Camag plate scanner. GC-MS analysis was carried out as per the methodology reported by CitationLeitao and De Oliveira (2001).
Treatment
Rats were randomly divided into 4 groups comprising of six animals each. Petroleum ether extract of Anacyclus pyrethrum (PEE) was administered orally as a suspension in arachis oil using a metal cannula.
Group I: Control, normal saline per ora (p.o.)
Group II: Negative control group, cyclophosphamide (CP) 30 mg/kg; intraperitoneally (i.p.)
Group III: Petroleum ether extract 50 mg/kg (PEE50) p.o.
Group IV Petroleum ether extract 100 mg/kg (PEE100) p.o.
Dosages were determined as 1/10 and 1/20 of LD50, after performing a systematic toxicity assay. A fresh animal set was used for each experiment unless specified.
In vivo phagocytosis using the carbon clearance method
The non-specific immunity characterized by the change in macrophage phagocytic activity via the reticuloendothelial system was determined by carbon clearance test (CitationBiozzi et al., 1953). The animals of respective groups received PEE50, PEE100 or CP treatment as previously mentioned for 14 days prior to experimentation. On day 14, 3 h before extract treatment, all the groups were administered with 0.1 mL carbon suspension (Pellikan Tuschea Ink, Hamburg, Germany) intravenously through the tail. Blood samples were collected from the retro-orbital plexus just after challenge with carbon particles (time 0) and at regular intervals i.e. 5,10, 15, 20, 25 and 30min followed by challenge. The blood samples collected were lysed with 2 mL 0.1% v/v acetic acid, and absorbance was recorded at 675 nm (Cintra GBC, DB-UV, Gottingen, Germany) (CitationThakur et al., 2007). The slope (K) of each time–concentration curve, was drawn by plotting absorbance as ordinate against time as abscissa was determined. The overall phagocytic index for each respective group was determined using the following formula:
where, Ksample represents the slope for various treatments (PEE50, PEE100 or CP), and Kcontrol represents the slope for untreated control group animals.
SRBC-induced delayed type hypersensitivity reaction (DTH response)
The effect of extracts on antigen-specific cellular immunity was evaluated by measuring footpad swelling as an indicator for delayed type hypersensitivity response. Treatment for 14 days as mentioned earlier was carried to challenge with SRBC. On day 0, all the groups were immunized by subcutaneous administration of 1 mL of SRBC cell suspension, which was equivalent to 5 × 109 SRBC per mL into the right hind foot pad. On day 15, all groups were challenged by subcutaneously injecting 0.5 mL of SRBC cell suspension into the left hind foot pad, and the thickness of the left hind foot pad was measured after 24 and 48 h of challenge using a plethysmometer. The difference between the thickness of the left hind foot just before and after challenge in mL was taken as a measure of DTH (CitationLagrange et al., 1974).
Neutrophil adhesion test
The method described by CitationWilkonson (1978) was used for evaluating the effect of PEE of A. pyrethrum roots on neutrophil adhesion. After 14 days of treatment to all four groups, blood samples were collected in heparinized vials by retro-orbital puncture, and total as well as differential leukocyte count was determined. After initial counts, the blood samples were incubated with 80 mg per mL of nylon fibers at 37°C for 15 min. The incubated samples were further analyzed for total and differential leukocyte count. The product of total leukocyte count and percentage neutrophil, known as neutrophil index, was determined for each animal of each respective group using the formula reported by CitationFulzele et al. (2002).
Non-specific immunity determined by survival rate against fungal infection
Animals of all four groups (Group I-IV) as defined previously were treated as control or with extract for 14 days prior to the challenge with Candida albicans infection. On day 15 animals of all groups were injected with 5 × 107 viable C. albicans cells and observed daily for mortality over a period of 7 days (CitationThakur et al., 2007).
Cyclophosphamide-induced immunosuppression
Cyclophosphamide-induced immunosuppression was assessed after cyclophosphamide treatment with or without extract administration. Test animals were divided into six groups of six rats each.
Group I: Control (without drug treatment)
Group II: Cyclophosphamide (CP) (30 mg/kg body weight i.p. twice weekly)
Group III: Cyclophosphamide (30 mg/kg body weight i.p. twice weekly) and PEE of A. pyrethrum (50 mg/kg body weight, daily)
Group IV: Cyclophosphamide (30 mg/kg body weight i.p. twice weekly) and PEE of A. pyrethrum (100 mg/ kg daily)
Group V: PEE of A. pyrethrum (50 mg/kg daily)
Group VI: PEE of A. pyrethrum (100 mg/kg daily)
On day 14, blood samples were collected from the retro-orbital plexus in heparinized vials and evaluated for hematological parameters. The changes in RBC, WBC, hemoglobin and platelet counts were determined (CitationZiauddin et al., 1996).
Humoral response to SRBC (Hemagglutination antibody titer value)
Six groups of animals received drug administration for 14 days as described earlier in the protocol for cyclophosphamide-induced immunosuppression. On day 0, paralleling the aforementioned treatment, these animals were immunized by injecting 0.5 mL of 5 × 109 SRBC/mL, i.p. and were challenged by injecting an equal volume of SRBC (0.5 mL of 5 × 109 SRBC/mL) on day 7. Blood samples were collected by retro-orbital puncture on day 14 for antibody titer (CitationAtal et al., 1986). Hemagglutination antibody (HA) titer was determined by using the micro-titration technique described by CitationDamre et al. (2003). In brief, 40 µL of 0.1% (w/v) bovine serum albumin (BSA) solution in normal saline was pipetted out in wells of micro-titration plates. To this solution 40 µL serum from treated and control group animals was added, which was later diluted serially (two-fold dilution). To the resulting mixture 20 µL of 0.1% suspension of SRBC in BSA-saline was added and incubated initially at 37°C followed by 4°C incubator for 60 min each. The value of the highest serum dilution causing visible hemagglutination was considered the antibody titer (CitationDamre et al., 2003).
For the detection of antibody formation, on day 0 all animals were immunized by i.p. administration of 0.2 mL of 1% w/v BSA in phosphate buffered saline (PBS). After immunization a drug regimen of 14 days as described in the cyclophosphamide-induced immunosuppression model was followed. On day 7 blood samples were collected and antibody IgG levels of immunized animals were measured by simple indirect enzyme linked immuno sorbent assay (ELISA) and recorded as primary antibody levels. On day 14, after the last dose, animals were challenged with 0.2 mL of 1% w/v BSA, and on day 21 blood samples were collected and subjected for ELISA to determine IgG levels which were recorded as secondary antibody levels.
The method reported by CitationMichalek et al. (1983) was used with slight modification. In brief, the wells of ELISA plates were coated with 100 µL of 1% w/v BSA (bovine serum albumin) in PBS and incubated at 37°C for 1 h. Unbound BSA was removed by washing three times with PBS T (phosphate buffered saline Tween) solution. Aliquots of 25 mL of 1000-fold diluted serum samples were added to corresponding wells and incubated for 1 h. The unbound serum antibodies were removed by washing three times with PBS T solution. Aliquots of 50 mL of rabbit anti-rat IgG HRP were added to all the wells and the plate was incubated for 1 h. All wells were washed three times to remove the unbound materials and 50 µL of substrate (TMB H2O2) was added and incubated for 5 min. The enzyme substrate reaction was stopped by adding 50 mL of 5N H2SO4. Optical density (OD) was measured at 450 nm (Elisa reader, Eppendorf, Germany) and results calculated as log10 values.
Results
Chromatographic analysis
Toluene:ethyl acetate (97:3 v/v) gave the best chromatographic resolution, and spots were visualized by derivatization with anisaldehyde in sulfuric acid followed by heating at 110°C for 5 min. The HPTLC fingerprint showed 10 distinct peaks. GC-MS of the PEE indicated the presence of a compound with molecular weight 223. The fragmentation pattern of this particular compound matched with the fragmentation pattern reported for pellitorine (CitationGulland & Hopton, 1930).
Carbon clearance test
Administration of PEE of A. pyrethrum roots enhanced phagocytic activity in albino rats, a phagocytic index of 1.7 was observed in PEE50. The index was 1.8 in PEE100, whereas it was 0.47 in animals receiving CP. The results therefore indicate an improved non-specific immunity by treatment with PEE of A. pyrethrum ().
Table 1. Effect of petroleum ether extract of Anacyclus pyrethrum on non-specific and specific immune function.
Neutrophil adhesion test
PEE treatment increased the process of marginalization of cells in blood vessels and the percentage neutrophil adhesion, which was significantly increased with PEE100 treatment. The percentage of neutrophil adhesion in the cases of PEE50 and PEE100 was 17.2 and 23.15, respectively, which in the case of control animals was 15.3 and was markedly reduced in CP-treated groups because of pronounced immunosuppression ().
Table 2. Effect of petroleum ether extract of Anacyclus pyrethrum on neutrophil adhesion in rats.
Delayed type hypersensitivity
The DTH response measured by footpad thickness in the hind paw is an indicator of cell mediated immunity. The percentage change in DTH response found after 24 h in the PEE50 and PEE100 groups was 33.2 and 63.8, respectively. It was 18.2 in the CP-treated group compared to control animals. PEE100 significantly (P < 0.01) stimulated the cell-mediated immune response ().
Survival rate studies
A general test for the evaluation of immune protection by drugs is the study of the survival rate of animals against Candida albicans infection. After 7 days of infection, the survival rate was ~33% in the control group whereas it was 83% in PEE100 and 66% in PEE50. The results suggest that PEE100 is most effective amongst the treatments with test materials in preventing mortality of rats due to Candida albicans infection. The results further support potentiation of non-specific immune response on treatment with PEE of A. pyrethrum roots ().
Cyclophosphamide-induced immuno suppression
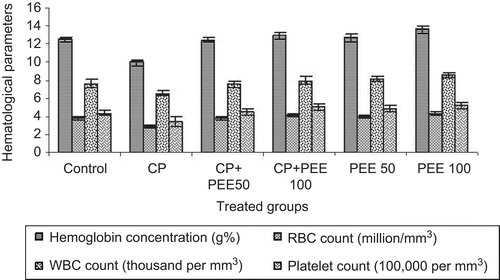
The effect of drug on immune-compromised animals was evaluated by determining blood profiles after CP administration. CP control animals showed a significant decrease in hematological parameters as compared to the control group. In contrast, a significant improvement was recorded (P < 0.01) in the extract-treated groups when compared with the CP-treated group, indicating immune protection effects of the extract. The PEE of A. pyrethrum had a potent hematopoietic activity. After 14 days the group receiving PEE100 showed maximum recovery and stimulation of blood RBC (4.1 million/mm3), hemoglobin (13 g), WBC (7.95 thousand/mm3) and platelet count (504.9 thousand/mm3) as compared to the cyclophosphamide-treated group in which the RBC count was found to be 2.91 million/mm3, hemoglobin 10 g, WBC count of 6.53 thousand/mm3 and platelet count of 347.6 thousand/mm3 ().
Effect on humoral response to SRBC (HA titer)
Administration of CP resulted in significantly decreased secondary HA titer 85.3 ± 13.4 as compared to normal control (149.3 ± 26.9). When compared with the CP-treated group, animals receiving CP + PEE100 showed maximum recovery observed in terms of HA titer value. On day 14 the antibody titer observed in CP + PEE50 and CP + PEE100 groups was 160 ± 32.6 and 245.3 ± 62.74, respectively, which was significantly higher compared to the CP group. PEE50 and PEE100 also showed increased antibody titer of 469.3 ± 122.1 and 640.0 ± 128.0, respectively, which was significantly higher (P < 0.01) compared to the control group ().
Table 3. Effect of petroleum ether extract of Anacyclus pyrethrum on HA titer value and IgG level after immunization in albino rats.
Effect on immunoglobulin (IgG) level
Secondary antibody responses in immunized animals were measured by simple indirect ELISA. Administration of CP resulted in significant decrease (P <0.01) in IgG level (1.60 ± 0.017) when compared to control animals (1.74 ± 0.015). On the contrary, CP + PEE100 and CP + PEE50 groups showed dose-dependent recovery with IgG levels of 1.83 ± 0.006 and 1.79 ± 0.011, respectively. The PEE50 and PEE100 treatment also showed increased IgG level (2.02 ± 0.031 and 2.12 ± 0.032, respectively) when compared to control group ().
Discussion
Ayurveda is a holistic system of medicine in which the underlying approach to health is not only the treatment of the disease but also the treatment of the cause along with it. Ayurvedic rasayana therapy uses herbs for improving overall resistance of the body against common infections and pathogens besides providing specific effects. The evidence gathered from this study supports conventional claims for the use of A. pyrethrum as a putative, protective and immunomodulatory agent (CitationCSIR, 2002; CitationPatwardhan et al., 2005). The petroleum ether extract of A. pyrethrum roots emerged as an effective immunostimulant principle. Boosting of non-specific immunity was validated by carbon clearance test, and survival rate evaluation against fungal infection. Carbon clearance test is an indicator of in vivo phagocytic activity of macrophages. Enhanced opsonization of microorganisms leading to their rapid clearance from the blood is reflected by the increased survival rate of extract-treated animals in the Candida albicans infection group. Enhancement of percentage neutrophil adhesion has been correlated with the ability of neutrophils to stimulate non-specific immune response due to the peripheral movement of neutrophils and consequently immune activation (CitationRoitt et al., 1993). The extracts were also effective in enhancing the humoral and cell-mediated immune responses.
Augmentation of the humoral response is evidenced by increased antibody production in response to SRBC challenge during post-immunization drug treatment. Enhanced responsiveness is an indication of macrophages and B-lymphocyte subsets involved in antibody synthesis. During responses, sensitized T-lymphocytes, when challenged by the antigen, are converted to lymphoblasts and secrete lymphokines, attracting more scavenger cells to the site of reaction (CitationFulzele et al., 2002). Since drugs augmented the circulating antibody titer, it was thought worthwhile to evaluate their effect on cyclophosphamide induced immunosuppression. A high degree of cell proliferation renders the bone marrow a sensitive target, particularly to cytotoxic drugs. In fact, bone marrow is the organ most affected during any immunosuppression therapy within this class of drug. Loss of stem cells and inability of the bone marrow to regenerate new blood cells results in thrombocytopenia and leukopenia (CitationAgrawal et al., 1999). In the case of cyclophosphamide-induced immunosuppression, plant extract was able to bring back the levels of WBC, RBC, and other blood parameters responsible for protection against cyclophosphamide. The protective effect offered by PEE of A. pyrethrum against myelosuppression induced by cyclophosphamide was comparable with studies on other drugs, e.g. Chlorophytum borivilianum Santapau & Fernandes and Withania somnifera (L.) Dunal (CitationZiauddin et al., 1996; CitationThakur et al., 2007).
Petroleum ether extract of A. pyrethrum investigated in the present study was found to contain alkylamide as revealed by phytochemical characterization. Alkylamides have been reported to possess potent immunostimulating activity and hence the alkylamide content may be implicated for the observed effects for A. pyrethrum petroleum ether extract. Alkylamides have been described as producing a strong stimulation of phagocyte function as well as lipoxygenase inhibiting activity (CitationMorazzoni et al., 2005). Similarly, alkylamides of Echinacea purpurea (L.) Moench have been shown to stimulate alveolar macrophage (CitationGoel et al., 2002). In our study there was a remarkable improvement in phagocytic function evident from rapid clearance of carbon particles. This could be correlated with the presence of alkylamides in petroleum ether extract of A. pyrethrum. CitationRehman et al. (1999) reported the effectiveness of alkylamide-containing extract in increasing the production of antigen-specific immunoglobulin G and M. Since IgG, IgM are distinctly associated with cell mediated immunity, hence an increase in their level suggests an increase in the ability to improve specific immune response. In our study, an increase in IgG level was observed which again may be attributed to the presence of alkylamide in the light of earlier observation. Apart from this, alkylamides have been reported to improve non-specific immune response, percentage survival rate against C. albicans infection (CitationMorazzoni et al., 2005) and suppression of IL-2 and Cox-2 inhibitors (CitationHinz et al., 2007). All these results provide valid support to strengthen the claims for immunostimulant activity of A. pyrethrum.
Conclusion
Akarkara is advocated as a rasayana drug in Ayurvedic medicine. Besides other actions, rasayana are believed to act by boosting immunity. Alkylamide may be providing an important contribution in the overall activity postulated for this herb. To the best of our knowledge, this is the first report of overall immune potentiation by administration of petroleum ether extract of A. pyrethrum. Echinacea purpurea has established itself as immunomodulator in various studies and alkylamide and polymeric carbohydrates (mainly inulin-type fructans) have been shown to contribute to the observed activity. The alkylamides of A. pyrethrum are chemically similar to the alkylamides of E. purpurea. The polymeric carbohydrates of A. pyrethrum are also mainly inulin-type fructans, thus providing a close chemical resemblance between the two plants. Therefore, we suggest that further studies may prove the traditional claim attributed to this drug.
Declaration of interest
Funding by the All India Council for Technical Education, New Delhi, India in the form of National Doctoral fellowship is duly acknowledged.
References
- Agrawal R, Diwanay S, Patki P, Patwardhan B (1999): Studies on immunomodulatory activity of Withania somnifera (Ashawagandha) extracts in experimental immune inflammation. J Ethnopharmacol 67: 27–35.
- Atal CK, Sharma ML, Kaul A, Khajuria A (1986): Immunomodulating agents of plant origin: Preliminary screening. J Ethnopharmacol 18: 133–141.
- Bendjeddou D, Lalaoui K, Satta D (2003): Immunostimulating activity of the hot water soluble polysaccharide extracts of Anacyclus pyrethrum, Alpinia galangal and Citrullus colocynthis. J Ethnopharmacol 88: 155–160.
- Bentley R, Trimen H (2004): Medicinal Plants Volume 3. New York: Informa Healthcare.
- Biozzi G, Benacerraf B, Halpern BN (1953): Quantitative study of the granulopoetic activity of reticulo endothelial system II: A study of kinetics of the RES relationship between weight of organs and their activity. Br J Exp Biol 34: 441–456.
- Crombie L (1954): Isolation and structure of an N-isobutyl-dienediynamide from pellitory (Anacyclus pyrethrum DC.). Nature 174: 832–833.
- CSIR (2002): The Wealth of India. New Delhi, India: Council for Scientific and Industrial Research Publishers.
- Dahanukar SA, Thatte UM (1997): Current status of Ayurveda in phytomedicine. Phytomedicine 4: 359–368.
- Damre AS, Gokhale AB, Phadke AS, Kulkarni KR, Saraf MN (2003): Studies on the immunomodulatory activity of flavonoidal fraction of Tephrosia purpurea. Fitoterapia 74: 257–261.
- Fulzele SV, Burchandani PM, Kanoje VM, Joshi SB, Dorle AK (2002): Immunostimulant activity of Ashtamangal ghrita in rats. Indian J Pharmacol 34: 194–197.
- Goel V, Chang C, Slama JV, Barton R, Bauer R, Gahler R, Basu TK (2002): Alkylamides of Echinacea purpurea stimulate alveolar macrophage function in normal rates. Int Immunopharmacol 2: 381–387.
- Gulland JM, Hopton GU (1930): Pellitorine, the pungent principle of Anacyclus pyrethrum. J Chem Soc (London) 6–11. DOI: 10.1039/JR9300000006.
- Hajto T, Hostanska K, Berki T, Palinkas L, Boldizsar F, Nemeth P (2005): Oncopharmacological perspectives of a plant lectin (Viscum album agglutinin-I): Overview of recent results from in vitro experiments and in vivo animal models, and their possible relevance for clinical applications. Evidence Based Complement Altern Med 2: 59–67.
- Hinz B, Woelkart K, Bauer R (2007): Alkamides from Echinacea inhibit cyclooxygenase12 activity in human neuroglioma cells. Biochem Biophys Res Commun 360: 441–446.
- Lagrange PH, Mackaness GB, Miller TE (1974): Potential of T cell mediated immunity by selective suppression of antibody formation with cyclophosphamide. J Exp Med 139: 1529–1539.
- Leitao da-Cunha EV, De Oliveira Chaves MC (2001): Two amides from Piper tuberculatum fruits. Fitoterapia 72: 197–199.
- Michalek SM, Morisaki I, Harmon CC, Hamada S, Mcghee JR (1983): Effective immunity to dental caries: Gastric intubation of Streptococcus mutans whole cells or cell walls induces protective immunity in gnetobiotic rats. Infect Immun 39: 645–654.
- Ministry of Health and Family Welfare (2004): The Ayurvedic Pharmacopoeia of India. New Delhi: Ministry of Health and Family Welfare.
- Morazzoni P, Cristoni A, Pierro FDi, Avanzini C, Ravarino D, Stornello S, Zucca M, Musso T (2005): In vitro and in vivo immune stimulating effects of a new standardized Echinacea angustifolia root extract (Polinacea). Fitoterapia 76: 401–411.
- Mukerji B (1953): The Indian Pharmaceutical Codex Vol.11 Indigenous Drugs. New Delhi: Council for Scientific and Industrial Research.
- Patwardhan B, Warude D, Pushpangadan P, Bhatt N (2005): Ayurveda and traditional Chinese medicine; A comparative overview. Evidence Based Complement Altern Med 2: 465–473.
- Puri HS (2003): “Rasayana”− Ayurvedic Herbs for Longevity and Rejuvenation. London: Taylor and Francis.
- Ramnath V, Rekha PS, Sujatha KS (2008): Amelioration of heat stress induced disturbances of antioxidant defense system in chicken by Brahma Rasayana. Evidence Based Complement Altern Med 5: 77–84.
- Rehman J, Dillow JM, Carter SM, Chor J, Le B, Maisel AS (1999): Increased production of antigen1 specific immunoglobulines G and M following in vivo treatment with the medicinal plants Echinacea angustifolia and Hydrastis canadensis. Immunol 68: 391–395.
- Roitt I, Brostoff J, Male D (1993): Immunology, third edition. London: Mosby.
- Thakur M, Bhargava S, Dixit VK (2007): Immunomodulatory activity of Chlorophytum borivilianum Sant. F. Evidence Based Complement Altern Med 4: 419–423.
- Thakur M, Dixit VK (2008): A review on some important medicinal plants of Chlorophytum spp. Pharmacog Rev 2: 168–172.
- Wilkonson PC (1978): Neutrophil adhesion test, in: Vane JR, Ferraria SH, eds. Handbook of Experimental Pharmacology. Berlin: Springer Verlag, pp. 109–110.
- Ziauddin M, Phansalkar N, Patki P, Diwanay S, Patwardhan B (1996): Studies on immunomodulatory effects of Ashwagandha. J Ethnopharmacol 50: 69–76.