Abstract
Context: Rosa laevigata Michx. (Rosaceae), widespread in China, contains many valuable nutrients and has long been used as food and medicine in Chinese folklore. Nowadays, due to its favorable property of coloring, the brown pigment of R. laevigata has an attractive potential as an available additive in food.
Objective: The aims of this study were to optimize the extraction process of brown pigment from R. laevigata and investigate its antioxidant activities on the basis of its abilities to scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, hydroxyl radical and superoxide radical.
Materials and methods: Extraction conditions of brown pigment from R. laevigata were investigated through an orthogonal design of L9 (3)4 assay. Ethanol concentration, extraction temperature, time, and ratio of material to solvent were the main factors affecting the extraction rate. Subsequently, the antioxidant activity of brown pigment was assessed using DPPH method, while hydroxyl radicals and superoxide free radicals were respectively determined by the Fenton-RhB (Rhodamine B) system and using the pyrogallol-luminol system.
Results: The optimum extraction conditions were determined: temperature 70°C, ethanol concentration was 60%, extraction time 2 h and ratio of material to solvent was 1:6. Brown pigment showed a good radical scavenging activity, and exhibited a concentration-dependent inhibition of hydroxyl radical and superoxide free radical at low concentrations. When the concentration of brown pigment was 1 mg/mL, the scavenging percentage of hydroxyl radical reached 67.33%.
Discussion and conclusion: The brown pigment of R. laevigata could potentially be used as a promising natural antioxidant in the food and pharmaceutical industries.
Introduction
Edible pigments are important food additives used to improve the quality and appearance of food (CitationMadkins & Schsefer, 1998; CitationZhang et al., 2006). Therefore, they have been widely applied in the food industry in recent years as well as in the pharmaceutical and cosmetics trades. Edible pigments are divided into synthetic colorants and natural colorants according to their source. By comparison with natural colorants, synthetic colorants have a number of advantages, such as great color fastness, stable performance, high brightness, convenient usage, low costs, etc. However, with the development of medical toxicology and biology in recent years, it was found that some synthetic colorants exhibit chronic toxicity and carcinogenicity (CitationHallagan et al., 1995), hence, the application and exploration of natural colorants have been given more attention.
Most natural pigments are anthocyanins, flavonoids, or carotenoids (CitationTimberlake & Henry, 1986). Long-term practice has proved that most natural pigments possess relatively high safety; moreover, some contain essential nutrient substances such as vitamins or their close chemical analogues, carrying a wide range of biological activities and antioxidant activities vital for the human body (CitationChen & Ding, 2002; CitationLinda et al., 2004; CitationDean & Kopsell, 2006). A number of natural pigments can activate human immune cells and increase body immunity which plays an important role in the treatment and prevention of many diseases (CitationWan et al., 1997; CitationKravchenko et al., 2003). As more attention is paid to the safety of edible pigments all over the world, advocating green and returning to nature have become development trends of the food industry, bringing great opportunities for the exploration and application of natural pigments (CitationSchoefs, 2004).
Rosa laevigata Michx. (Rosaceae), containing considerable vitamins C, B1, B2, carotene, saccharide, and lipid, is widely distributed over the east, south and southwest of China (CitationMin, 2001; CitationZhou et al., 2006), and has a long history of being used as food and medicine in Chinese folklore (CitationGao et al., 1993; CitationXiao, 1999; CitationYuan et al., 2008). Recent studies have also shown that R. laevigata has antioxidation, fat suppression, anti-inflammatory, antibacterial, immunomodulatory and cholesterol lowering effects (CitationHe, 2001; CitationZhao et al., 2003). Some studies have been conducted and it was found that extract of R. laevigata could remove a great part of NO2- under simulated gastric juice conditions (CitationXie et al., 2001). It was also reported that flavonoid compounds in R. laevigata can even prevent linoleic acid from oxidant itself, indicating their high antioxidant activities (CitationChen & Zhang, 2005).
The aim of this work was to optimize extraction parameters of brown pigment derived from R. laevigata by employing an orthogonal test design. In addition, the antioxidant activities of brown pigment were assessed using the DPPH method, the hydroxyl radicals using the Fenton-RhB system, and the superoxide free radical using the pyrogallol-luminol system to get more bioactive functional information of the brown pigment from R. laevigata as a potential available additive for food industry.
Materials and methods
Materials
The dry fruit drugs of R. laevigata were purchased from a local drug store (Guangzhou, Guangdong Province, China). It was authenticated by Gang Hao, Department of Botany, South China Agricultural University and a voucher specimen of R. laevigata is deposited in his Specimen Bank of Medicinal Plants Medicinal Plants. R. laevigata in this investigation grew in Hezhou, Guangxi province, harvested in the middle of November. Then sun-dried, the burr was removed, washed and immersed in the water for several minutes. Then the nucleolus was removed and sun-dried again. The dry fruit drugs were transferred immediately to the laboratory where they were peeled and crushed into fine powder by pulverizer to be prepared as testing samples.
The luminol was purchased from Merck, (Beijing, China). Tripyridyltriazine (TPTZ) and 1,1-diphenyl-2-picrylhydrazyl (DPPH) was purchased from Sigma (Guangzhou, China). Other chemicals used were all analytical grade.
Equipment and apparatus
The following instruments were used: LDS-10 low speed centrifuge (Jintan Science Analysis Instruments, Wuxi, China); SHZ-D water circulated vacuum pump (Yuhua Instruments, Gongyi City, China); RE52A Rotary Evaporator (Shanghai Yarong Biochemical Instrument Factory, China); UV-1700 UV-VIS spectrophotometer (Unico Instruments, Shanghai); Cary Eclipse fluorescence spectrophotometer (Varian, USA); BS200s electronic analytical balance (Shanghai Balance Instrument Factory).
Extraction of brown pigment
The R. laevigata fruits were soaked in petroleum ether for 12 h at room temperature with a liquid/solid ratio 15:1. After that, the petroleum ether was poured out and a volume of fresh petroleum ether was added to the sample and soaked for a further 20–24 h with liquid/solid ratio 10:1 until the color of petroleum ether got lighter. Then collected the residue after filtration and volatilized the residual solvent. After the addition of 80% ethanol with liquid/solid ratio 5:1, the residue was heated in 90°C water bath for 1.5 h and centrifuged (15 min, 3000 rpm), then poured the supernatant carefully. The precipitate was re-extracted to remove any oligosaccharide. Then, collecting the precipitate and vaporizing the ethanol to give a dry powder for the following experiment.
The pretreated sample (10 g) was extracted with ethanol (concentration ranging from 20 to 70%) and the extraction time ranging from 0.5 to 3 h (0.5, 1, 1.5, 2, 2.5 and 3 h), while the temperature of the water bath ranged from 30 to 90°C and the ratio of material to solvent ranged from 1:2 to 1:14 (CitationSun et al., 2009). The pretreated R. laevigata was extracted twice. The sample solution with brown color was collected by filtration, and then absorbance of the solution was measured using a spectrophotometer at 390 nm (CitationYu et al., 1995).
Optimization of extraction conditions
An orthogonal L9 (3)4 test design was used to investigate the optimal extraction condition of brown pigment from R. laevigata () (CitationGuo et al., 2007a). As seen from , the experiment was carried out with four factors and three levels, namely extraction temperature (50, 70, 90°C), ethanol concentration (80, 60, 40%), extraction time (2, 4, 6 h), and ratio of material to solvent (1:6, 1:8, 1:10). The range of each factor level was based on the results of single factor tests. The extraction rate of brown pigment was the dependent variable.
Table 1. Main factors and their level settings used for the optimization extraction of brown pigment.
Scavenging activity on DPPH radical
The scavenging activity of brown pigment on DPPH radical was determined according to the reported method (CitationPan et al., 2009; CitationOliveira et al., 2009) with some modifications. In brief, the brown pigment was dissolved in 95% ethanol to give a final concentration of 0.01%. Then 2 mL of 2 × 10−4 mol/L DPPH in absolute ethanol was added to 2 mL of 0.01% brown pigment solution. The solution was then mixed vigorously and allowed to stand at room temperature for 30 min until the reaction reached steady state. The absorbance value was measured at 517 nm using a UV-1700 UV-VIS spectrophotometer (CitationChandrasekar et al., 2006). For the control, the assay was conducted in the same manner but 95% ethanol was added instead of DPPH solution. The blank was prepared in the same manner, excepting that 95% ethanol was used instead of sample solution. The scavenging activity of brown pigment on DPPH radical was calculated according to the following equation:
where A is the absorbance of sample, A1 is the absorbance of the control and A0 is the absorbance of the blank. The scavenging activity of butylated hydroxytoluene (BHT) and vitamin C was also assayed for comparison.
Scavenging activity on hydroxyl radical
A fluorometric method was adopted to evaluate the scavenging activity of brown pigment on hydroxyl radical using Fenton-RhB system (CitationYu et al., 2008; CitationPan et al., 2009). The brown pigment aqueous solution was added to media containing 6 mL Tris-HCl buffer solution (pH 5.5), 19.2 μL 1.92 × 10−3 mol/L RhB, and 1.28 mL 0.64 × 10−3 mol/L FeSO4, 0.48 mL 4.08 × 10−3 mol/L H2O2 was added last. After gentle stirring for 5 min, the test solution was then diluted to 10 mL with distilled water. The absorption of RhB was measured at 550 nm after standing for 30 min (CitationKi et al., 2007; CitationYu et al., 2008). The scavenging activity of brown pigment on hydroxyl radical was calculated according to the following equation:
where Fs is the absorption of RhB with brown pigment aqueous solution and Fenton reagent, F0 is the absorption of RhB in the presence of the Fenton reagent, and F is the absorption with FeSO4 and buffer only.
Scavenging activity on superoxide free radical
The scavenging activity of brown pigment on superoxide free radical was determined by a chemoluminescence (CL) method (CitationGuo et al., 2007b) in the pyrogallol-luminol system.
The luminescent reaction was initiated by adding 2 mL 1 × 10−3 mol/L luminal solution, sample solution (brown pigment aqueous solution ranging from 0.05 to 1 × 10−3 mol/L) and 20 μL distilled water to 20 μL 10 × 10−3 mol/L pyrogallol. After rapid stirring, a light intensity of 5 s was recorded. The control was performed in the same manner in the mixture without the sample solution. All tests were performed in triplicate and the results were centered. The scavenging activity of brown pigment on superoxide free radical was calculated according to the following equation:
where CL0 is the light intensity of control, CL is light intensity of sample solution.
Statistical analysis
The data were presented as mean ± standard deviation (SD). Statistically significant differences between groups were evaluated using Student’s t-test. Statistical significance was set at p <0.05, P values <0.05 were regarded as significant and P values <0.01 as extremely significant.
Results and discussion
Single factor test
As far as the ethanol concentration is concerned, the extraction rate of brown pigment affected by different ethanol concentration ranging from 20 to 70% can be seen in , when ratio of material to solvent, extraction temperature and extraction time were fixed at 1:6, 70°C and 1 h. The result implied the extraction rate of brown pigment increased with the increasing ethanol concentration. When ethanol concentration was above 60%, no significant increase in the extraction rate was observed.
Figure 1. Effect of different ethanol concentrations (A), temperatures (B), and ratios of material to solvent (C) and time (D) on extraction rate of brown pigment. The results are presented as mean ± SD of three independent experiments, and error bars show the standard deviations of duplicate determinations.
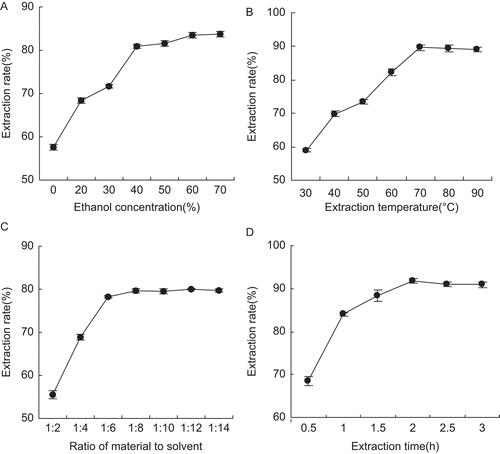
The extraction rate of brown pigment affected by different extraction temperatures ranging from 30 to 90°C was seen in , when ethanol concentration, ratio of material to solvent and extraction time were fixed at 60%, 1:6 and 1 h. With the increase of the extraction temperature from 30 to 70°C, the extraction rate of brown pigment increased quickly. In addition, the extraction rate increased little when extraction temperature surpassed 70°C. Therefore, the suitable extraction temperature for higher extraction rate of the brown pigment was considered to be 70°C.
The extraction rate of brown pigment affected by the different ratio of material to solvent ranging from 1:2 to 1:14 is shown in , when ethanol concentration, extraction temperature and extraction time were fixed at 60%, 70°C and 1 h, respectively. The result showed the extraction rate of brown pigment increased with the increasing ratio of material to solvent up to 1:8, above which there was no significant increase. Therefore, 1:8 was selected as the optimum ratio of material to solvent in consideration of the cost.
The extraction rate of brown pigment affected by different extraction times ranging from 0.5 to 3 h was seen in , when ethanol concentration, extraction temperature and ratio of material to solvent were fixed at 60%, 70°C and 1:6. The extraction rate of brown pigment increased with the extraction time and reached the peak at 2 h. However, the extraction rate had no noticeable enhancement when extraction time increased from 2 to 3 h. Thus, 2 h was selected as the optimum extraction time.
Optimization of extraction parameters
Various parameters play an important role in the optimization of the experimental conditions for the development of a solvent extraction method (CitationJing et al., 2009). On the basis of the single factor test, the combination effect of ethanol concentration, temperature, time and ratio of material to solvent on the extraction of brown pigment from R. laevigata were evaluated through an orthogonal experiment L9 (3)4 (CitationGuo et al., 2007a) in order to determine the most suitable extraction conditions (). The values of K and R were calculated by statistical software. It can be seen in that ethanol concentration plays the most important role in the extraction of brown pigment, and the order of importance that influenced extraction rate was found to be ethanol concentration > extraction temperature > extraction time > ratio of material to solvent according to the R value.
Table 2. L9 (3) 4 Matrix as the experimental design for the optimization extraction of brown pigment.
The optimum combination of variables was A2B3C1D1, i.e., extraction temperature 70°C, ethanol concentration was 60%, extraction time 2 h and ratio of material to solvent was 1:6.
Scavenging activity on DPPH radical
The DPPH radical is considered as a model of a lipophilic radical. A chain reaction in lipophilic radicals is initiated by the lipid autoxidation. The DPPH radical scavenging activity was determined by the decrease in its absorbance at 517 nm, which is induced by antioxidants (CitationShruti et al., 2009). As can be seen from , the antioxidant activity of brown pigment was evaluated by DPPH radical assay with BHT and vitamin C as positive controls. At the concentration of 0.01%, brown pigment, BHT and vitamin C all showed significant scavenging activity on DPPH radical. Brown pigment exhibited a DPPH scavenging activity of 55.68 ± 0.24%, and BHT and vitamin C showed 79.35 ± 0.37% and 96.55 ± 0.2% respectively, which means the antioxidant capacity of brown pigment was 68.89% that of BHT, and 57.12% that of vitamin C.
Table 3. The DPPH scavenging activity of brown pigment, BHT and vitamin C.
Scavenging activity on hydroxyl radical
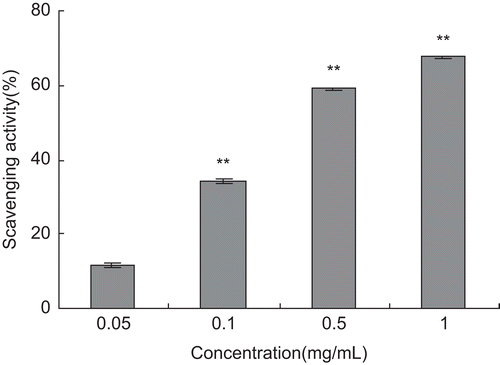
The scavenging activity of brown pigment on the hydroxyl radical was investigated in the Fenton-RhB system (CitationYu et al., 2008). shows that brown pigment was effective in scavenging hydroxyl radical, exhibiting a quite strong concentration-dependent inhibition of hydroxyl radical at low concentration. The scavenging activity of brown pigment on hydroxyl radical extraction increased with the increasing concentration. The scavenging activity on hydroxyl radical of differentiating concentration demonstrated extremely significant difference compared with that of 0.05 mg/mL (P <0.01). The scavenging activity of brown pigment on hydroxyl radical (1 mg/mL) was more than five times that of 0.05 mg/mL (67.66 ± 0.4% versus 11.73 ± 0.73%, P <0.01).
Figure 2. The scavenging activity of brown pigment on the hydroxyl radical. The scavenging activity on hydroxyl radical increased considerably in direct proportion to the differentiation concentration, the results are presented as mean ± SD of three independent experiments, and error bars show the standard deviations of duplicate determinations (**P <0.01).
Scavenging activity on superoxide free radical
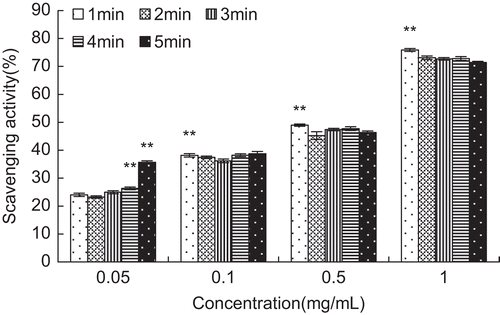
Pyrogallol can be autoxidized to generate superoxide anion in alkaline conditions. Luminol is excited by superoxide anion in this reaction, in the meantime, as the decay from the excited state is getting back to the ground state, it is accompanied with chemiluminescence (CitationGuo et al., 2007b). In the present study, chemoluminescence (CL) method in the pyrogallol-luminol system was applied to measure the scavenging activity of brown pigment on superoxide free radical. It can be seen from , as the concentration of brown pigment increased, the scavenging activity on superoxide free radical increased rapidly (P < 0.01). At 1, 2, 3, 4, 5 min, the scavenging activity of brown pigment on superoxide free radical reached significant difference respectively after the concentration up to 0.1 mg/mL or more than 0.1 mg/mL compared with that of 0.05 mg/mL (P < 0.01). In addition, at 0.05 mg/mL, the scavenging activity of brown pigment on superoxide free radical was significantly different statistically after differentiation for more than 3 min compared with that of 1 min (P < 0.01); the scavenging activity of brown pigment on superoxide free radical at 5 min was more than 1.4 times that of 1 min (35.65 ± 0.53 versus 24.03 ± 0.58, P < 0.01).
Figure 3. The scavenging activity of brown pigment on superoxide free radical. As the time and concentration of brown pigment were different, the scavenging activity changed. The results are presented as mean ± SD of three independent experiments, and error bars show the standard deviations of duplicate determinations (**P <0.01).
Conclusion
In the present study, the extraction conditions of brown pigment from R. laevigata were optimized, and the antioxidant activity of brown pigment has been evaluated. The optimal performance of extraction was obtained under extraction temperature 70°C, extraction time 2 h, and 60% ethanol solution with a ratio of material to solvent 1:6. In addition, brown pigment has a strong antioxidant potential according to the in vitro evaluation of its DPPH radical scavenging activity, hydroxyl radical scavenging activity and superoxide free radical scavenging activity, indicating that brown pigment from R. laevigata might be a valuable natural antioxidant resource and has possible application in food industries or other related fields.
Declaration of interest
This project was supported by National Key Technology R&D Program 2006BAD27B04 of China. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Chandrasekar D, Madhusudhana K, Ramakrishna S, Diwan PV. (2006). Determination of DPPH free radical scavenging activity by reversed-phase HPLC: A sensitive screening method for polyherbal formulations. J Pharm Biomed Anal, 40, 460–464.
- Chen NF, Zhang L. (2005). Study on the antioxidant property of flavonoid compound in Rosa laevigata Michx. Forest By-Prod Spec China, 5, 2–4.
- Chen XL, Ding XW. (2002). The effects of RLE on lardy autoxidation. J Chongqing Coll Educ, 15, 53–56.
- Dean AK, Kopsell DE. (2006). Accumulation and bioavailability of dietary carotenoids in vegetable crops. Trends Plant Sci, 11, 499–507.
- Gao Y, Cheng WM, Li GY. (1993). Chemical constituents of Rosa laevigata Michx. Chin J Chin Material Medica, 18, 426–428.
- Guo LY, Cho SY, Kang SS, Lee SH, Baek HY, Kima YS. (2007a). Orthogonal array design for optimizing extraction efficiency of active constituents from Jakyak-Gamcho decoction, the complex formula of herbal medicines, Paeoniae radix and Glycyrrhizae radix. J Ethnopharmacol, 113, 306–311.
- Guo SS, Deng QC, Xiao JS, Xie BJ, Sun ZD. (2007b). Evaluation of antioxidant activity and preventing DNA damage effect of pomegranate extracts by chemiluminescence method. J Agr Food Chem, 55, 3134–3140.
- Hallagan JB, Allen DC, Borzelleca JF. (1995). The safety and regulatory status of food, drug and cosmetics colour additives exempt from certification. Food Chem Toxicol, 33, 515–528.
- He HY. (2001). Progress in researches of biological functions of Rosa Michx. and health food made from it. Beverage Ind, 1, 28–29.
- Jing LJ, Cui GW, Feng Q, Xiao YS. (2009). Orthogonal test design for optimization of the extraction of polysaccharides from Lycium barbarum and evaluation of its anti-athletic fatigue activity. J Med Plants Res, 3, 433–437.
- Ki SK, Noriko Y, Hyun YK, Takuya O, Yasuo S. Takako Y. (2007). Increase in the free radical scavenging activities of American ginseng by heat processing and its safety evaluation. J Ethnopharmacol, 113, 225–232.
- Kravchenko LV, Morozov1 SV, Beketova1 NA, Deryagina1 VP, Avreneva1 LI, Tutel’yan1 VA. (2003). Antioxidant status of rats receiving lycopene in different doses. Bul Exp Biol Med, 135, 353–357.
- Linda SE, Kurt AR, Xiao DL, Margaret JB, Edward JK. (2004). Anthocyanin antioxidants from edible fruits. Food Chem, 84, 23–28.
- Madkins B, Schsefer B. (1998). Colors: New ideas about old pet food colors. Pet Food Ind, 98, 24–27.
- Min YJ. (2001). The preliminary investigation and study on the reserve of wild Rosa leavigata Michx in the Wanxi Dabieshan mountain area. J Biol, 18, 28–29.
- Oliveira I, Coelho V, Baltasar R, Pereira JA, Baptist P. (2009). Scavenging capacity of strawberry tree (Arbutus unedo L.) leaves on free radicals. Food Chem Toxicol, 47, 1507–1511.
- Pan YM, Zhu ZR, Huang ZL, Wang HS, Liang Y, Wang K, Lei Q, Liang M. (2009). Characterisation and free radical scavenging activities of novel red pigment from Osmanthus fragrans seeds. Food Chem, 112, 909–913.
- Schoefs B. (2004). Determination of pigments in vegetables. J Chromatogr A, 1054, 217–226.
- Shruti S, Archana M, Vivek KB, Savita S. (2009). In vitro antioxidant activity and total phenolic content of ethanolic leaf extract of Stevia rebaudiana Bert. Food Chem Toxicol, 47, 2338–2343.
- Sun YX, Li YJ, Li MQ, Tong HB, Yang XD, Liu JC. (2009). Optimization of extraction technology of the Anemone raddeana polysaccharides (ARP) by orthogonal test design and evaluation of its anti-tumor activity. Carbohyd Polymers, 75, 575–579.
- Timberlake CF, Henry BS. (1986). Plant pigments as natural food colours. Endeavour, 10: 31–36.
- Wan HC, Cao G, Prior RL. (1997). Oxygen radical absorbing capacity of anthocyanins. J Agr Food Chem, 45, 304–309.
- Xiao RG. (1999). The exploitation and application of Jinyinzi (Rosa laevigata Michx.) in Tibet. Tibetan J Med, 20, 10–11.
- Xie XM, Ding XW, Chen JQ. (2001). Study on extracts of Rosa laevigata Michx. to remove NO2-. Food Sci, 1, 30–33.
- Yu F, Xu D, Lei R, Li N, Li KA. (2008). Free-radical scavenging capacity using the Fenton reaction with rhodamine B as the spectrophotometric indicator. J Agr Food Chem, 56, 730–735.
- Yu YG, Zeng CG, Duan LD. (1995). Research on the extraction of Rosa laevigata Michz and its ability. J Shaoyang Coll, 8, 347–349.
- Yuan JQ, Yang XZ, Miao JH, Tang CP, Ke CQ, Zhang JB, Ma XJ, Ye Y. (2008). New triterpene glucosides from the roots of Rosa laevigata Michx. Molecules, 13, 2229–2237.
- Zhang HC, Zhan JX, Su KM, Zhang YX. (2006). A kind of potential food additive produced by Streptomyces coelicolor: Characteristics of blue pigment and identification of a novel compound, λ-actinorhodin. Food Chem, 95, 186–192.
- Zhao YT, Guo XM, Li FZ. (2003). Anti-oxidative activity of polysaccharide from Rosa laevigata Michx. J Biol, 20, 23–24.
- Zhou HT, Chen SJ, Yang Y. (2006). Progress on development and utilization of Rosa laevigata Michx. Pr Forest Technol, 3, 29–31.