Abstract
Context: The Asteraceae family has been of interest to researchers due to the presence of polyphenolic compounds, mainly flavonoids, which demonstrated antiviral activity.
Objective: The hydroethanol extract of the aerial parts of Acanthospermum australe (Loefl.) Kuntze (Asteraceae) and its fractions, were evaluated in vitro for their potential cytotoxic and antiviral activity against bovine herpesvirus and human poliovirus.
Materials and methods: The sulforhodamine B colorimetric assay were used to evaluate the capacity of the hydroethanol extract and fractions to inhibit the lytic activity of herpes and poliovirus in infected cell cultures and their influence on the viability of uninfected cell cultures.
Results and discussion: A progressive increase in the antiviral effect against herpesvirus was observed in the course of the purification process of the extract. The hydroethanol extract had a 50% antiviral effective concentration (EC50) at 70 μg/mL and 36 μg/mL for herpes and poliovirus, respectively, and it exhibited no cytotoxicity. The fractions F3 (dichloromethane) and F4 (dichloromethane: ethyl acetate (1:1 v/v)) both showed EC50 at 6.25 μg/mL against herpesvirus, and these fractions showed cytotoxic concentrations (CC50) at 12.7 and 11.7 μg/mL, respectively. These fractions had no effect against poliovirus in the concentrations tested. From the bioactive F3, a diterpene lactone (acanthoaustralide-1-O-acetate) was isolated at a concentration of 0.5% and from F4 two flavonoids (quercetin and chrysosplenol D) were isolated at concentrations of 0.14 and 0.24%, respectively.
Conclusion: The present study reports for the first time the antiviral activity of extracts and fractions from A. australe aerial parts.
Introduction
Infectious viral diseases are an important and serious public-health problem worldwide because of the development of viral resistance to prophylactic measures, mainly in immunodeficient patients, who are more susceptible to viral and microbial infections (CitationKuiken et al., 2003). Plants contain a variety of chemical constituents that inhibit the replication cycle of various types of viruses. Compounds of natural origin are interesting as possible sources of drugs to control viral infections (CitationVlietinck & Berghe 1991; CitationCowan 1999; CitationJassim & Naji 2003; CitationOnozato et al., 2009).
The Asteraceae is a cosmopolitan family of herbs, containing 1,100 genera and 25,000 species; in Brazil, there are about 180 genera. The family Asteraceae is one of the most-often investigated in screening studies for evaluation of antiviral activity in vitro (CitationSemple et al., 1999; CitationPalomino et al., 2002). This interest is due to the presence of polyphenolic compounds, mainly flavonoids, in the great majority of Asteracean species, and the demonstrated antiviral activity of this class of compounds (CitationVlietinck & Berghe 1991; CitationCowan 1999).
The genus Acanthospermum includes four species: A. austral (Loefl.) Kuntze, A. hispidum DC., A. humile DC. and A. xanthioides DC. A. hispidum DC. has been much used in research on biological activity, based on the popular use of this species in many parts of the world (CitationSummerfield et al., 1997; CitationNoumi & Dibakto 2000; CitationFleischer et al., 2003). A strong antiviral activity in vitro and weak cytoxicity in cell cultures were attributed to the species A. hispidum in research that used alpha herpesvirus (CitationSummerfield et al., 1997) and HIV-1-RT (CitationAli et al., 2002). Moreover, previous studies using A. hispidum extracts demonstrated their antimicrobial activity against Gram-positive bacteria (CitationFleischer et al., 2003), and important activity against Plasmodium falciparum (Carvalho & Krettli 1991; Carvalho et al., 1991; Brandão et al., 1992; Sanon et al., 2003).
Acanthospermum australe is an annual shrub widely distributed in South America. In Brazil, where it is popularly known as “carrapichinho” or “carrapicho-de-carneiro,” it grows vigorously in agricultural fields, pasture and fallow soil. Its aerial parts are used in folk medicine as a tonic, diaphoretic, eupeptic, vermifuge, antidiarrheal, antimalarial, antigonorrheal, febrifuge, and antianemic (CitationLorenzi & Matos 2002). CitationShimizu et al. (1987) reported the oral use of A. australe in the treatment of blood stagnations, rheumatisms and arthritis, and its topical use on swellings and hemorrhages; in their study, the ethanol crude extract of A. australe was analyzed for inhibitory activity against the aldose reductase enzyme in mice. Previous phytochemical investigations of A. australe have led to the isolation of germacranolides, melampolides, diterpene lactones and 6-methoxyflavonoids (CitationBohlmann et al., 1979, Citation1981; CitationMatsunaga et al., 1996). From aerial parts of A. australe, CitationMatsunaga et al. (1996) isolated a geracronolide with antitumor activity, denominated acanthostral. In a previous study, we isolated from aerial parts of A. australe a diterpene lactone named acanthoaustralide acetate and the flavonoids quercetin and chrysosplenol D (CitationMartins et al., 2006), which were previously isolated by CitationDebenedetti et al. (1987) and CitationShimizu et al. (1987). Other studies have reported activity of A. australe extract against Plasmodium falciparum in mice (Carvalho et al., 1991; CitationKrettli et al., 2001). More recently, eight melampolides of the acanthospermal type were isolated from an ethanol extract of A. australe, and some of them displayed slight antibiotic activity against the Gram-positive bacteria Staphylococcus aureus and Bacillus subtilis (CitationSanchez et al., 2009).
The aim of the present study was to evaluate the in vitro antiviral activity of a crude extract and fractions from A. australe against bovine herpesvirus type 1 and poliovirus. The cytotoxic activity of this plant on HEp-2 cells was also evaluated.
Materials and methods
Plant material
Acanthospermum australe (Loefl.) Kuntze (Asteraceae) was collected in January 2003 in Campo Grande, Mato Grosso do Sul, Brazil. It was collected and identified by Prof. Dra. Ubirazilda Maria Resende. A voucher specimen HUM 10.508 deposited and authenticated in the Herbarium of the Federal University of Mato Grosso do Sul, Brazil.
Plant extraction
The aerial parts (stem, leaves, flowers and fruits) from A. australe, were dried at room temperature and powdered (679 g). The extract was prepared by exhaustive maceration in ethanol:water (9:1) at room temperature. The hydroethanol extract was filtered, concentrated under vacuum at 40°C, lyophilized and stored at -20°C, yielding 96.9 g of the crude hydroethanol extract (HE).
Fractionation and isolation of constituents
The hydroethanol extract (HE) (90 g) was chromatographed in a vacuum silica-gel column (silica gel 150 g) and eluted with hexane (1000 mL), hexane:dichloromethane (1:1 v/v) (1000 mL), dichloromethane (1400 mL), dichloromethane: ethyl acetate (1:1 v/v) (500 mL), ethyl acetate (500 mL), methanol (1400 mL) and methanol:water 9:1 v/v (1800 mL), afforded 7 fractions (F1 = 4.92 g), (F2 = 5.29 g), (F3 = 2.74 g), (F4 = 7.96 g), (F5 = 2.34 g), (F6 = 48.69 g), (F7 = 1.68 g). This extract and fractions were assayed for them antiviral activity against bovine herpesvirus type 1 and poliovirus as described below.
The fraction F3 (1.16 g) was rechromatographed on a silica-gel column chromatograph (silica gel 60 70-230 mesh) using hexane, hexane:dichloromethane (98:2, 95:5, 90:10, 80:20, 50:50 v/v), dichloromethane, dichloromethane:ethyl acetate (98:2, 95:5, 90:10, 80:20, 50:50 v/v), ethyl acetate, ethyl acetate:acetone (50:50 v/v), methanol and methanol:water (90:10 v/v), afforded 13 fractions (D1-D13). The fraction D9 (241 mg) was rechromatographed by column chromatography on silica gel 60 (230-240 mesh) and eluted with hexane; hexane:dichloromethane (1:1 v/v); dichloromethane; dichloromethane:ethyl acetate (96:4 and 90:10 v/v) and methanol, yielding 9 fractions (D9A-D9I). The sub-fraction D9C was identified as a diterpene lactone acanthoaustralide-1-O-acetate (3) (5.6 mg; 0.5% yield) ().
Figure 1. Structure of the compounds quercetin (1), crysosplenol D (2) and acanthoaustralide-1-O-acetate (3) isolated of the Acanthospermum australe.
The fraction F4 (2.12 g) was purified by gel filtration on Sephadex LH-20 column using chloroform:methanol (50:50 v/v) afforded 9 fractions (F4A-F4I). The fraction F4B (43 mg) was rechromatographed by Sephadex LH-20 eluted with ethanol:water (90:10 v/v) yielding four fractions (F4B1-F4B4). The sub-fraction F4B2 was identified as quercetin (1) (3 mg; 0.14% yield) (). The fraction F4F (73 mg) rechromatographed on silica gel 60 (230-240 mesh) and eluted with: chloroform, chloroform:ethyl acetate (98:2, 95:5, 90:10, 80:20 and 50:50 v/v), ethyl acetate and methanol, yielding 9 fractions (F4F1-F4F9). The sub-fraction F4F3 was identified as chrysosplenol D (2) (5 mg; 0.24% yield) (). Structures were established with the use of spectroscopic methods (UV, EI-MS, 1H NMR, 13C NMR, H-HCOSY, gNOE, TOCSY, HMBC and by comparison them with literature data.
Structure elucidation
The structure of the isolated compounds were identified by analyses of NMR Spectra (One-dimensional 1H, 13C and DEPT; Bidimensional COSY 1H × 1H, HMQC and HMBC) were obtained in a MERCURY-300BB, using DMSO deuterated solvent and TMS as the internal standard, at room temperature. The CG-MS analyses were performed in an Electronic Impact-Mass Spectrometry – Micromass Quattro LC.
Medium, virus and cells culture
The HEp-2 cells (carcinoma of human larynx cells) were cultivated in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma Chemical Co., St. Louis, Missouri, USA), containing Eagle’s balanced salt solution supplemented with 10% of fetal bovine serum (FBS), 50 μg/ml of gentamicine, 2 mM of l-glutamine (Gibco Invitrogen Corporation, New York, USA) and 0.37% of NaHCO3. The cells were incubated at 37°C in a humidified atmosphere with 5% CO2.
Viruses used in the antiviral assays were bovine herpesvirus type 1 and human poliovirus, obtained from the State University of Londrina. The virus titres were determined by the 80% tissue culture-infective dose (TCID80) determined by the colorimetric method of sulforhodamine B (SRB) assay, performed using the technique described by CitationPapazisis et al. (1997) with a few modifications. Briefly, cells were fixed with 10% v/v of trichloroacetic acid, at 4°C for 1 h and the plate was gently washed five times with tap water and then dried at room temperature. Next, 50 μl of 0.4% w/v SRB stain (in 1% v/v aqueous acetic acid) was added to each well. After 30 min, unbounded SRB was removed by washing 4 times with acetic acid (1% v/v), followed by addition of 150 μl of 10 mM Tris base (Sigma-Aldrich, Irvine, U.K.) in each well to dilute the dyed bounded cells. The plate was shaken for 15 min on a gyratory shaker, and the optical density (OD) at 530 nm read in a microplate reader (Bio-Tek FL-600 Microplate Fluorescence Reader).
Cytotoxicity assay
The HEp-2 cells were seeded onto 96-well microtiter plates at a concentration of 2.5 104 cells per well and incubated for 24 h in DMEM containing 5% of FBS; this time is sufficient to proliferate the cells, forming a monolayer. Different concentrations (12.5 to 100 μg/mL) of crude extract and fractions A. australe were added on the cells monolayer in triplicate. In all tests, 0.5% dimethyl sulfoxide (DMSO; Sigma Chemical Co., St. Louis, Mo., U.S.A.), a concentration that was used to dissolve the highest dose of the samples but was proven to have no effect on cell proliferation, and the medium alone were used as controls. After incubation at 37°C with 5% of CO2 for 48 h, the cellular growth was evaluated by the sulforhodamine B assay. The absorbance was read at 530 nm in a microplate reader (Power Wave XS, Bio-Tek). The absorbance of each individual well, minus the blank value, was calculated automatically. Each experiment was performed in triplicate on three different occasions, and the percentage of viable cells was calculated in relation to controls cultured in medium alone. The 50% Cytotoxicity Concentration (CC50) was defined as the concentration of the sample which reduces the OD530 to 50%, compared with untreated cells. The CC50 was determined by logarithm regression analysis of the data obtained.
Antiviral activity
HEp-2 cells grown in 96-well microtiter plates (2.5 × 104 cells per well) and incubated for 24 h. On the confluent monolayer, 25 μL of virus suspension, corresponding to TCID80, was added and incubated for 1 h at 37°C. Next, 100 μL of the extract and fractions in different concentrations (6.25 to 100 μg/mL) were added on the culture cells and the plates were incubated for 48 h. In all tests, 0.5% dimethyl sulfoxide (DMSO; Sigma Chemical Co., St. Louis, Mo., U.S.A.), a concentration that was used to dissolve the highest dose of the samples but was proven to have no effect on cell proliferation, untreated infected cells and untreated uninfected cells were used as controls. Following incubation, cell viability was evaluated by the SRB colorimetric technique. The absorbance was read at 530 nm in a microplate reader (Power Wave XS, Bio-Tek). The assays were performed in triplicate on three separate occasions. The 50% antiviral effective concentration (EC50) was defined as the inhibitory concentration which achieves 50% protection of treated infected cells, compared with untreated infected cells. The EC50 was determined by logarithm regression analysis of the data obtained.
Statistical analysis
All experiments were performed in triplicate. The means and standard deviations of at least three experiments were determined. Statistical analysis of the differences between mean values obtained for experimental groups was done by means of Student’s t-test. P values of 0.05 or less were regarded as significant.
Results and Discussion
From the aerial parts of A. australe the active antiviral fractions F3 (dichloromethane) and F4 (dichloromethane: ethyl acetate (1:1 v/v)) were obtained. Fractionation of F3 led to purification of the compound identified as a diterpene lactone acanthoaustralide-1-O-acetate at a concentration of 0.5%. From the fractionation of F4, two flavonoids were isolated, which were identified as quercetin and chrysosplenol D, at concentrations of 0.14 and 0.24%, respectively (CitationMartins et al., 2006). The structures were identified by analysis of their spectroscopic data and by comparison with literature data (CitationHerz & Kalyanaraman 1975; CitationBohlmann et al., 1981, Citation1984; CitationSemple et al., 1999; CitationMatsunaga 1996; CitationMartins et al., 2006).
Medicinal plants with potential antiviral activity have been widely studied, with promising results. Several members of the Asteraceae have shown antiviral activity (Robin et al., Citation2001; CitationBettega et al., 2004; CitationOoi et al., 2006; CitationRomero et al., 2006; CitationOnozato et al., 2009). In previous studies, the species Acanthospermum hispidum showed strong antiviral activity in vitro against α-herpesvirus (CitationSummerfield et al., 1997) and HIV-1-RT (CitationAli et al., 2002). It also showed weak cytotoxicity in cell cultures.
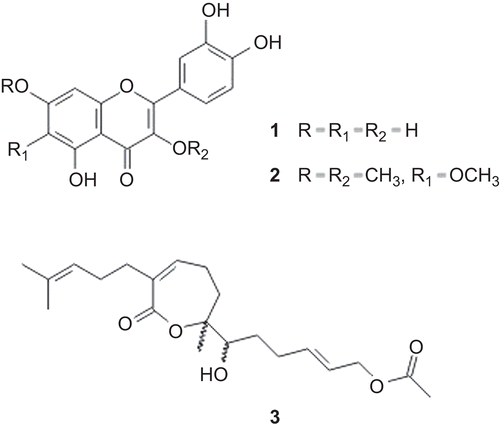
The results of the antiviral evaluation are shown in and and . The EC50, CC50 and SI of the crude extract and fractions (F1-F7) are shown in in order to compare their antiviral activity against bovine herpesvirus type 1 and poliovirus, cytotoxic effects on HEp-2 cells, and the selectivity index. The cytotoxicity to HEp-2 cells of the samples tested and their activity against the virus were compared using the selectivity index (SI) ratio (CC50/EC50). When this value is greater than 1, the sample is more selective for activity against the virus than the cells; when the value is less than 1, the sample is more selective for activity against HEp-2 cells.
Table 1. Antiviral activity (EC50), cytotoxicity (CC50) and selective index (SI) of hydroethanolic extract (HE) and fractions from Acanthospermum australe against bovine herpesvirus type 1 and poliovirus.
Figure 2. Antiviral activity of the hydroethanolic extract (HE) and fractions (F1-F7) from Acanthospermum australe against herpesvirus (A) and poliovirus (B). The antiviral activity was determined by a sulforhodamine B colorimetric assay. The values represent the mean ± S.D. of at least three experiments performed in triplicate. The bars indicate standard deviations. All results were significant at p≤ 0.05 (compared to the control group, Student’s t-test).
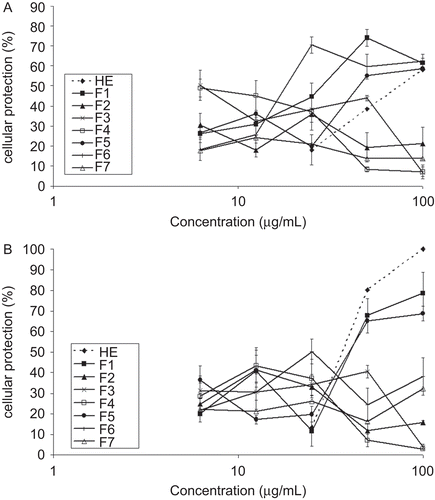
Figure 3. Cytotoxic effects of hydroethanolic extract (HE) and fractions (F1-F7) of the Acanthospermum australe on HEp-2 cells. The HEp-2 cells were treated with different concentrations of these samples and incubated in DMEM medium supplemented with 5% of heat-inactivated fetal bovine serum for 48 h. Non-treated cells were used as the control. The bars indicate standard deviations. All results were significant at p≤ 0.05 (compared to control group, Student’s t-test).
The hydroethanol extract (HE) of Acanthospermum australe showed a 50% antiviral effective concentration (EC50) at 70 µg/mL for herpesvirus, and it showed no cytotoxic effects to HEp-2 cells, with a cytotoxic concentration (CC50) more than 100 µg/mL. Fractionation of the hydroethanol extract yielded seven fractions (F1-F7), and all of them were tested for antiviral activity. Of these fractions, F3 and F4 showed more significant antiviral activity against herpesvirus, with EC50 values of 6.25 µg/mL for both (, ). The observed decline in antiviral activity for fractions F3 and F4 can be explained, based on the cytotoxicity values (, ), because the toxic concentrations for 50% of the cells (CC50) at 12.7 and 11.7 µg/mL, respectively, were only two times higher than the EC50 values, although they displayed a satisfactory selectivity index. The fractions F3 and F4 showed the highest cytotoxic effects on HEp-2 cells of all samples tested (, ).
With respect to the antiviral activity on the poliovirus, fractions F3 and F4 did not protect 50% of the cells against virus infection in any of the concentrations tested ( and ). The extract (HE) proved to be more active than fractions F3 and F4 against poliovirus, which may be due to the synergic action of the present compounds in hydroethanol extract, mainly polyphenolic compounds, whose antiviral activity was reported previously by several researchers (CitationVlietinck & Berghe 1991; CitationSemple et al., 1999; CitationCowan 1999). In addition, the extract (HE) was more active against poliovirus than herpesvirus, as demonstrated by EC50 values of 36 and 70 µg/mL, respectively. Moreover, this extract showed no cytotoxic effects on HEp-2 cells, with CC50 values more than 100 µg/mL. Thus, the hydroethanol extract showed important antiviral activity against the poliovirus, and it had no cytotoxic effects on cells. On the other hand, fraction F6 was the most active of all against the poliovirus, with EC50 at 25 µg/mL.
From fraction F3 a diterpene lactone acanthoaustralide-1-O-acetate (3) was isolated, and from fraction F4 the flavonoids quercetin (1) and chrysosplenol D (2) were isolated. The antiviral activity of flavonoids and their methoxyl derivatives have been described in the literature (CitationVlietinck & Berghe 1991; CitationJassim & Naji 2003). CitationSemple et al. (1999) described the activity of the flavonoid chrysosplenol C against poliovirus in a culture of monkey kidney cells, obtaining excellent cellular protection (EC50 of 0.27 µg/mL). The antiviral activity of flavonoids, as well as polyphenolic compounds, seems to be attributable to the connection of those substances to the protein structures of the viral capsule, which inhibits the adsorption of the viral particles in the cells of the host, as well as promoting the blockade of RNA synthesis. The mechanism of antiviral activity of terpenoids was attributed to the inhibition of the synthesis of viral DNA, through mechanisms mediated by the cell membrane (CitationJassim & Naji 2003).
Although aqueous and methanol extracts of Acanthospermum hispidum, according to CitationSummerfield et al. (1997) and CitationAli et al. (2002), have demonstrated a wide spectrum of antiviral activity for different types of particles in tests in vitro, this activity was not attributed to any particular chemical compounds present in this species.
Conclusions
The present study reports for the first time the antiviral activity of extracts and fractions from aerial parts of A. australe. The antiviral evaluation revealed the antiviral activity of the hydroethanol extract and fractions (F3 and F4) of the crude extract. The flavonoids and diterpene lactones present in these fractions seem to be involved in antiviral activity. However, further experimental studies will be necessary to study the antiviral activity of the compounds isolated from fractions F3 and F4 and to elucidate the antiviral mechanisms.
Declaration of interest
This study was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Capacitação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Programa de Pós-graduação em Ciências Farmacêuticas from Universidade Estadual de Maringá.
References
- Ali H, König GM, Khalid SA, Wright AD, Kaminsky R. (2002). Evaluation of selected Sudanese medicinal plants for their in vitro activity against hemoflagellates, selected bacteria, HIV-1-RT and tyrosine kinase inhibitory, and for citotoxicity. J Ethnopharmacol, 83, 219–28.
- Bettega JM, Teixeira H, Bassani VL, Barardi CR, Simoes CM. (2004). Evaluation of antiherpetic activity of standartized extracts of Achyrocline satureioides. Phytother Res, 18, 819–23.
- Bohlmann F, Jakupovic J, Zdero C, King RM, Robinson H. (1979). Neue melampolide und cis,cis-germacranolide aus vertretern der subtribus melampodiinae. Phytochemistry, 18, 625–30.
- Bohlmann F, Jakupovic J, Dhar AK, King RM, Robinson H. (1981). Two sesquiterpene and three diterpene lactones from Acanthospermum australe. Phytochemistry, 20, 1081–83.
- Bohlmann F, Schmeda-Hirschmann G, Jakupovic J. (1984). Neue melampolide aus Acanthospermum australe. Planta Med, 50(37), 37–9.
- Cowan MM. (1999). Plant products as antimicrobial agents. Clin Microbiol Rev, 12, 564–82.
- Debenedetti S, Martino V, Palacios P, Coussio JD. (1987). 6-Methoxy flavonoids from Acanthospermum australe. J Nat Prod, 50, 325.
- Fleischer TC, Ameade EPK, Sawer IK. (2003). Antimicrobial activity of the leaves and flowering tops of Acanthospermum hispidum. Fitoterapia, 74, 130–2.
- Herz W, Kalyanaraman PS. (1975). Acanthospermal A and acanthospermal B two new melapolides from Acanthospermum species. J Org Chem, 40, 3486–91.
- Jassim SAA, Naji MA. (2003). Novel antiviral agents: A medicinal plant perspective. J Appl Microbiol, 95, 412–27.
- Krettli AU, Andrade-Neto VF, Brandão MGL, Ferrari WMS. (2001). The search for new antimalarial drugs from plants used to treat fever and malaria or plants ramdomly selected: A review. Mem I Oswaldo Cruz, 96, 1033–42.
- Kuiken T, Fouchier R, Rimmelzwaan G, Osterhaus A. (2003). Emerging viral infections in a rapidly changing world. Curr Opin Biotechnol, 14, 641–46.
- Lorenzi H, Matos FJA. (2002). Plantas Medicinais no Brasil: Nativas e Exóticas, Instituto Plantarum, São Paulo, p.127.
- Martins LRR, Cortez LER, Dias-Filho BP, Nakamura CV, Ferreira AG, Cortez DAG. (2006). Atribuição dos deslocamentos químicos dos átomos de 1H e 13C do acetato de acantoaustralida. Braz J Pharmacogn, 16, 490–6.
- Matsunaga K, Saitoh M, Ohizumi Y. (1996). Acanthostral, a novel antineoplastic cis,cis,cis-germacranolide from Acanthospermum australe. Tetrahedron Lett, 37, 1455–6.
- Noumi E, Dibakto TW. (2000). Medicinal plants used for peptic ulcer in the Bangangte region, western Cameroon. Fitoterapia, 71, 406–12.
- Onozato T, Nakamura C V, Cortez DAG, Dias-Filho BP, Ueda-Nakamura T. (2009). Tanacetum vulgare: Antiherpes virus activity of crude extract and the purified compound parthenolide. Phytother Res, 23, 791–3.
- Ooi LS, Wang H, He Z, Ooi VE. (2006). Antiviral activities of purified compounds from Youngia japonica (L.) DC (Asteraceae, Compositae). J Ethnopharmacol, 106, 187–91.
- Palomino SS, Abad MJ, Bedoya LM, García J, Gonzales E, Chiriboga X, Bermejo P, Alcami J. (2002). Screening of South American plants against human immunodeficiency virus: Preliminary fractionation of aqueous extract from Baccharis trinervis. Biol Pharm Bull, 25, 1147–50.
- Papazisis KT, Geromichalos GD, Dimitriadis KA, Kortsaris AH. (1997). Optimization of the sulforhodamine B colorimetric assay. J Immunol Methods, 208, 151–8.
- Robin V, Irurzum A, Amoros M, Boustie J, CarrascoL. 2001. Antipolivirus flavonoids from Psiadia dentata. Antivir Chem Chemother, 12, 283–91.
- Romero MR, Serrano MA, Vallejo M, Efferth T, Alvarez M, Mrin JJ. (2006). Antiviral effect of arthemisinin from Artemisia annua against a model member of the Flaviviridae family, the bovine viral diarrhea virus (BVDV). Planta Med, 72, 1169–74.
- Sanchez M, Kramer F, Bargardi S, Palermo AJ. (2009). Melampolides from Argentinean Acanthospermum australe. Phytochem Lett, 2, 93–95.
- Semple SJ, Nobbs SF, Pyke SM, Reynolds GD, Flower RLP. (1999). Antiviral flavonoid from Pterocaulon sphacelatum, an Australian Aboriginal medicine. J Ethnopharmacol, 68, 283–8.
- Shimizu M, Horie S, Arisawa M, Hayashi T, Suzuki S, Yoshizaki M, Kawasaki M, Terashima S, Tsuji H, Wada S, Ueno H, Morita N, Berganza LH, Ferro E, Basualdo I. (1987). Chemical and pharmaceutical studies on medicinal plants in Paraguay. I. Isolation and identification of lens aldose reductase inhibitor from “tapecué”, Acanthospermum australe O.K.. Chem Pharm Bull, 35, 1234–7.
- Summerfield A, Keil GM, Mettenleiter TC, Rziha HJ, Saalmüller A. (1997). Antiviral activity of an extract from leaves of the tropical plant Acanthospermum hispidum. Antivir Res, 36, 55–62.
- Vlietinck AJ, Berghe DAV. (1991). Can ethnopharmacology contribute to the development of antiviral drugs? J Ethnopharmacol, 32, 141–53.