Abstract
Context: Xanthine oxidase (XO) is a key enzyme in the pathophysiological homeostasis of hyperuricemia. It catalyzes the oxidation of hypoxanthine to xanthine and then to uric acid, the reaction involves the formation of free radical intermediates and superoxide byproducts.
Objectives: This study was undertaken to investigate the antioxidant, antihyperuricemic, and xanthine oxidase inhibitory potentials of Hyoscyamus reticulatus L. (Solanaceae) extract.
Materials and methods: The antioxidant potency was measured using the ABTS•+ scavenging capacity system, which includes Trolox as a standard. The xanthine oxidase inhibitory activity of the extract was quantitated in vitro by measuring the decline in the catalytic rate of xanthine oxidase following incubations with the plant extracts and using xanthine as a substrate. The hypouricemic potential of the extract was evaluated using an in vivo model for hyperuricemia. We tested three different doses of the extract and allopurinol was used as standard antihyperuricemic positive control.
Results: H. reticulatus aqueous extract exhibited significant antioxidant scavenging properties (533.26 μmol TE/g dry extract weight) and inhibitory effect on xanthine oxidase activity (IC50 12.8 μg/mL). Furthermore, oral administration of the aqueous extract significantly reduced serum urate levels in oxonate-induced hyperuricemic mice in a dose-dependent manner.
Discussion and conclusion: Our results suggest that the aqueous extract of H. reticulatus aerial parts might have great potential as an antioxidant and a hypouricemic agent. Our lab is currently identifying the active compounds in the extract to which the biological activities could be attributed.
Introduction
Free radicals may be defined as any chemical species capable of existing with one or more unpaired outer shell electrons. They are extremely reactive and generally highly unstable (CitationMartinez-Cayuela, 1995). Free radicals have been involved in the pathophysiology and progression of a vast array of diseases. These include aging, coronary heart disease, inflammation, stroke, Parkinson’s disease, diabetes mellitus, rheumatism, liver disorders, renal failure, cancer and Alzheimer’s disease (CitationDormandy, 1983; CitationCos et al., 1998; CitationCheng et al., 2003; CitationDew et al., 2005; CitationLoscalzo, 2008; CitationMoller et al., 2008). The term “reactive oxygen species (ROS)” often describes free radicals such as ·O2− (superoxide anion), ·OH− (hydroxyl radical), H2O2 (hydrogen peroxide), and 1O2 (singlet oxygen). These reactive species can pose a detrimental risk on cellular integrity and functions. Their effects include a wide spectrum of cellular injuries such as peroxidation of polyunsaturated fatty acids in biological membranes (CitationHalliwell, 1994; CitationLoscalzo, 2008; CitationMoller et al., 2008), DNA damage (CitationAmoroso et al., 2008; CitationLi et al., 2008; CitationMartinez & Andriantsitohaina, 2009), protein damage, and oxidation of enzymes in the human body (CitationBartold et al., 1984; CitationVarani et al., 1985; CitationMartinez-Cayuela, 1995; CitationMartinez & Andriantsitohaina, 2009). The best defensive strategy against the damaging effects of free radicals is to inhibit their formation. Unfortunately, the endless supply of free radicals via endogenous and exogenous sources makes it practically impossible to implement such strategy. Examples of exogenous sources include ionizing radiation, tobacco smoke, pesticides, pollutants, and drugs. Endogenously, free radicals are produced virtually in all cells, as metabolic byproducts, via a number of intracellular systems. These include small cytoplasmic molecules, cytoplasmic proteins, membrane enzymes, peroxisomes, mitochondrial electron transport systems, and microsomic electron transport systems (CitationMartinez-Cayuela, 1995).
Xanthine oxidase (XO) is an important biological generator of oxygen free radicals (CitationMatata & Elahi, 2007). XO catalyzes the metabolism of hypoxanthine to xanthine and then xanthine to uric acid in the presence of molecular oxygen. In the process, superoxide anion and hydrogen peroxide are generated (CitationHo et al., 1999). XO has been shown to be a key player in the etiology and progression of gouty arthritis. The hall mark of the disease is hyperuricemia (high serum levels of uric acid), which leads to uric acid deposition in the joints. Hyperuricemia is present in about 5-30% of the general population. The rate seems to be increasing worldwide, however. The clinical outcome is a chronic and painful inflammation that worsens with prolonged escalation of serum levels of uric acid. Further, it has been shown that hyperuricemia is implicated in acute gout attacks, tophaceous gout, gouty nephropathy and nephrolithiasis (CitationWatanabe et al., 2002; CitationVazquez-Mellado et al., 2004; CitationForbes et al., 2008). Therefore, inhibition of uric acid formation through the XO pathway cannot be over emphasized as a promising strategy to combat hyperuricemia and its complications (CitationGiorgi et al., 2009).
A legitimate challenge, however, is the availability of a XO inhibitor that is both safe and effective for long term clinical use. To find such inhibitor, botanical sources were relentlessly investigated to fish out any clinically applicable XO inhibitors (CitationKong et al., 2000; CitationSweeney et al., 2001; CitationZhu et al., 2004; CitationZhao et al., 2006; CitationWang et al., 2008).
Our current manuscript summarizes a significant amount of work that was undertaken to screen plant species native to Jordan to investigate their potential use as natural XO inhibitors. After initial screening, we proceeded to further investigate the more promising herbal extracts. Hence, the in vitro and in vivo XO inhibitory activity of the aqueous extract of Hyoscyamus reticulatus L. (Solanaceae) aerial parts collected from northern region of Jordan was further investigated. H. reticulatus, commonly known as henbane, is a medicinal plant rich in tropane alkaloids, mainly hyoscyamine and scopolamine, widely used for their mydriatic, antispasmodic, anticholinergic, analgesic and sedative properties. In addition, the plant has traditional uses and claimed benefits in asthma, gastric ulcers and Parkinson’s disease, however, erroneous use of this plant would be toxic (CitationMateus et al., 1998). Other secondary metabolites detected in Hyoscyamus species include: flavonoids, chlorogenic acid, tannins, and coumarins (CitationHoppe, 1975) These metabolites have been suggested to have significant anti-inflammatory and free radical scavenging (antioxidant) activities (CitationFunabiki et al., 1999). In this study, we investigated the hypouricemic effects of H. reticulatus aqueous extract in an in vivo model for hyperuricemia. Hyperuricemia was induced by treating the BALB/c mice with oxonate. Phenolic content of the plant extract, the antioxidant and the xanthine oxidase inhibitory activities were quantitated.
Materials and methods
Chemicals
Xanthine oxidase (EC 1.1.3.22) isolated from cow’s milk (1.3 units/mL), xanthine, allopurinol, gallic acid, Folin-Ciocalteu reagent, and the antioxidant assay kit [myoglobin, 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid (ABTS), hydrogen peroxide, and Trolox were all obtained from Sigma (USA).
Preparation of H. reticulatus extracts
The aerial parts of H. were collected from the northern region of Jordan in 2008, and were authenticated by K. Tawaha, Department of Medicinal Chemistry and Pharmacognosy, School of Pharmacy, University of Jordan, Amman, Jordan. A voucher specimen was deposited in the Department of Pharmaceutical Sciences/Faculty of Pharmacy, University of Jordan. The plant raw materials were cleaned and air-dried at room temperature. The dried aerial parts of H. reticulatus were minced into small pieces. Leafy material (20 g) was extracted by incubating the extract with 100 mL of ultra-purified water for 60 min at 85°C with continuous shaking (CitationTawaha et al., 2007). This extract was filtered and subsequently freeze-dried and the lyophilized particles were kept in air-tight brown bottles until used.
Animals
Male BALB/c mice (20–25 g), approximately 10 weeks old, were used in this study. The animals were obtained from the University of Jordan Animal Facility, a nationally accredited facility, and fed standard chow pellets and water ad libitum. All mice were acclimated for one week before commencing the experiments. All the animals were treated according to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH, 1996). The experimental procedures were approved by the ethical committee in the Faculty of Pharmacy/University of Jordan.
Hyperuricemia model in mice
The uricase inhibitor potassium oxonate was used to induce hyperuricemia in Balb/c mice as previously described (CitationStavric et al., 1975; CitationZhu et al., 2004; CitationZhao et al., 2006). Briefly, mice were injected intraperitoneally with potassium oxonate at a dose of 250 mg/kg. All injections were given 1 h before treatments to increase serum and hepatic urate levels.
Animal treatments
All the animals were fasted for 6 h prior to treatments. The lyophilized H. reticulatus extract and allopurinol, at various concentrations were dissolved in phosphate buffer saline solution (PBS, pH = 7.4). The final form was either a solution or a suspension depending on the solubility in PBS. The mice were divided into six groups, five animals in each group. The first group was the no-treatment control and did not receive any potassium oxonate injections. The second group was the hyperuricemia group. They were treated with potassium oxonate, but did not receive further treatment. Both of the aforementioned groups were given PBS to eliminate any vehicle-related variations. The four treatment groups (groups I-IV) received the plant extracts at doses of 200, 400, and 600 mg/kg and allopurinol at 20 mg/kg dose as a positive control for hypouricemic effects, respectively (CitationZhao et al., 2006). The doses given to the mice were calculated based on the body weight measured immediately prior to each dose. All treatments were given orally once daily for 5 days.
Uric acid assay
One hour after the final treatment, whole blood samples were collected from the retro-orbital plexus of the eye of each mouse in the six groups. The blood was allowed to clot for 30 min at room temperature and then centrifuged at 3000 rpm for 5 min to obtain the serum. The serum was stored at −20°C until assayed. The uric acid level was determined by the phosphotungstic acid method, as described previously (CitationCarroll et al., 1971).
Determination of total antioxidant activity
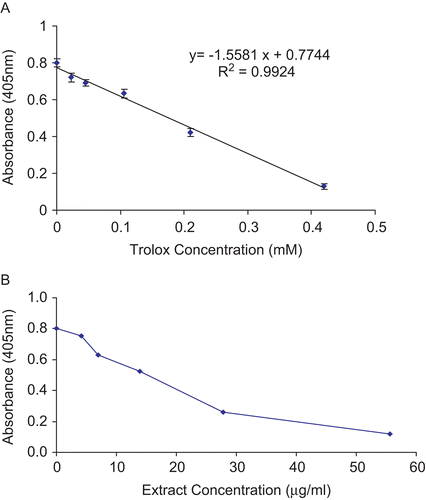
The antioxidant activity was measured using a common antioxidant assay kit (Sigma, USA). Briefly, the ABTS•+ scavenging capacity of each H. reticulatus extract was quantitated by comparing the antioxidant potential to the commercially available, water soluble vitamin E analog, Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxilic acid) as described previously (CitationMiller & Rice-Evans, 1997; CitationRe et al., 1999). A standard curve for ABTS•+ scavenging capacity was obtained using six escalating concentrations of Trolox (0.00-0.42 mM). All of the measurements were done in triplicates. Aqueous extracts of the plant in a concentration range of 0–60 μg/mL were used in this assay. The reactive mixture was allowed to stand at room temperature for 5 min and the absorbance was recorded immediately at 405 nm. The antioxidant activity of Trolox suppressed the production of the ABTS radical in a concentration-dependent manner, and the color intensity decreased proportionally (). The ABTS•+ scavenging capacity of the crude aqueous H. reticulatus extracts was calculated as Trolox equivalents (TE, μmol Trolox equivalents per g dry weight of plant), using the following formula:
where X is the amount of antioxidant of test sample (μmol) relative to Trolox standard; is the average absorbance of three replicates of the test sample at 405 nm; Intercept is the intercept of the Y axis by the Trolox standard curve (); Slope is slope of the standard curve. Both the intercept and the slope were obtained from equation of the linear regression of the standard curve (); D is the dilution factor, fold dilution of the original sample (only applicable if the sample was diluted prior to adding to the well); V is the volume of the original sample (μL).
Figure 1. (A) Concentration-response curve for the absorbance at 405 nm for ABTS•+ as a function of standard Trolox solution. Data are expressed as mean of three replicates ± SEM. (B) ABTS•+ scavenging capacities of five concentrations of H. reticulatus aqueous extract. Data are expressed as the mean of three replicates.
Measurement of total phenolic content
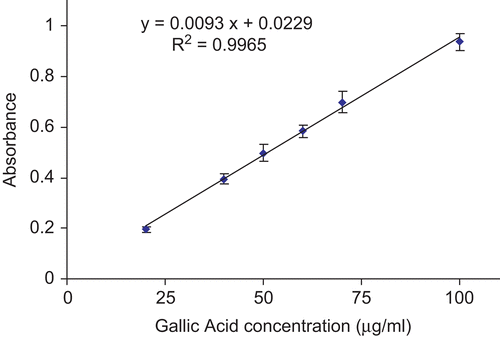
The total phenolic content was measured using the Folin-Ciocalteu’s reagent method as described previously (CitationSingleton & Rossi, 1965; CitationTawaha et al., 2007). The reagent is a mixture of phosphomolybdate and phosphotungstate that is frequently utilized in the colorimetric assays of phenolic and polyphenolic antioxidants. Briefly, in this assay, 500 μL of the aqueous plant extract (1.5 mg/mL) was mixed with 0.25 mL of Folin-Ciocalteu’s reagent and 2.0 mL of saturated sodium carbonate (75 g/L). The mixture was incubated at 30°C for 1.5 h. The absorbance was then measured at 767 nm. Gallic acid in 80% methanol was used as the standard for the calibration curve. All measurements were done in triplicates. The total phenolic content was expressed as mg gallic acid equivalents (GAE) per gram of tested dry sample, using the equation obtained from the linear regression of concentration-response curve of the gallic acid standard ().
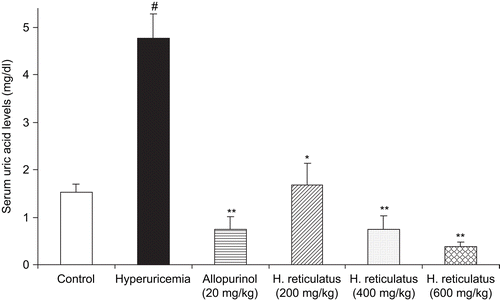
Figure 2. Effects of H. reticulatus aqueous extract and allopurinol on serum urate levels in the hyperuricemic mice pretreated with the potassium oxonate. Data represent mean values (± SEM) of urate levels in serum (mg/dL) in the groups of animals (n = 5). #P <0.001 vs. normal control group, *P <0.01, **P <0.001 versus hyperuricemic control group.
Xanthine oxidase activity
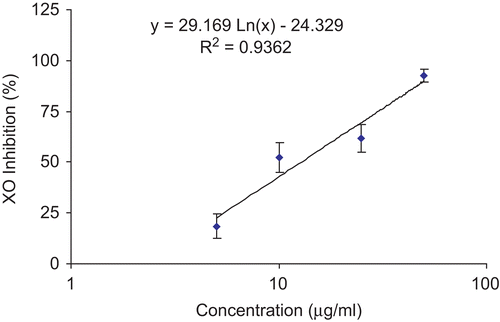
The xanthine oxidase inhibitory activity was quantitated as previously described (CitationChiang et al., 1994; CitationKong et al., 2001; CitationSweeney et al., 2001). The substrate and the enzyme solutions were prepared immediately before use. The reaction mixture was composed of 80 mM sodium pyrophosphate buffer (pH = 8.5), 120 mM xanthine and 0.1 IU of xanthine oxidase. Four concentrations of freeze-dried plant extract were used for this assay. The frozen extracts were dissolved and diluted in the reaction mixture, containing everything but the xanthine oxidase. The concentrations were 5, 10, 25 and 50 μg/mL. Xanthine oxidase was added, then incubated at 25°C as described previously. The absorption at 295 nm correlated with the amount of uric acid in the solution. The initial rate of uric acid formation was calculated for each extract concentration. Then IC50 was calculated (the concentration that inhibits 50% of the XO enzyme activity). All of the incubations and measurements were done in triplicates. A negative control (blank) was prepared by adding purified water to the reaction mixture instead of the aqueous plant extract. Allopurinol, a known inhibitor of XO, was used as a positive control in this assay. XO inhibitory activity was calculated as follows:
where test inclination is the linear change in absorbance per minute of test material, and blank inclination is the linear change in absorbance per minute of blank.
Statistical analysis
All of the incubations and measurements in this work were done in triplicates. Results were expressed as mean ± standard error of the mean (SEM). The statistical significance of the differences between the groups was calculated using Student’s t-test for independent means. Two-tailed values of P < 0.05 were considered significant.
Results
Dose-dependent lowering of hyperuricemia by H. reticulatus aqueous extracts in the Balb/c-potassium oxonate hyperuricemic mouse model
Injection of the mice with potassium oxonate caused hyperuricemia, as indicated by drastic increase in urate levels in serum (). Potassium oxonate, a selective competitive uricase inhibitor, blocks the effect of hepatic uricase and produces hyperuricemia in rodents (CitationStavric et al., 1975; CitationZhao et al., 2006; CitationWang et al., 2008). The Balb/c-potassium oxonate mouse has been widely applied to study the pathophysiology of hyperuricemia and therapeutic modulators of uric acid formation. The serum urate dose-dependent lowering effects of H. reticulatus aqueous extracts were investigated after a 5-day treatment regimen as described in Materials and methods. A significant difference was observed in serum urate levels between groups that received the aqueous extract at the doses of 200, 400, or 600 mg/kg and the hyperuricemic control group ( and ). Allopurinol, at 20 mg/kg, also significantly reduced serum urate levels in the hyperuricemic mice. The 20 mg/kg dose was chosen to ensure relevance to the human allopurinol starting dose of 100-200 mg daily. Interestingly, at the aforementioned dose allopurinol was found to be more potent than the 200 mg/kg H. reticulatus aqueous extract, but less potent than the 600 mg/kg dose. There was no statistically significant difference in serum urate levels between mice treated with the 400 mg/kg extract and allopurinol. Our data confirmed that the serum urate levels inversely correlated with the H. reticulatus aqueous extract dose. The data suggest a dose-dependent hyperuricemic lowering effect of the plant.
Table 1. Average serum urate levels of oxonate-Balb/c mice after oral administration of three doses of H. reticulatus extract concentrations (200, 400 and 600 mg/kg).
Antioxidant activity
The concentration-response curves for ABTS•+, as a function of six concentrations of the Trolox standard (0.0, 0.023, 0.045, 0.105, 0.21, and 0.42 mM) with r2 of 0.9924 (see Materials and methods), are shown in . Concentration-dependent ABTS•+ scavenging capacities of H. reticulatus aqueous extracts were measured at five different concentrations (). The plant had a pronounced antioxidant activity of 533.26 ± 93.2 μmol TE/g expressed in terms of Trolox equivalent antioxidant capacity (μmol Trolox equivalents per g dry weight of plant) as described in Materials and methods.
Total phenolic content
A linear calibration curve of gallic acid, in the range of 20-100 μg/mL with R2 value of 0.9965, was constructed (). The total phenolic content was expressed in units of mg of gallic acid equivalent (GAE) per g freeze-dried extract. The total phenolic content of H. reticulatus aqueous extract was 38.1 ± 0.5 mg GAE/g freeze-dried plant extract.
Inhibitory effect on xanthine oxidase activity
The H. reticulatus aqueous extract exhibited an inhibitory effect on the xanthine oxidase induced superoxide/uric acid formation in a concentration-dependent manner (). At concentrations of 5–50 μg/mL, the percentage of enzyme inhibition ranged from 18 to 93%, respectively (). The IC50 value of the plant extract was 12.8 μg/mL. Allopurinol, on the other hand, showed an IC50 value of 4.6 μg/mL.
Discussion
The decrease in the efficacy to neutralize reactive oxygen species (ROS) can lead to detrimental cellular and biological outcomes. Aerobic organisms are protected from ROS toxicity by their natural antioxidant defense systems involving enzymatic and non-enzymatic mechanisms. An imbalance between the amount of ROS and the antioxidant defense systems is a common feature in a broad spectrum of human pathologies. Therefore, it is pivotal to fortify the endogenous antioxidant defense systems with external sources of potent antioxidants in order to maintain a healthier and more prolonged living (CitationHalliwell, 1994; CitationSchinella et al., 2002). Plants, in general, are the primary reservoir of the antioxidants consumed by humans.
In this study, the antioxidant and XO inhibition activities of the aqueous extract of a Jordanian plant, H. reticulatus, was investigated. An electron transfer-based antioxidant assay was applied to evaluate the antioxidant activities of the extract. The plant extract exhibited excellent antioxidant activity as measured by the ABTS•+ scavenging assay (, see Results). Our results show that the antioxidant capacity of the H. reticulatus extract is dose-dependent. Several Hyoscyamus species of different origins have been previously reported to posses antioxidant activities (CitationAlali et al., 2007; CitationRamadan et al., 2007; CitationTawaha et al., 2007). The antioxidant capacity measured in the ABTS•+ scavenging assay indicates the presence of water soluble antioxidant compounds in the plant extract. The antioxidant activity of Hyoscyamus species has been suggested to be attributed to its content of phenolic compounds (CitationAlali et al., 2007). A plethora of previously published phytochemical research indicated that many of the pharmacological effects of Hyoscyamus species could be attributed to its tropane alkaloids; hyoscyamine and scopolamine. However, the biological activities of the other secondary metabolites detected in Hyoscyamus plants including flavonoids (rutin), chlorogenic acid, tannins and coumarins, should not be overlooked (CitationHoppe, 1975). The latter compounds have favorable water solubility and can be extracted in an aqueous medium. Their chemical structures predicted strong antioxidant activities. Indeed, rutin has been shown to exhibit significant anti-inflammatory and free radical scavenging (antioxidant) activities (CitationFunabiki et al., 1999). Further, a compelling body of research has put chlorogenic acid, tannins and coumarins among the elite members of the free radical neutralizing phytochemicals (CitationKontogiorgis et al., 2006; CitationKoleckar et al., 2008). Thus, it is quite plausible to attribute the antioxidant activity or at least part of it in aqueous extracts H. reticulatus to those very same phytochemicals. Further investigation, however, is warranted to confirm this theory.
A tight correlation between antioxidant activities and total phenolic contents of plant extracts was illustrated by various research groups (CitationZheng & Wang, 2001; CitationCai et al., 2004; CitationAlali et al., 2007; CitationTawaha et al., 2007). In order to verify the correlation between the phenolic content and the antioxidant activity of H. reticulatus, we measured total phenolic content of the H. reticulatus aqueous extract using Folin-Ciocalteu assay. The total phenolic content was 38.1 mg GAE/g dry extract weight. It is generally recognized the antioxidant activities of phenolic compounds are predominantly due to their redox potentials, which enable them to function as reducing agents, hydrogen donors, and singlet oxygen quenchers. In addition to their antioxidant activities, a number of phenolic compounds have been reported to have metal-chelating potentials (CitationRice-Evans et al., 1995).
Finally, the current study shows that H. reticulatus aqueous extract was also able to inhibit XO activity, in vitro, in a marked dose-dependent manner (, IC50 value of 12.8 μg/mL). To confirm the clinical significance of this inhibition we proceeded with an in vivo study to evaluate the effects of the extract on serum urate levels. Indeed, oral administration of H. reticulatus aqueous extract was able to significantly reduce serum urate levels in a dose-dependent manner (, see Results). Uric acid synthesis has been shown to be mainly a hepatic process; therefore, the hypouricemic effect of H. reticulatus extract could be explained, at least in part, by blocking of liver XO activities.
Conclusion
H. reticulates was evaluated for its potential antioxidant and XO inhibition activities. Our results showed that H. reticulatus aqueous extract exhibited significant antioxidant scavenging properties (533.26 μmol TE/g dry extract weight) and xanthine oxidase inhibitory activity (IC50 12.8 μg/mL). Furthermore, oral administration of the aqueous extract significantly reduced serum urate level in the oxonate-induced hyperuricemic mice in a dose-dependent manner. The antioxidant activity of the plant extract in this work suggests that the H. reticulatus aqueous extract could be further investigated for possible applications in ischemic injury, aging and to reduce or ameliorate oxidative stress in tissues. The XO inhibitory action and the hypouricemic effect of the plant extract could be quite promising in our quest to discover new therapeutic options in the management of gout or other XO-induced diseases.
Our lab is currently identifying the active compounds in the extract to which the biological activities could be attributed.
Declaration of interest
This project was sponsored by the Deanship of Scientific Research at the University of Jordan (grant No.1070) and it was conducted during the sabbatical leave of Dr. Bustanji. The authors wish to thank the Deanship of Scientific Research at the University of Jordan for their generous funds.
References
- Alali FQ, Tawaha K, El-Elimat T, Syouf M, El-Fayad M, Abulaila K, Nielsen SJ, Wheaton WD, Falkinham JO, Oberlies NH (2007): Antioxidant activity and total phenolic content of aqueous and methanolic extracts of Jordanian plants: An ICBG project. Nat Prod Res 21: 1121–1131.
- Amoroso A, Crespan E, Wimmer U, Hubscher U, Maga G (2008): DNA polymerases and oxidative damage: Friends or foes? Curr Mol Pharmacol 1: 162–170.
- Bartold PM, Wiebkin OW, Thionard JC (1984): The effect of oxygen-derived free radicals on gingival proteoglycans and hyaluronic acid. J Periodont Res 19: 390–400.
- Cai Y, Luo Q, Sun M, Corke H (2004): Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74: 2157–2184.
- Carroll JJ, Coburn H, Douglass R, Babson AL (1971): Simplified alkaline phosphotungstate assay for uric acid in serum. Clin Chem 17: 158–160.
- Cheng HY, Lin TC, Yu KH, Yang CM, Lin CC (2003): Antioxidant and free radical scavenging activities of Terminalia chebula. Biol Pharm Bull 26: 1331–1335.
- Chiang HC, Lo YJ, Lu FJ (1994): Xanthine oxidase inhibitors from the leaves of Alsophila spinulosa (Hook) Tryon. J Enzyme Inhib 8: 61–71.
- Cos P, Ying L, Calomme M, Hu JP, Cimanga K, Van Poel B, Pieters L, Vlietinck AJ, Vanden Berghe D (1998): Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J Nat Prod 61: 71–76.
- Dew TP, Day AJ, Morgan MR (2005): Xanthine oxidase activity in vitro: Effects of food extracts and components. J Agric Food Chem 53: 6510–6515.
- Dormandy TL (1983): An approach to free radicals. Lancet 2: 1010–1014.
- Forbes JM, Coughlan MT, Cooper ME (2008): Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 57: 1446–1454.
- Funabiki R, Takeshita K, Miura Y, Shibasato M, Nagasawa T (1999): Dietary supplement of G-rutin reduces oxidative damage in the rodent model. J Agric Food Chem 47: 1078–1082.
- Giorgi A, Bombelli R, Luini A, Speranza G, Cosentino M, Lecchini S, Cocucci M (2009): Antioxidant and cytoprotective properties of infusions from leaves and inflorescences of Achillea collina Becker ex Rchb. Phytother Res 23: 540–545.
- Halliwell B (1994): Free radicals and antioxidants: A personal view. Nutr Rev 52: 253–265.
- Ho KY, Huang JS, Tsai CC, Lin TC, Hsu YF, Lin CC (1999): Antioxidant activity of tannin components from Vaccinium vitis-idaea L. J Pharm Pharmacol 51: 1075–1078.
- Hoppe HA (1975): Drogenkunde Band 1 Angiospermien, eighth edition, Berlin, New York, DeGryter, pp. 596–598.
- Koleckar V, Kubikova K, Rehakova Z, Kuca K, Jun D, Jahodar L, Opletal L (2008): Condensed and hydrolysable tannins as antioxidants influencing the health. Mini Rev Med Chem. 5, 436–447.
- Kong LD, Abliz Z, Zhou CX, Li LJ, Cheng CH, Tan RX (2001): Glycosides and xanthine oxidase inhibitors from Conyza bonariensis. Phytochemistry 58: 645–651.
- Kong LD, Cai Y, Huang WW, Cheng CH, Tan RX (2000): Inhibition of xanthine oxidase by some Chinese medicinal plants used to treat gout. J Ethnopharmacol 73: 199–207.
- Kontogiorgis CA, Savvoglou K, Hadjipavlou-Litina DJ (2006): Antiinflammatory and antioxidant evaluation of novel coumarin derivatives. J Enzyme Inhib Med Chem 21: 21–29.
- Li L, Jiang L, Geng C, Cao J, Zhong L (2008): The role of oxidative stress in acrolein-induced DNA damage in HepG2 cells. Free Radical Res 42: 354–361.
- Lin CC, Huang PC, Lin JM (2000): Antioxidant and hepatoprotective effects of Anoectochilus formosanus and Gynostemma pentaphyllum. Am J Chin Med 28: 87–96.
- Loscalzo J (2008): Membrane redox state and apoptosis: Death by peroxide. Cell Metab 8: 182–183.
- Martinez-Cayuela M (1995): Oxygen free radicals and human disease. Biochimie 77: 147-161.
- Martinez MC, Andriantsitohaina R (2009): Reactive nitrogen species: Molecular mechanisms and potential significance in health and disease. Antioxid Redox Signal 11: 669–702.
- Matata BM, Elahi MM (2007): Sources of reactive oxidants species in biology and disease. Oxid Stress: 23–38.
- Mateus L, Cherkaoui S, Christen P, Veuthey JL (1998): Capillary electrophoresis for the analysis of tropane alkaloids: Pharmaceutical and phytochemical applications. J Pharm Biomed Anal 18: 815–825.
- Miller NJ, Rice-Evans CA (1997): Factors influencing the antioxidant activity determined by the ABTS•+ radical cation assay. Free Radical Res 26: 195–199.
- Moller MN, Lancaster JR, Denicola A (2008): The interaction of reactive oxygen and nitrogen species with membranes. Curr Top Membr 61: 23–42.
- NIH (1996): Guide for the Care and Use of Laboratory Animals, (NIH Publication No. 85–23 revised). Bethesda, MD, US National Institutes of Health.
- Ramadan MF, Zayed R, El-Shamy H (2007): Screening of bioactive lipids and radical scavenging potential of some Solanaceae plants. Food Chem 103: 885–890.
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999): Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26: 1231–1237.
- Rice-Evans CA, Miller NJ, Bolwell PG, BramLey PM, Pridham JB (1995): The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res 22: 375–383.
- Schinella GR, Tournier HA, Prieto JM, Mordujovich de Buschiazzo P, Rios JL (2002): Antioxidant activity of anti-inflammatory plant extracts. Life Sci 70: 1023–1033.
- Singleton VL, Orthofer R, Lamuela-Raventos RM (1999): Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Meth Enzymol 299: 152–178.
- Singleton VL, Rossi JA (1965): Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16: 144–158.
- Stavric B, Clayman S, Gadd RE, Hebert D (1975): In vivo effects in the rat induced by chlorprothixene and potassium oxonate. Pharmacol Res 7: 117–124.
- Sweeney AP, Wyllie SG, Shalliker RA, Markham JL (2001): Xanthine oxidase inhibitory activity of selected Australian native plants. J Ethnopharmacol 75: 273–277.
- Tawaha K, Alali FQ, Gharaibeh M, Mohammad M, El-Elimat T (2007): Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem 104: 1372–1378.
- Varani J, Fligiel SE, Till GO, Kunkel RG, Ryan US, Ward PA (1985): Pulmonary endothelial cell killing by human neutrophils: Possible involvement of hydroxyl radical. Lab Invest 53: 656–663.
- Vazquez-Mellado J, Alvarez Hernandez E, Burgos-Vargas R (2004): Primary prevention in rheumatology: The importance of hyperuricemia. Best Pract Res Clin Rheumatol 18: 111–124.
- Wang SY, Yang CW, Liao JW, Zhen WW, Chu FH, Chang ST (2008): Essential oil from leaves of Cinnamomum osmophloeum acts as a xanthine oxidase inhibitor and reduces the serum uric acid levels in oxonate-induced mice. Phytomedicine 15: 940–945.
- Watanabe S, Kanellis J, Nakagawa T, Han L, Ohashi R, Lan H, Feng L, Johnson RJ (2002): Reducing uric acid as a means to prevent cardiovascular and renal disease. Expert Opin Ther Pat 12: 193–199.
- Zhao X, Zhu JX, Mo SF, Pan Y, Kong LD (2006): Effects of Cassia oil on serum and hepatic uric acid levels in oxonate-induced mice and xanthine dehydrogenase and xanthine oxidase activities in mouse liver. J Ethnopharmacol 103: 357–365.
- Zheng W, Wang SY (2001): Antioxidant activity and phenolic compounds in selected herbs. J Agric Food Chem 49: 5165–5170.
- Zhu JX, Wang Y, Kong LD, Yang C, Zhang X (2004): Effects of Biota orientalis extract and its flavonoid constituents, quercetin and rutin on serum uric acid levels in oxonate-induced mice and xanthine dehydrogenase and xanthine oxidase activities in mouse liver. J Ethnopharmacol 93: 133–140.