Abstract
Context: Traditional Chinese herbal medicines have attracted considerable attention in many countries with treatment of several end-stage liver diseases.
Objective: The present study investigated the protective effects of baicalin on hepatotoxicity and hepatic fibrosis and explored the role of transforming growth factor β1 (TGFβ1) and peroxisome proliferator activated receptors γ (PPARγ) on the rat liver injury model.
Materials and methods: The rat liver injury model was introduced by subcutaneous injection of carbon tetrachloride (CCl4) for 8 weeks. At week 5, rats were treated with baicalin of different doses or silymarin. Detection of biochemical indicators, histological analysis, and enzyme-linked immunosorbent assays were employed to evaluate severity of liver inflammation, and western blotting and RT-PCR assay were performed to evaluate TGFβ1 and PPARγ pathway related proteins and gene expression.
Results: The administration of baicalin could significantly improve histological changes of CCl4 treated rat livers and return biochemical indicators for liver injury to nearly baseline level. In addition, the increased expression of TGFβ1 was markedly suppressed by baicalin, and decreased expression of PPARγ was also dramatically elevated by baicalin as well. The hepatoprotective effects of baicalin may be conferred by elevating the level of PPARγ contributing to down-regulation of TGFβ1 signaling pathway and suppression of hepatic stellate cell activation.
Conclusions: The studies demonstrated that baicalin is a potent and promising antifibrotic drug in the treatment of hepatic fibrosis.
Introduction
Carbon tetrachloride (CCl4) is a well-known hepatotoxicant, and has been used to establish liver injury models in rodents, including acute (CitationYokogawa et al., 2004) and chronic (CitationTsai et al., 2008) conditions, through generating trichloromethyl free radicals by cytochrome P450 2E1 in vivo (CitationJeong, 1999; CitationRecknagel et al., 1989). CCl4 also promotes release of various inflammatory factors including tumor necrosis factor α (TNFα) and transforming growth factor β1 (TGFβ1) (CitationGrasl-Kraupp et al., 1998; CitationSimeonova et al., 2001). Previous research has confirmed that hepatic fibrosis is related to the activation of HSC and excessive production and deposition of extracellular matrix (ECM) (CitationBataller & Brenner, 2005; CitationGressner & Weiskirchen, 2006). In the progress of hepatic fibrosis, increased activity of TGFβ1, an important inflammatory factor, may lead to increased production and deposition of collagen, which finally induces severe hepatic fibrosis (CitationSanderson et al., 1995). Thus, TGFβ1 is a major contributing factor in CCl4-induced hepatic fibrosis.
Peroxisome proliferator-activated receptors (PPARs) are members of the hormone nuclear receptor superfamily, and three types of PPARs have been identified: PPARα, PPARβ/δ, and PPARγ (CitationEvans, 1988), in which PPARγ has been known to have evident anti-inflammatory properties. The activation of PPARγ could inhibit the release of TGFβ1 (CitationChang et al., 2008; CitationGressner et al., 2008; CitationKawai et al., 2009; CitationLee et al., 2006). Thus, suppressing PPARγ might be a favorable strategy in the treatment of liver diseases.
Baicalin (7-d-glucuronic acid,5,6-dihydroxyflavone), a bioactive flavonoid isolated from the root of Scutellaria baicalensis Georgi (baical skullcap root, Scutellaria L., Labiatae), has many biological functions, including hepatoprotective effects (CitationLiu et al., 2007; CitationPark et al., 2008; CitationWan et al., 2008; CitationZhao et al., 2005). Up to now, the hepatoprotective effects of baicalin associated with PPARγ have not been well investigated. In the present study, we investigated the protective effects of baicalin on CCl4-induced liver injury and fibrosis, and explore the role of PPARγ and TGFβ1.
Materials and methods
Animals and experimental treatments
Male Sprague Dawley (SD) rats weighing 180∼200 g were randomly divided into six groups. All rats were fed with rodent chow and kept at 22∼24°C under a 12 h dark-light cycle. Rats had free access to a standard diet and drinking water. Experiments were performed in accordance with the standards established by the Guide for the Care and Use of Laboratory Animals of Zhejiang University, and approved by the local ethics committee. The whole laboratory procedure was carried out under the permission and surveillance of the ethics committee. Rats in group 1 were subcutaneously injected with only the vehicle edible oil (1 mL/kg body weight). Rats in group 2 to 6 were subcutaneously injected with CCl4 dissolved in edible oil [1:1 (v/v)] (1 mL/kg body weight) twice a week for 8 consecutive weeks to induce liver injury. At week 5, rats in these groups were orally given baicalin or silymarin daily for 4 weeks. Rats in group 2 were treated with normal saline (1 mL/100 g body weight), those in group 3 with high dose baicalin (100 mg/kg), those in group 4 with intermediate dose baicalin (50 mg/kg) and those in group 5 with low dose baicalin (25 mg/kg). However, rats in group 6 (positive control group) were orally given silymarin (200 mg/kg). Forty-eight hours after CCl4 treatment, rats were sacrificed and blood and livers were immediately obtained. The left liver lobe was removed and cut into two parts. One part was fixed in 10% formalin, and the other part was rapidly put into liquid nitrogen for PCR and western blotting analysis.
Detection of biochemical indicators for liver injury
The serum albumin (ALB), total protein (TP), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) levels were measured with diagnostic kits (Olympus Diagnostics, Beijing, China) using an Olympus AU400 Automatic Biochemistry Analyzer (Olympus Diagnostica, Mishima, Japan) according to the manufacturer’s instructions. The hydroxyproline level was detected with a hydroxyproline test kit (Jiancheng Bioengineering Institute, Nanjing, China).
Enzyme-linked immunosorbent assays
Levels of liver or serum TNFα and TGFβ1 were quantified using corresponding ELISA kits purchased from Boster (Boster, Wuhan, China) according to the protocols provided by the manufacturer.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, California). The total RNA was reversely transcribed into cDNA by AMV reverse transcriptase (Promega, Madison, Wisconsin). Primers used for amplification are listed in , and the mRNA levels were normalized by GAPDH level.
Table 1. Primers and annealing temperatures used for RT-PCR.
Western blotting analysis
Liver tissues were lysed with lysis buffer (pH 7.4) containing 50 mM Tris HCl, 150 mM NaCl, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 2 mM ethylenediaminetetraacetic acid (EDTA), 1% Triton X-100, 1 mM phenylmethyl-sulfonyl fluoride (PMSF), 1 μg/mL aprotinin and 1 μg/mL leupeptin. The protein concentration was determined by the Bradford assay (Applygen Technologies, Beijing, China). Total proteins (50 μg) were loaded onto 10% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE gel electrophoresis). PPARγ protein was detected using primary antibodies against PPARγ (1:200, Santa Cruz) and secondary antibodies (1:10 000, Santa Cruz) conjugated to horseradish peroxidase (HRP). β-actin (Santa Cruz) was used as an internal control. Enhanced chemiluminescence (ECL) detection (Amersham Bioscience) was performed and images were captured for analysis.
Histological analysis
The post-fixed liver tissues were embedded in paraffin, and cut into 5 μm sections. The sections were stained with Azan to quantify the degree of fibrosis.
Statistical analysis
Data are expressed as mean ± SD. Statistical analyses were performed using one-way analysis of variance (ANOVA) and Dunnett t-test. A value of P <0.05 was considered statistically significant.
Results
Effect of baicalin in CCl4-induced liver injury
As shown in , compared with group 1, the activities of serum ALT, AST, and ALP were significantly elevated in CCl4 treated rats (group 2) (P <0.01). After treatment with baicalin at 100 and 50 mg/kg, the levels of ALT, AST, and ALP were markedly reduced (P <0.01 and P <0.05, respectively) when compared with group 2, but no significant difference was found between the low dose baicalin-treated rats (group 5) and untreated rats (group 2). In addition, the contents of serum ALB and TP were significantly reduced in rats injected with CCl4 (P <0.01), and baicalin attenuated the hepatotoxic effects of CCl4. These results confirmed the hepatoprotective effects of baicalin in the CCl4-induced liver injury rat model.
Table 2. Effect of baicalin on serum biochemical indicators in the rat model with CCl4-caused hepatic injury. Values are expressed as mean ± SD (n = 6).
Baicalin suppressed hepatic fibrosis
Hydroxyproline is an amino acid, existing almost exclusively in collagens. Measurement of hydroxyproline in the fibrotic tissues has been regarded as a favorable method to quantify fibrosis and to evaluate the effectiveness of novel potentially antifibrotic agents (CitationFu et al., 2008). As shown in , the content of hydroxyproline in the livers of CCl4-treated rats (group 2) was significantly higher than that in normal rats (P <0.01), and significant reduction of hydroxyproline content was observed in the baicalin treated rats (group 3 and 4) (P <0.01 versus group 2).
Table 3. Determination of the content of hepatic hydroxyproline in the rat model with CCl4-caused hepatic injury. Values are expressed as mean ± SD (n = 6).
Histological analysis indicated severe hepatic steatosis, necrosis, and fibrotic septa in CCl4-treated rats (group 2) (). Baicalin treatment attenuated the degree of the CCl4-induced pathological changes in a dose-dependent manner (). A larger blue area (fibrosis) was seen in sections of CCl4-treated rats (). The effects of baicalin on fibrosis were evaluated by digitization of the blue-stained area (). In the baicalin-treated groups, the area of hepatic fibrosis was significantly decreased (P <0.01 versus group 2).
Figure 1. The representative photographs of Azan-staining (×100). (A) normal group, (B) CCl4 control group, (C) CCl4 and positive control group, (D) CCl4 and high dose baicalin treated group, (E) CCl4 and intermediate dose baicalin treated group, (F) CCl4 and low dose baicalin treated group.
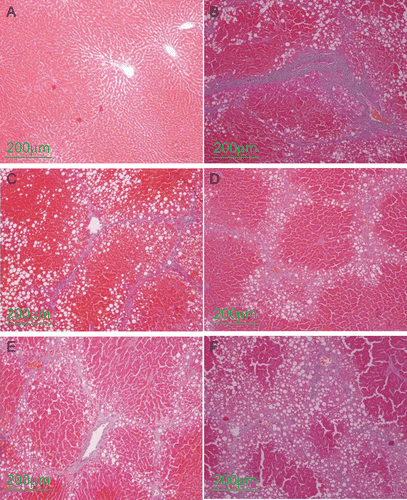
Figure 2. Quantitative analysis of liver fibrosis using Azan-staining. (B) CCl4 control group, (C) CCl4 and high dose baicalin treated group, (D) CCl4 and intermediate dose baicalin treated group, (E) CCl4 and low dose baicalin treated group, (F) CCl4 and positive control group. Data were expressed as mean ± SD (n = 6). The level of significance was established at *P < 0.05, or **P < 0.01.
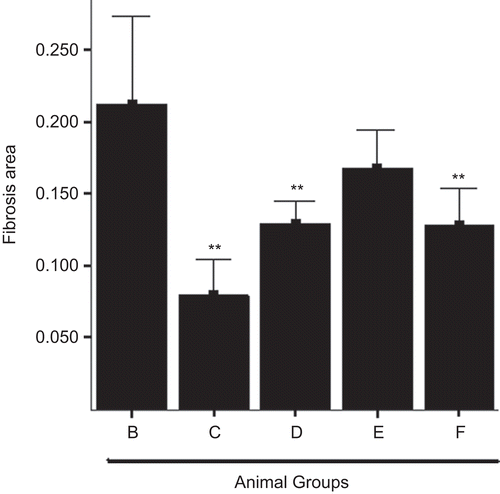
These results confirmed the anti-fibrotic effects of baicalin in CCl4-induced liver injury model, which was in a dose-dependent manner, and even better than that of silymarin.
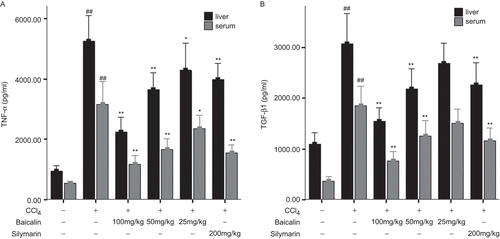
Baicalin suppressed release of inflammatory factors
The process of hepatic fibrosis was accompanied by inflammatory responses, thus resulting in release of numerous cytokines including pro-inflammatory cytokines TNF-α and pro-fibrogenic cytokines TGF-β1 (CitationBahcecioglu et al., 2008; CitationStalnikowitz & Weissbrod, 2003). As shown in , compared with the normal group (group 1), the levels of TNF-α and TGF-β1 in the CCl4 treated rats (group 2) were significantly elevated in the liver and serum. Baicalin treatment dramatically reduced the levels of TNF-α and TGF-β1 in the liver and serum in a dose-dependent manner.
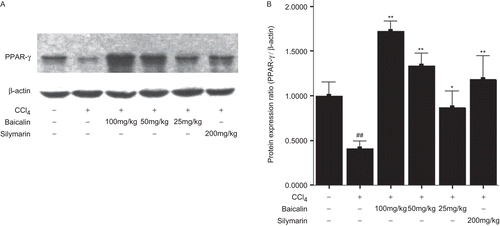
Baicalin abrogated the expression of TGF-β1 related proteins and increased the expression of PPARγ mRNA and protein
Compared with the normal rats (group 1), CCl4 treatment significantly induced the mRNA expression of TGF-β1 related proteins. As shown in , the mRNA levels of TGF-β1, Tβ-RI (type I receptors for TGF-β), Tβ-RII (type II receptors for TGF-β), SMAD3 were significantly increased by approximately 4.2-, 3.5-, 3.0- and 3.6-fold, respectively, in the CCl4 treated rats (group 2). Baicalin treatment markedly reduced the increased expression which was in a dose-dependent manner. In addition, the mRNA levels of α-SMA, a marker of activated HSC, and αI(I) collagen were significantly increased by CCl4 which was also attenuated by baicalin.
Figure 4. Expression of TGF-β1 related genes and PPARγ in the liver. (A) primary results of PCR, (B) semiquantitative results of PPARγ, (C) semiquantitative results of TGFβ1, TβRI and TβRII, (D) semiquantitative results of SMAD3, α-SMA and αI(I) collagen. Data were expressed as mean ± SD (n = 3). *P < 0.05 and **P < 0.01 versus untreated group; #P < 0.05 and ##P < 0.01 versus normal group.
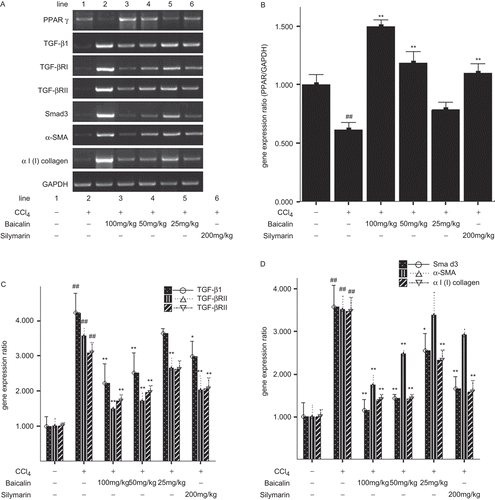
Thereafter, we detected the expression of PPARγ mRNA and protein. As shown in , and , the expression of PPARγ mRNA and protein was markedly reduced by 39% and 59%, respectively, in the CCl4-treated rats (group 2), but it was dramatically elevated after treatment with baicalin in a dose-dependent manner. However, the expression of PPARγ mRNA and protein was still at a relatively low level even after treatment with high dose and intermediate dose baicalin.
Discussion
CCl4-induced hepatic fibrosis model has been extensively used in screening hepatoprotective drugs. The metabolism of CCl4 in the liver results in the production of free radicals including CCl3 (CitationJeong, 1999; CitationRecknagel et al., 1989) causing hepatocyte necrosis, inducing inflammation, and further promoting progression of hepatic fibrosis. In the present study we demonstrated that baicalin could decrease the activities of serum ALT, AST and ALP, improve the histology of the liver, and attenuate hepatic inflammation induced by CCl4. Furthermore, other quantitative assays including histopathological analysis (Azan-staining) (CitationOyagi et al., 2006) and hydroxyproline detection (CitationSakaida et al., 1996 ) were performed to confirm the attenuated hepatic fibrosis by baicalin. Histopathological analyses demonstrated that baicalin significantly reduced the Azan-stained area in the liver. Additionally, the increased content of hydroxyproline in the liver induced by CCl4 was markedly reduced by baicalin in a dose-dependent manner.
TGFβ1, released from Kupffer cells and activated HSC (CitationFriedman, 1999), plays an important role in hepatic fibrosis. Activation of HSC induced by over-expressed TGF-β1 results in increased synthesis and decreased degradation of ECM which transdifferentiates HSC into α-SMA-positive myofibroblasts (CitationCarpino et al., 2005; CitationLi & Friedman, 1999; CitationMeindl-Beinker & Dooley, 2008). In the present study, baicalin significantly reduced the elevated mRNA levels of TGFβ1-related proteins both in serum and liver induced by CCl4 which was in a dose-dependent manner. TβRI and TβRII, receptors for TGF-β1, are expressed in activated HSC (CitationZheng & Chen, 2006), and have structural similarity to serine/threonine kinases, presenting as homodimers in the absence of ligands (CitationFriedman et al., 1994). The binding of ligand to TβRII will form hetero-tetrameric complexes of TβRII and TβRI. Then, TβRII kinases phosphorylate TβRI which then induces autophosphorylation and phosphorylation of SMADs (SMAD2 and SMAD3) (CitationGressner & Weiskirchen, 2006). SMADs are the members of signaling molecules superfamily, and downstream molecules of the TGFβ1 signal pathway. SMAD3 is the key mediator in the pathogenic effects of TGFβ1 in fibrosis (CitationGressner et al., 2008; CitationRoberts et al., 2003). CitationLiu et al. (2006) reported that naringenin, a SMAD3-specific inhibitor, could exert antifibrogenic effects by directly down-regulating SMAD3 protein expression and its phosphorylation through TGF-β1 signaling pathway. In our study, the mRNA levels of TβRI, TβRII and SMAD3 were significantly increased after CCl4 treatment which was down-regulated by baicalin treatment. In addition, elevated mRNA levels of α-SMA and αI(I) collagen induced by CCl4 were reduced by baicalin as well. These findings above suggested that baicalin could inhibit HSC activation and TGFβ1 releasing.
PPARγ is a transcriptional factor belonging to the ligand-activated nuclear receptors superfamily, acting as an anti-inflammatory molecule by inhibiting inflammatory responses (CitationAlleva et al., 2002; CitationChung et al., 2000; CitationJiang et al., 1998; CitationStraus et al., 2000). In the liver, PPARγ is expressed in various cell types including Kupffer cells, hepatocytes and HSC (CitationOrfila et al., 2005; CitationMiyahara et al., 2000). After CCl4 treatment the number of hepatocytes was decreased; on the contrary, that of ED2-positive Kupffer cell expressing PPARγ was significantly increased. However, the number of PPARγ-immunopositive cells were decreased (CitationOrfila et al., 2005),which leading to the expression of PPARγ protein was decreased remarkably. In addition, studies indicated that HSC activation was accompanied by a dramatic reduction of the level of PPARγ and its activity (CitationMiyahara et al., 2000; CitationGalli et al., 2000), Stimulation of PPARγ activity by its potential agonists could inhibit HSC proliferation and α1(I) collagen expression (CitationMiyahara et al., 2000), which were all confirmed by our study. Furthermore, growing researches have established that there was a negative relationship between PPARγ and TGFβ1 (CitationChang et al., 2008; CitationFu et al., 2008; CitationGressner et al., 2008; CitationKawai et al., 2009; CitationLee et al., 2006). Activation of PPARγ inhibited the expression of TGFβ1 receptors, leading to the interruption of the TGFβ1 signaling pathway in activated HSCs (CitationZheng & Chen, 2006). CitationZhao et al. (2006) further certified that PPARγ agonists could induce a dose-dependent inhibition of the TGF-β1/SMAD3-signaling pathway in HSCs. In our study, the expression of PPARγ mRNA and protein was markedly reduced after CCl4 treatment which was dramatically increased by baicalin in a dose-dependent manner. These results suggested that baicalin protected the rat liver against CCl4-induced hepatic fibrosis by up-regulation of PPARγ resulting in suppression of the TGFβ1 signaling pathway.
In conclusion, our results demonstrated that baicalin could protect rat liver against CCl4-induced injury by suppressing liver inflammation, reducing the severity of hepatic fibrosis and inhibiting HSC activation, contributing to partial recovery of liver function, in a dose-dependent manner. Furthermore, increased expression of PPARγ by baicalin inhibited the TGFβ1 signaling pathway, which played a critical role in the hepatoprotective effects of baicalin. Our results suggested that baicalin might be a potent and promising anti-fibrotic drug in the treatment of hepatic fibrosis.
Declaration of interest
This study was supported by a project from Department of Science and Technology, Zhejiang Province, China (No. 2005C13027).
References
- Alleva DG, Johnson EB, Lio FM, Boehme SA, Conlon PJ, Crowe PD. (2002). Regulation of murine macrophage proinflammatory and anti-inflammatory cytokines by ligands for peroxisome proliferator-activated receptor-gamma: Counter-regulatory activity by IFN-gamma. J Leukoc Biol, 71, 677–685.
- Bahcecioglu IH, Koca SS, Poyrazoglu OK, Yalniz M, Ozercan IH, Ustundag B, Sahin K, Dagli AF, Isik A. (2008). Hepatoprotective effect of infliximab, an anti-TNF-alpha agent, on carbon tetrachloride-induced hepatic fibrosis. Inflammation, 31, 215–221.
- Bataller R, Brenner DA. (2005). Liver fibrosis. J Clin Invest, 115, 209–218.
- Carpino G, Morini S, Ginanni Corradini S, Franchitto A, Merli M, Siciliano M, Gentili F, Onetti Muda A, Berloco P, Rossi M, Attili AF, Gaudio E. (2005). Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig Liver Dis, 37, 349–356.
- Chang HJ, Lee JH, Hwang KJ, Kim MR, Chang KH, Park DW, Min CK. (2008). Transforming growth factor (TGF)-beta1-induced human endometrial stromal cell decidualization through extracellular signal-regulated kinase and Smad activation in vitro: Peroxisome proliferator-activated receptor gamma acts as a negative regulator of TGF-beta1. Fertil Steril, 90, 1357–1365.
- Chung SW, Kang BY, Kim SH, Pak YK, Cho D, Trinchieri G, Kim TS. (2000). Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated mouse macrophages via direct interactions between peroxisome proliferator-activated receptor-gamma and nuclear factor-kappa B. J Biol Chem, 275, 32681–32687.
- Evans RM. (1988). The steroid and thyroid hormone receptor superfamily. Science, 240, 889–895.
- Friedman SL. (1999). Cytokines and fibrogenesis. Semin Liver Dis, 19, 129–140.
- Friedman SL, Yamasaki G, Wong L. (1994). Modulation of transforming growth factor beta receptors of rat lipocytes during the hepatic wound healing response. Enhanced binding and reduced gene expression accompany cellular activation in culture and in vivo. J Biol Chem, 269, 10551–10558.
- Fu Y, Zheng S, Lin J, Ryerse J, Chen A. (2008). Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Mol Pharmacol, 73, 399–409.
- Galli A, Crabb D, Price D, Ceni E, Salzano R, Surrenti C, Casini A. (2000). Peroxisome proliferator-activated receptor gamma transcriptional regulation is involved in platelet-derived growth factor-induced proliferation of human hepatic stellate cells. Hepatology, 31, 101–108.
- Grasl-Kraupp B, Rossmanith W, Ruttkay-Nedecky B, Müllauer L, Kammerer B, Bursch W, Schulte-Hermann R. (1998). Levels of transforming growth factor beta and transforming growth factor beta receptors in rat liver during growth, regression by apoptosis and neoplasia. Hepatology, 28, 717–726.
- Gressner AM, Weiskirchen R. (2006). Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-beta as major players and therapeutic targets. J Cell Mol Med, 10, 76–99.
- Gressner OA, Lahme B, Rehbein K, Siluschek M, Weiskirchen R, Gressner AM. (2008). Pharmacological application of caffeine inhibits TGF-beta-stimulated connective tissue growth factor expression in hepatocytes via PPARgamma and SMAD2/3-dependent pathways. J Hepatol, 49, 758–767.
- Jeong HG. (1999). Inhibition of cytochrome P450 2E1 expression by oleanolic acid: Hepatoprotective effects against carbon tetrachloride-induced hepatic injury. Toxicol Lett, 105, 215–222.
- Jiang C, Ting AT, Seed B. (1998). PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature, 391, 82–86.
- Kawai T, Masaki T, Doi S, Arakawa T, Yokoyama Y, Doi T, Kohno N, Yorioka N. (2009). PPAR-gamma agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-beta. Lab Invest, 89, 47–58.
- Lee SJ, Yang EK, Kim SG. (2006). Peroxisome proliferator-activated receptor-gamma and retinoic acid X receptor alpha represses the TGFbeta1 gene via PTEN-mediated p70 ribosomal S6 kinase-1 inhibition: Role for Zf9 dephosphorylation. Mol Pharmacol, 70, 415–425.
- Li D, Friedman SL. (1999). Liver fibrogenesis and the role of hepatic stellate cells: New insights and prospects for therapy. J Gastroenterol Hepatol, 14, 618–633.
- Liu LL, Gong LK, Wang H, Xiao Y, Wu XF, Zhang YH, Xue X, Qi XM, Ren J. (2007). Baicalin protects mouse from concanavalin A-induced liver injury through inhibition of cytokine production and hepatocyte apoptosis. Liver Int, 27, 582–591.
- Liu X, Wang W, Hu H, Tang N, Zhang C, Liang W, Wang M. (2006). SMAD3 specific inhibitor, naringenin, decreases the expression of extracellular matrix induced by TGF-beta1 in cultured rat hepatic stellate cells. Pharm Res, 23, 82–89.
- Meindl-Beinker NM, Dooley S. (2008). Transforming growth factor-beta and hepatocyte transdifferentiation in liver fibrogenesis. J Gastroenterol Hepatol, 23, S122–127.
- Miyahara T, Schrum L, Rippe R, Xiong S, Yee HF Jr, Motomura K, Anania FA, Willson TM, Tsukamoto H. (2000). Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem, 275, 35715–35722.
- Orfila C, Lepert JC, Alric L, Carrera G, Béraud M, Pipy B. (2005). Immunohistochemical distribution of activated nuclear factor kappaB and peroxisome proliferator-activated receptors in carbon tetrachloride-induced chronic liver injury in rats. Histochem Cell Biol, 123, 585–593.
- Oyagi S, Hirose M, Kojima M, Okuyama M, Kawase M, Nakamura T, Ohgushi H, Yagi K. (2006). Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol, 44, 742–748.
- Park SW, Lee CH, Kim YS, Kang SS, Jeon SJ, Son KH, Lee SM. (2008). Protective effect of baicalin against carbon tetrachloride-induced acute hepatic injury in mice. J Pharmacol Sci, 106, 136–143.
- Recknagel RO, Glende EA Jr, Dolak JA, Waller RL. (1989). Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther, 43, 139–154.
- Roberts AB, Russo A, Felici A, Flanders KC. (2003). SMAD3: A key player in pathogenetic mechanisms dependent on TGF-beta. Ann N Y Acad Sci, 995, 1–10.
- Sakaida I, Matsumura Y, Kubota M, Kayano K, Takenaka K, Okita K. (1996). The prolyl 4-hydroxylase inhibitor HOE 077 prevents activation of Ito cells, reducing procollagen gene expression in rat liver fibrosis induced by choline-deficient l-amino acid-defined diet. Hepatology, 23, 755–763.
- Sanderson N, Factor V, Nagy P, Kopp J, Kondaiah P, Wakefield L, Roberts AB, Sporn MB, Thorgeirsson SS. (1995). Hepatic expression of mature transforming growth factor beta 1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci USA, 92, 2572–2576.
- Simeonova PP, Gallucci RM, Hulderman T, Wilson R, Kommineni C, Rao M, Luster MI. (2001). The role of tumor necrosis factor-alpha in liver toxicity, inflammation, and fibrosis induced by carbon tetrachloride. Toxicol Appl Pharmacol, 177, 112–120.
- Kershenobich Stalnikowitz D, Weissbrod AB. (2003). Liver fibrosis and inflammation. Ann Hepatol, 2, 159–163.
- Straus DS, Pascual G, Li M, Welch JS, Ricote M, Hsiang CH, Sengchanthalangsy LL, Ghosh G, Glass CK. (2000). 15-Deoxy-delta 12,14-prostaglandin J2 inhibits multiple steps in the NF-kappa B signaling pathway. Proc Natl Acad Sci, 97, 4844–4849.
- Tsai JH, Liu JY, Wu TT, Ho PC, Huang CY, Shyu JC, Hsieh YS, Tsai CC, Liu YC. (2008). Effects of silymarin on the resolution of liver fibrosis induced by carbon tetrachloride in rats. J Viral Hepat, 15, 508–514.
- Wan JY, Gong X, Zhang L, Li HZ, Zhou YF, Zhou QX. (2008). Protective effect of baicalin against lipopolysaccharide/d-galactosamine-induced liver injury in mice by up-regulation of heme oxygenase-1. Eur J Pharmacol, 587, 302–308.
- Yokogawa K, Watanabe M, Takeshita H, Nomura M, Mano Y, Miyamoto K. (2004). Serum aminotransferase activity as a predictor of clearance of drugs metabolized by CYP isoforms in rats with acute hepatic failure induced by carbon tetrachloride. Int J Pharm, 269, 479–489.
- Zhao C, Chen W, Yang L, Chen L, Stimpson SA, Diehl AM. (2006). PPARgamma agonists prevent TGFbeta1/SMAD3-signaling in human hepatic stellate cells. Biochem Biophys Res Commun, 350, 385–391.
- Zhao Y, Li H, Gao Z, Xu H. (2005). Effects of dietary baicalin supplementation on iron overload-induced mouse liver oxidative injury. Eur J Pharmacol, 509, 195–200.
- Zheng S, Chen A. (2006). Curcumin suppresses the expression of extracellular matrix genes in activated hepatic stellate cells by inhibiting gene expression of connective tissue growth factor. Am J Physiol Gastrointest Liver Physiol, 290, G883–893.