Abstract
Context: This study describes the antispasmodic, bronchodilator, and cardiovascular-modulatory activities of Hypericum perforatum Linn. (Hypericaceae) fractions and constituents.
Aim of study: Pharmacological investigation of H. perforatum fractions and active principles.
Materials and methods: H. perforatum extract fractions [petroleum spirit (HpPet), chloroform (HpCHCl3), ethyl acetate (HpEtAc), and aqueous (HpAq)] and its compounds (hyperforin, hypericin, and hyperoside) were studied in various isolated tissue preparations.
Results: In rabbit jejunum, HpCHCl3, HpEtAc and HpAq, like papaverine, inhibited both spontaneous and K+ (80 mM)-induced contractions at similar concentrations, whereas HpPet was relatively potent against K+, as verapamil. All fractions caused rightward of Ca2+ concentration-response curves (CRCs), similar to verapamil. HpCHCl3, HpEtAc, and HpAq shifted isoprenaline-inhibitory CRCs to left, like papaverine, while HpPet was devoid of any such effect, as verapamil. In guinea-pig trachea, HpCHCl3, HpEtAc, and HpAq equipotently relaxed carbachol and K+-induced contractions and shifted the isoprenaline-curves to the left, whereas HpPet was more effective against K+, without potentiating isoprenaline effect. When tested in rabbit aorta, all fractions exhibited vasoconstrictor and vasodilator effects, except HpEtAc, which did not produce vasoconstriction. In guinea-pig atria HpCHCl3, HpEtAc, and HpAq initially caused cardiac stimulation, followed by inhibition, similar to papaverine, whereas HpPet, like verapamil, caused only cardiac suppression. Hyperforin, hypericin, and hyperoside showed a similar pattern of spasmolytic effect to verapamil.
Discussion and conclusion: Thus, all tested fractions of H. perforatum exhibit a combination of Ca2+ antagonist and phosphodiesterase-inhibition, except petroleum spirit which was devoid of later mechanism. The compounds tested showed only Ca2+ channel blocking effect.
Introduction
Hypericum perforatum Linn. (Hypericaceae), commonly known as “St. John’s wort”, is used in folk medicine for the treatment of multiple disorders, including asthma, colic, and diarrhea. Phytochemical studies revealed the presence of multiple chemicals in the plant, such as ascorbic acid, brenzcatechin, cadinine, carotene, carotenoids, choline, emodinantherol, epicatechin, gurjunene, hyperforin, hypericin, hyperoside, imanin, isohypericin, isoquercitrin, limonene, mannitol, myristic acid, pectin, phenol, phlobaphene, phloroglucinol, protohypericin, pseudohypericin, pyrogallol, quercetin, quercitrin, resorcynol, rutin, saponin, tannins, amino acids, biflavones, essential oils, flavonols, glycosides, naphthodianthrones, phenylpropanes, proanthocyanidins, and xanthones (CitationKhan & Gilani, 2009a).
Hypericum perforatum has been known to exhibit a wide range of pharmacological activities. We have previously observed the Ca2+ channel blocking (CCB) and phosphodiesterase inhibitory (PDEI) effects of Hypericum perforatum hydro-ethanol extract (CitationGilani et al., 2005a). The present study was carried out to investigate the pharmacological effects of petroleum spirit, chloroform, ethyl acetate, and aqueous fractions, obtained from the crude extract of Hypericum perforatum and to see if any separation of the Ca2+ antagonist and PDEI components occur among the resultant fractions. Apart from the fractions, some of the known commercially available pure compounds of Hypericum perforatum namely: hyperforin, hypericin, and hyperoside, were also studied.
Materials and methods
Plant material and extraction
The aerial parts (stem + leaves) of Hypericum perforatum were collected from the northern areas of Pakistan (Gillyat) in 2002. The plant was identified with the help of a taxonomist, Professor Dr. Muhammad Ibrar, at the Department of Pharmacy, University of Peshawar, Peshawar, North West Frontier Province. A voucher specimen (PUP 012529) was submitted to the Herbarium of the Department of Botany of the same university. The plant material was cleaned, shade-dried and coarsely ground. The powdered material was extracted with 70% aqueous-ethanol by cold maceration for three days with occasional shaking. It was filtered through a muslin cloth and then through a Whatman qualitative grade 1 filter paper. This procedure was repeated twice and the combined filtrate was evaporated in a rotary evaporator under reduced pressure (–760 mmHg) to a thick, semi-solid mass of dark brown color, i.e., crude extract of Hypericum perforatum (HpCr), yielding approximately 18.52% w/w.
Activity-guided fractionation was carried out by using solvents of increasing polarity (CitationGilani et al., 2006). Approximately 22.6 g of HpCr was dissolved in about 150 mL of distilled water. Petroleum spirit was added to it and shaken vigorously in a separating funnel. The mixture was allowed to separate into two layers. The petroleum spirit layer (upper) was removed and the extraction with petroleum spirit was repeated twice more. All of the petroleum spirit layers were combined and evaporated in the rotary evaporator to give the petroleum spirit fraction (HpPet, 10.2%). The other separated layer (lower) was taken in a separating funnel; chloroform was added to it and separated as above. The chloroform layer (lower) was collected three times and evaporated in the rotary evaporator to give the chloroform fraction (HpCHCl3, 22.12%). The other layer (upper) was again taken into a separating funnel; ethyl acetate was added to it, separated and evaporated with the rotary evaporator to get the ethyl acetate fraction (HpEtAc, 9.3%). The remaining lower layer was collected and evaporated to obtain the aqueous fraction (HpAq, 56.2%).
Chemicals
Acetylcholine chloride, carbachol (CCh), isoprenaline hydrochloride, papaverine hydrochloride, phenylephrine hydrochloride (PE), verapamil (Sigma Chemicals, St Louis, MO), hyperforin, hyperoside (ChromaDex, Santa Ana, CA), and hypericin obtained from Planta Natural Products, Vienna, Austria. Stock solutions of all chemicals were prepared in distilled water except hyperforin, hypericin and hyperoside, which were dissolved in 10% dimethyl sulfoxide and further dilutions were made fresh in distilled water. The vehicle used for solubilization had no effect in the control experiments. Chemicals used for making physiological salt solutions were: calcium chloride, glucose, magnesium chloride, magnesium sulfate, potassium dihydrogen phosphate, sodium bicarbonate, sodium dihydrogen phosphate from Merck, Darmstadt, Germany, while ethylene diamine tetra-acetic acid, potassium chloride (KCl) from Sigma, and sodium chloride from BDH Laboratory supplies, Poole, UK. All chemicals used were of analytical grade.
Animals
Rabbits (1–1.2 kg) and guinea-pigs (500–550 g) of local breed and either sex were used for this study housed at the Animal House of the Aga Khan University, maintained at 23°–25°C and were given a standard diet and tap water. Rabbits starved for 24 h were sacrificed by a blow on back of the head and guinea-pigs by cervical dislocation. Experiments performed complied with the rulings of the Institute of Laboratory Animal Resources, Commission on Life Sciences, CitationNational Research Council (1996) and approved by the Ethical Committee of the Aga Khan University.
Phytochemical screening
Preliminary analysis for the detection of various phytochemical classes, such as alkaloids, saponins, coumarins, sterols, terpenes, flavonoids, tannins and anthraquinones was done according to the standard procedures (CitationGilani et al., 2007). Alkaloids were detected by using Dragendorff’s reagent. Presence of saponins was detected based on the appearance of froth upon vigorous shaking of diluted samples. The observation of yellow florescence under ultraviolet light on examination of filter paper previously exposed to the vapors from boiling plant material indicated the presence of coumarins. For the detection of sterols and terpenes, plant material was treated with petroleum ether and subsequently extracted with chloroform. The appearance of green to pink (for sterols) and pink to purple colors (for terpenes) was then noted after treatment of the chloroform layer with acetic anhydride and concentrated hydrochloric acid in succession. Plant material was noted as positive for flavonoids when it gave a yellow color with aluminum chloride reagent and for tannins, when green or black was produced with aqueous ferric chloride. Lastly, for detecting anthraquinones, the extract was dissolved in 1% HCl, then in benzene and later if the extract showed pink, violet, or red with ammonium hydroxide.
Rabbit jejunum
The rabbit abdomen was opened and the jejunum was dissected out, kept in normal Tyrode’s solution and cleaned of mesenteries (CitationGilani et al., 2010). Each segment of about 2 cm length was suspended in a 10 mL tissue bath containing Tyrode’s solution (pH 7.4), maintained at 37°C and aerated with a mixture of 95% O2 and 5% CO2 (carbogen). The composition of Tyrode’s solution was (mM): NaCl: 136.9, KCl: 2.7, MgCl2•6H2O: 0.5, NaHCO3: 11.9, NaH2PO4.2H2O: 0.32, CaCl2: 1.8, and glucose: 5.05. One end of the segment was attached to the metal tissue hook and the other was attached by a cotton thread to an isotonic Bioscience transducer, connected to a Student oscillograph (Harvard Apparatus, Holliston, MA). An initial load of 1 g was applied to each tissue and was allowed to equilibrate for 30 min before the addition of any drug. Following the equilibration period, each preparation was then stabilized with a sub-maximal concentration of ACh (0.3 μM) at 3 min interval until constant responses were recorded. Under these experimental conditions, rabbit jejunum exhibited spontaneous rhythmic contractions, allowing the testing of relaxant (spasmolytic) activity directly without the use of an agonist. The inhibitory effects of test substances were measured as the percentage change in jejunum spontaneous contractions.
For the determination of CCB effect, high K+ (80 mM) was used to depolarize the preparations. High K+ (> 30 mM) is known to cause smooth muscle contractions through the opening of voltage-dependent Ca2+ channels, thus allowing influx of extracellular Ca2+, causing a contractile effect (CitationMusha et al., 2005) and a substance causing inhibition of high K+-induced contraction is considered as a blocker of Ca2+ influx through l-type Ca2+ channels (CitationChan et al., 2006). Once a plateau of the induced contraction was achieved (usually within 7-10 min), the test material was then added in a cumulative fashion to obtain concentration-dependent inhibitory responses. To confirm the Ca2+ antagonist action of the test substance, the tissue was allowed to stabilize in normal Tyrode’s solution, which was then replaced with Ca2+-free Tyrode’s solution containing EDTA (0.1 mM) for 30 min in order to remove Ca2+ from the tissues. This solution was further replaced with K+-rich and Ca2+-free Tyrode’s solution, having the composition (mM): NaCl: 91.03, KCl: 50, MgCl2•6H2O: 0.50, NaHCO3: 11.9, NaH2PO4.2H2O: 0.32, glucose: 5.05, and EDTA-Na2•2H2O: 0.1. Following an incubation period of 30 min, control concentration-response curves (CRCs) of Ca2+ were obtained. When the control Ca2+ CRCs were found super-imposable (usually after two cycles), the tissue was pretreated with test drug for 1 h. The CRCs of Ca2+ were reconstructed in the presence of different concentrations of the test material to observe the Ca2+ antagonist effect (CitationGilani et al., 2008a). The PDEI effect was studied through constructing isoprenaline-induced inhibitory CRCs against CCh-induced contractions in the absence and presence of the test substance, as PDE-inhibitors are known to potentiate the isoprenaline effect (CitationGilani et al., 2005a).
Guinea-pig trachea
Trachea was dissected from guinea-pig and kept in normal Krebs solution. The tracheal tube was cut into rings, 2-3 mm wide, each containing about two cartilages. Each ring was opened by a longitudinal cut on the ventral side opposite the smooth muscle, forming a strip with smooth muscle in the center and cartilaginous portions on the edges (CitationKhan & Gilani, 2009b). The preparation was mounted in a 20 mL tissue bath containing Krebs solution (pH 7.4), at 37°C and aerated with carbogen. The composition of Krebs solution was (mM): NaCl: 118.2, NaHCO3: 25.0, CaCl2: 2.5, KCl: 4.7, KH2PO4: 1.2, MgSO4•7H2O: 1.2, and glucose: 11.7. A tension of 1 g was applied to the tracheal strips continuously throughout the experiment. The tissues were allowed to equilibrate for 1 h before the addition of any drug. The CCh (1 μM) was used for the stabilization. When sustained contractions of the spasmogens, such as CCh and/or K+ was obtained in the respective preparations, the relaxant effect of the test substance was assessed by adding in a cumulative fashion. As in jejunum, isoprenaline-CRCs were constructed in trachea as described previously (CitationAbdel-Haq et al., 2000). Isometric responses were recorded on a Grass model 7 Polygraph (Grass Instrument Company, Quincy, MA).
Rabbit aorta
The thoracic aorta from rabbit was removed and cut into 2-3 mm wide rings which were mounted individually in a 20 mL tissue bath containing Krebs solution, at 37°C and aerated with carbogen. A resting tension of 2 g was applied to each tissue and an equilibrium period of 1 h was given, after which the tissues were stabilized with PE (1 μM). The effect of the test drug was first determined on the resting base line of the tissue to see if it had any vasoconstrictor effect and later tested for ability to relax the contractions induced with PE and/or high K+. The inhibition of PE-induced contraction determines the blockade of Ca2+ influx through the receptor-operated calcium channels (CitationKhan & Gilani, 2008a). The changes in isometric tensions were measured via a force-displacement transducer (FT-03) using a Grass Model 7 Polygraph.
Guinea-pig atria
Right atrium isolated from the guinea-pig was mounted in a 20 mL tissue bath containing Krebs solution, at 32°C and aerated with carbogen. The tissue was allowed to beat spontaneously (due to a pacemaker) under a resting tension of 1 g (CitationKhan & Gilani, 2008b). An equilibrium period of 45 min was given before the application of any drug. Control responses of ACh (1 μM) and isoprenaline (1 μM) were obtained at least in duplicate. The drug-induced changes in force of atrial contractions were measured as the percentage change in base-line values. Tension changes in the tissue were recorded via force-displacement transducer (FT-03) using a Grass Model 7 Polygraph.
Statistical analysis
All the data expressed are mean ± standard error of mean (SEM, n = number of experiment) and the median effective concentrations (EC50) with 95% confidence intervals (CI). The CRCs were analyzed by non-linear regression using GraphPad (San Diego, CA). The statistical parameter applied was Student’s t-test; p <0.05 noted as significantly different.
Results
Phytochemical analysis
HpPet was found to contain flavonoids, tannins, and terpenes. HpCHCl3 was tested positive for anthraquinones, flavonoids, and tannins. HpEtAc showed the presence of coumarins, flavonoids, and sterols. HpAq was found to contain flavonoids, saponins, tannins, and terpenes.
Effect on jejunum
shows comparative inhibitory effects of HpPet, HpCHCl3, HpEtAc, HpAq, papaverine, and verapamil against spontaneous and K+ (80 mM)-induced contractions of isolated rabbit jejunum. HpPet, like verapamil was found to be more potent against K+ (80 mM)-induced contractions with EC50 value of 0.11 mg/mL (0.06–0.2, n = 4, 95%CI) compared to spontaneous contractions [0.83 mg/mL (0.54–1.2, n = 5)], while HpCHCl3, HpEtAc, and HpAq equipotently relaxed the spontaneous and K+ (80 mM)-induced contractions with EC50 values of 0.5 (0.32–0.9, n = 4) and 0.47 (0.26–0.82, n = 3), 0.04 (0.03–0.07, n = 4) and 0.05 (0.03–0.09, n = 3), 1.4 (0.91–2.0, n = 3) and 1.5 mg/mL (0.86–2.5, n = 3), respectively. Papaverine showed non-specific inhibitory response with respective EC50 values of 11.1 (10.1–12.7, n = 6) and 10.9 μM (9.7–12.0, n = 6), whereas verapamil was found more potent against K+ (80 mM)-induced contractions (P <0.05) with an EC50 value of 0.041 μM (0.03-0.07, n = 4), as compared to spontaneous contractions [0.37 μM (0.24–0.51, n = 4)]. HpPet (0.03–0.1 mg/mL), HpCHCl3 (0.1–0.3 mg/mL), HpEtAc (0.01–0.03 mg/mL), and HpAq (0.3–1.0 mg/mL) shifted the Ca2+ CRCs to the right with suppression of maximum response, similar to that caused by papaverine (3–10 μM) and verapamil (0.03–0.1 μM) as shown in . When tested for possible interaction with isoprenaline, pretreatment of the tissues with HpCHCl3 (0.03-0.1 mg/mL), HpEtAc (0.003–0.01 mg/mL), and HpAq (0.1–0.3 mg/mL) shifted the isoprenaline-induced inhibitory CRCs to the left, showing a potentiating effect. Papaverine (1.0–3.0 μM) also caused a similar leftward shift of the isoprenaline curves. HpPet (0.03–0.1 mg/mL), like verapamil (0.03–0.1 μM), did not alter the inhibitory response to isoprenaline ().
Figure 1. Concentration-dependent inhibitory effect on spontaneous and K+ (80 mM)-induced contractions of (A) petroleum spirit (HpPet), (B) chloroform (HpCHCl3), (C) ethyl acetate (HpEtAc), (D) aqueous (HpAq) fractions of Hypericum perforatum, (E) papaverine, and (F) verapamil in isolated rabbit jejunum preparations. Values shown are mean ± SEM, n = 3–6.
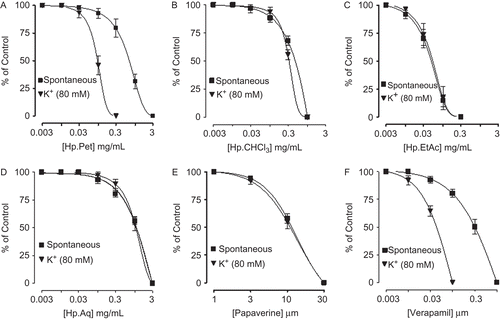
Figure 2. Concentration-response curves of Ca++ in the absence and presence of different concentrations of (A) petroleum spirit (HpPet), (B) chloroform (HpCHCl3), (C) ethyl acetate (HpEtAc), (D) aqueous (HpAq) fractions of Hypericum perforatum, (E) papaverine, and (F) verapamil in isolated rabbit jejunum preparations. Values shown are mean ± SEM, n = 3–5.
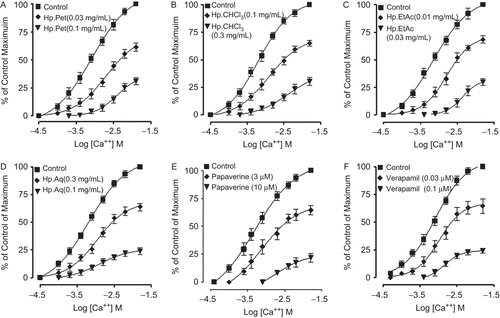
Figure 3. Inhibitory concentration-response curves of isoprenaline against carbachol (CCh)-induced contractions in the absence and presence of different concentrations of petroleum spirit (HpPet), chloroform (HpCHCl3), ethyl acetate (HpEtAc), aqueous (HpAq) fractions of Hypericum perforatum, papaverine, and verapamil in isolated rabbit jejunum preparations. Values shown are mean ± SEM, n = 3–4. The curves obtained by pretreatment of tissues with HpCHCl3, HpEtAc, HpAq, and papaverine are significantly different from the respective isoprenaline control curves (p <0.05), while that constructed in the presence of HpPet and verapamil are not significantly different (p >0.05), Student’s t-test.
Effect on trachea
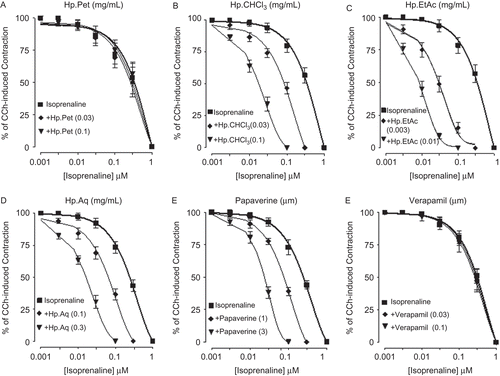
HpPet, HpCHCl3, HpEtAc, HpAq, papaverine, and verapamil were found devoid of any bronchoconstrictor effect, when screened on the tracheal resting baseline (data not shown). When tested against CCh (1 μM) and K+ (80 mM)-induced contractions, HpPet was more potent against K+ (80 mM)-induced contractions with EC50 value of 0.09 mg/mL (0.06–0.14, n = 3), compared to CCh-induced contractions (0.8 mg/mL (0.54-1.1, n = 3)), while HpCHCl3, HpEtAc, and HpAq caused relaxation of CCh (1 μM) and K+ (80 mM)-induced contractions with similar EC50 values of 0.16 (0.13–0.24, n = 3) and 0.15 (0.09–0.22, n = 4), 0.02 (0.008–0.03, n = 4) and 0.015 (0.007-0.02, n = 5), 0.37 (0.23–0.6, n = 3) and 0.39 mg/mL (0.25–0.7, n = 3), respectively. Papaverine relaxed the CCh (1 μM) and K+ (80 mM)-induced contractions at a similar concentration with respective EC50 values of 3.6 (2.0–4.2, n = 5) and 3.7 μM (2.6–5.7, n = 4), whereas verapamil was found more potent in its inhibitory effect against K+ (80 mM)-induced contractions with EC50 value of 0.05 μM (0.02–0.07, n = 3), when compared with CCh-induced contractions [0.43 μM (0.33–0.53, n = 3)] as shown in . Pretreatment of tracheal preparations with HpCHCl3 (0.03–0.1 mg/mL), HpEtAc (0.003–0.01 mg/mL), and HpAq (0.03–0.1 mg/mL) shifted the isoprenaline-CRCs to the left, similar to that caused by papaverine (1–3 μM), while HpPet (0.03–0.1 mg/ mL) and verapamil (0.03–0.1 μM) were found devoid of any such effect ().
Figure 4. Concentration-response curves showing effect of (A) petroleum spirit (HpPet), (B) chloroform (HpCHCl3), (C) ethyl acetate (HpEtAc), (D) aqueous (HpAq) fractions of Hypericum perforatum, (E) papaverine, and (F) verapamil on carbachol (CCh) and K+-induced contractions in isolated guinea-pig tracheal preparations. Values shown are mean ± SEM, n = 3–5.
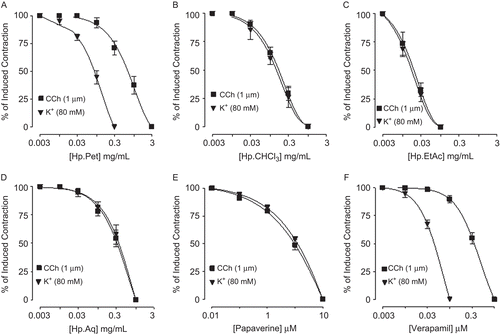
Figure 5. Inhibitory concentration-response curves of isoprenaline against carbachol (CCh)-induced contractions in the absence and presence of different concentrations of petroleum spirit (HpPet), chloroform (HpCHCl3), ethyl acetate (HpEtAc), aqueous (HpAq) fractions of Hypericum perforatum, papaverine, and verapamil in isolated guinea-pig tracheal preparations. Values shown are mean ± SEM, n = 3–4. The curves obtained by pretreatment of tissues with HpCHCl3, HpEtAc, HpAq, and papaverine are significantly different from the respective isoprenaline control curves (p <0.05), while that constructed in the presence of HpPet and verapamil are not significantly different (p >0.05), Student’s t-test.
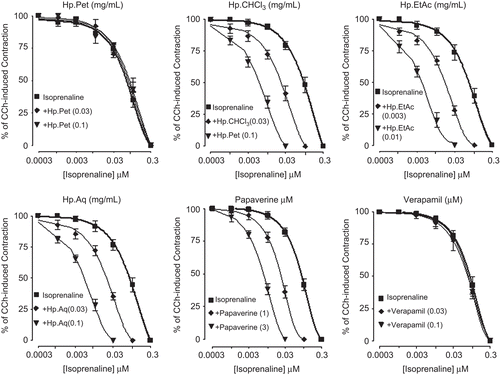
Effect on aorta
When tested on vascular preparations at resting tension, HpPet (0.3–5 mg/mL, n = 3), HpCHCl3 (0.3–1 mg/ mL, n = 3), and HpAq (3–5 mg/mL, n = 4) exhibited weak vasoconstrictor effect followed by vasodilation. This vasoconstrictor effect was resistant to pretreatment of tissues with phentolamine (1 μM). HpEtAc (0.01–1 mg/ mL, n = 4) was found devoid of vasoconstrictor effect (). HpPet, HpCHCl3, HpEtAc, and HpAq caused relaxation of PE (1 μM) and K+ (80 mM)-induced contractions with respective EC50 values of 2.4 (1.5–3.9, n = 3) and 0.9 (0.6–1.2, n = 3), 0.6 (0.3–1.3, n = 3) and 0.5 (0.2–1, n = 3), 0.08 (0.06-0.1, n = 4) and 0.05 (0.03–0.09, n = 4), 1.4 (0.8–2.5, n = 3) and 1.6 mg/mL (0.9–3.4, n = 3). Papaverine and verapamil, when tested on resting base line, were found devoid of any vasoconstrictor effect, but inhibited the PE (1 μM) and K+ (80 mM)-induced contractions with EC50 values of 6.0 (4.4–8.44, n = 3) and 8.3 (5.6–13.2, n = 3), 1.1 (0.8–2.9, n = 3) and 0.9 μM (0.52–1.63, n = 3), respectively ().
Figure 6. Concentration-response curves showing effect of (A) petroleum spirit (HpPet), (B) chloroform (HpCHCl3), (C) ethyl acetate (HpEtAc), (D) aqueous (HpAq) fractions of Hypericum perforatum (E) papaverine, and (F) verapamil on the resting base line and agonist-induced contractions in isolated rabbit aorta preparations. The vasoconstrictor effect was studied on resting basal tension and expressed as % of phenylephrine maximum (PE Max.), while the vasodilator effect was studied against PE and K+-induced contractions. Values shown are mean ± SEM, n = 3–4.
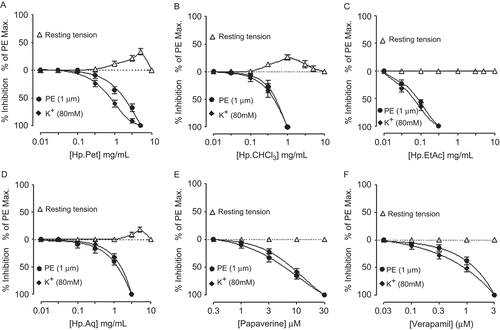
Effect on atria
HpPet was found devoid of any cardiotonic effect and caused cardiac-suppression with an EC50 value of 1.4 mg/ mL (0.75–2.9, n = 3). HpCHCl3 (0.03–0.1 mg/mL), HpEtAc (0.3–1 mg/mL), and HpAq (0.03–0.1 mg/mL) at first caused an increase in atrial force of contraction followed by an inhibitory effect at higher concentrations with respective EC50 values of 4.5 (2.5–8.6, n = 3), 4.8 (2.8–8.9, n = 3) and 7.6 mg/mL (6.2–8.1, n = 3). Papaverine produced cardiotonic effect at 3–30 μM followed by cardiac relaxation at next higher concentrations of 100–1000 μM with EC50 value of 452.0 μM (260.0–804.0, n = 3). Verapamil was found devoid of any cardiac stimulation, while caused inhibition of atrial force of contraction with EC50 value of 1 μM (0.64–1.5, n = 4), as shown in .
Figure 7. Effect of (A) petroleum spirit (HpPet), (B) chloroform (HpCHCl3), (C) ethyl acetate (HpEtAc), (D) aqueous (HpAq) fractions of Hypericum perforatum, (E) papaverine, and (F) verapamil on force of contraction of the spontaneously beating isolated guinea-pig right atrial preparations. Values shown are mean ± SEM, n = 3–4.
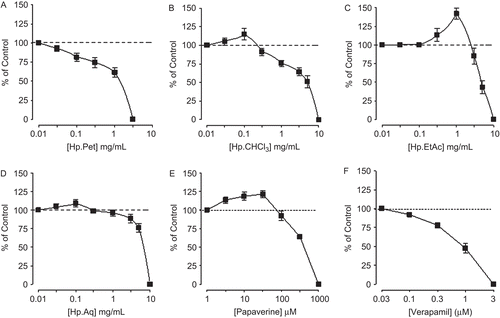
Effect of pure compounds
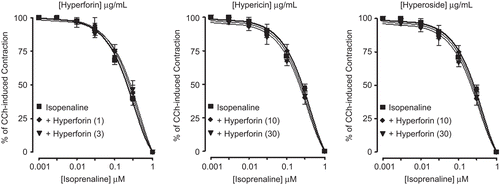
Hyperforin, hypericin, and hyperoside relaxed the spontaneous and K+ (80 mM)-induced contractions of rabbit jejunum with respective EC50 values of 3.4 (2–5.7, n = 2) and 0.31 (0.14–0.8, n = 2), 544.5 (261–1136, n = 2) and 94.4 (60.7–146.8, n = 2), 598.6 (294.3–1217, n = 2) and 118.1 μg/mL (67.9–241.7, n = 2), as shown in . All the three compounds failed to cause any potentiation of isoprenaline-inhibitory effect ().
Figure 8. Concentration-dependent inhibitory effect of the Hypericum perforatum constituents: (A) hyperforin, (B) hypericin, and (C) hyperoside on spontaneous and K+ (80 mM)-induced contractions in isolated rabbit jejunum preparations. Values shown are mean ± SEM, n = 2.
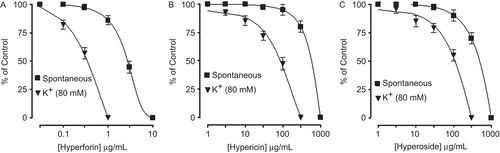
Figure 9. Inhibitory concentration-response curves of isoprenaline against carbachol (CCh)-induced contractions in the absence and presence of different concentrations of Hypericum perforatum pure compounds: hyperforin, hypericin, and hyperoside in isolated rabbit jejunum preparations. Values shown are mean ± SEM, n = 2. The curves constructed in the presence of hyperforin, hypericin, and hyperoside are not significantly different from the respective isoprenaline control curves (p >0.05), Student’s t-test.
Discussion
When tested in isolated jejunum, the chloroform, ethyl acetate and aqueous fractions of Hypericum perforatum inhibited both spontaneous and high K+-induced contractions with similar potency. Papaverine, a PDE and Ca2+ influx inhibitor (CitationKaneda et al., 2005), caused similar pattern of inhibitory effect with comparable potency against spontaneous and K+-induced contractions, while petroleum spirit fraction, like verapamil, a standard Ca2+ antagonist (CitationGilani et al., 2009) was relatively selective in its inhibitory effect on the K+-induced contractions, a typical characteristic of Ca2+ entry blockers (CitationGilani et al., 2005b). The ethyl acetate fraction was found to be the most potent, being 20-, 12- and 35-times more potent for its antispasmodic effect than the petroleum spirit, chloroform and aqueous fraction, respectively. All the plant fractions shifted the Ca2+ CRCs to the right, similar to verapamil and/or papaverine, thus confirming their Ca2+ antagonist effect. However, similar inhibitory patterns of the chloroform, ethyl acetate and aqueous fractions against spontaneous and K+-induced contractions, like that of papaverine, reflect that they may have additional mechanisms involved in the spasmolytic effect, like inhibition of PDE. This PDEI effect was further confirmed when these fractions potentiated the isoprenaline-induced relaxant effect, similar to that caused by papaverine, while the petroleum spirit fraction was found devoid of any such effect, as verapamil, suggesting that it possesses only Ca2+ antagonist effect without PDE-inhibition.
The chloroform, ethyl acetate and aqueous fractions, like papaverine caused tracheal relaxation pre-contracted with CCh and high K+ at similar concentrations, while the petroleum spirit fraction, like verapamil was found to be comparatively more potent against K+ than CCh-induced contractions, as expected from Ca2+ antagonists (CitationGilani et al., 2008b). Pretreatment of tracheal tissues with chloroform, ethyl acetate and aqueous fractions shifted the isoprenaline-induced inhibitory CRCs to the left, similar to that observed in gut preparations, indicating the presence of additional PDEI-like bronchodilatory substance(s). The petroleum spirit fraction, like verapamil, did not cause any potentiation of isoprenaline curves, suggesting the presence of only Ca2+ antagonist component(s).
When tested in aorta, the petroleum spirit, chloroform and aqueous fractions produced a combination of vasoconstrictor and vasodilator effects. The vasoconstrictor effect was not reproducible on the same preparation probably due to the presence of accompanied relaxant component(s). However, the vasoconstrictor effect was evident in each fresh preparation, which remained unaffected in the presence of phentolamine, α-adrenoceptor antagonist (CitationGhayur & Gilani, 2007), which suggests that the vasoconstrictor effect is not mediated through adrenergic receptor activation. The ethyl acetate fraction showed only non-specific vasodilator effects, such as those caused by the standard vasodilators, verapamil and papaverine, suggesting that the ethyl acetate fraction lacks the vasoconstrictor component(s), distributed in rest of the three fractions.
The PDE inhibitors increased intracellular level of cAMP through inhibition of PDE, considered relaxant in the smooth muscle and stimulant in the heart (CitationSaegusa et al., 2003). Interestingly, the chloroform, ethyl acetate and aqueous fractions produced positive inotropic effects in atria followed by cardiac inhibition at the next higher concentrations. A similar pattern of activity was observed with papaverine. The ethyl acetate fraction exhibited a marked cardiotonic effect, suggesting that the PDEI component might be concentrated in it. The petroleum spirit fraction, like verapamil was found devoid of any cardio-stimulant effect and only caused cardiac suppression.
Some of the constituents of Hypericum perforatum, such as hyperforin, hypericin and hyperoside showed verapamil-like inhibitory patterns, being more effective against K+-induced contractions, and failed to potentiate the isoprenaline effect, thus showing only Ca2+ antagonist effect. The test compounds were obtained in the following order of potency: hyperforin > hypericin > hyperoside. Hyperforin was found around 160 and 175 times more potent against spontaneous contraction and 300 and 380 times on K+-induced contractions, compared to hypericin and hyperoside respectively. The compounds could not be studied further due to their limited supply.
Conclusions
This study indicates that the petroleum spirit, chloroform, ethyl acetate and aqueous fractions of Hypericum perforatum extract exhibited antispasmodic, bronchodilator, vasoconstrictor, vasodilator, cardiotonic and cardio-depressant activities. All the tested fractions possess a combination of Ca2+ antagonist and PDEI components, except petroleum spirit, which was devoid of any PDEI effect. The Hypericum perforatum active principles, hyperforin, hypericin and hyperoside showed only CCB effect. Further studies are suggested to identify the constituent(s) responsible for the PDEI activity.
Declaration of interest
This study was supported by funds made available by the Pakistan Science Foundation and Higher Education Commission, Government of Pakistan. The authors report no conflicts of interest. The authors are responsible for the content and writing of the paper.
References
- Abdel-Haq H, Cometa MF, Palmery M, Leone MG, Silvestrini B, Saso L. (2000). Relaxant effect of Hydrastis canadensis and its major alkaloids on guinea-pig isolated trachea. Pharmacol Toxicol, 87, 218–222.
- Chan SS, Choi AO, Jones RL, Lin G. (2006). Mechanisms underlying the vasorelaxing effects of butylidenephthalide, an active constituent of Ligusticum chuanxiong in rat isolated aorta. Eur J Pharmacol, 537, 111–117.
- Ghayur MN, Gilani AH. (2007). α-Adrenergic receptor mediated hypertensive and vasoconstrictor effects of dietary radish leaves extract. J Health Sci, 53, 151–155.
- Gilani AH, Khan A, Subhan F, Khan M. (2005a). Antispasmodic and bronchodilator activities of St. John’s wort are putatively mediated through dual inhibition of calcium influx and phosphodiesterase. Fund Clin Pharmacol, 19, 695–705.
- Gilani AH, Shah AJ, Ghayur MN, Majeed K. (2005b). Pharmacological basis for the use of turmeric in gastrointestinal and respiratory disorders. Life Sci, 76, 3089–3105.
- Gilani AH, Khan A, Ghayur MN, Ali SF, Herzig JW. (2006). Antispasmodic effect of Rooibos tea (Aspalathus linearis) is mediated predominantly through K+ channel activation. Basic Clin Pharmacol Toxicol, 99, 365–373.
- Gilani AH, Bashir S, Khan A. (2007). Pharmacological basis for the use of Borago officinalis in gastrointestinal, respiratory and cardiovascular disorders. J Ethnopharmacol, 114, 393–399.
- Gilani AH, Khan A, Raoof M, Ghayur MN, Siddiqui BS, Vohra W, Begum S. (2008a). Gastrointestinal, selective airways and urinary bladder relaxant effects of Hyoscyamus niger are mediated through dual blockade of muscarinic receptors and Ca2+ channels. Fund Clin Pharmacol, 22, 87–99.
- Gilani AH, Khan A, Ali T, Ajmal S. (2008b). Mechanisms underlying the antispasmodic and bronchodilatory properties of Terminalia bellerica fruit. J Ethnopharmacol, 116, 528–538.
- Gilani AH, Mehmood MH, Janbaz KH, Khan A, Saeed SA. (2009). Gut modulatory and antiplatelet activities of Viscum cruciatum. Pharm Biol, 47, 955–961.
- Gilani SN, Khan A, Gilani AH. (2010). Pharmacological basis for the medicinal use of Zanthoxylum armatum in gut, airways and cardiovascular disorders. Phytother Res, 24, 553–558.
- Kaneda T, Takeuchi Y, Matsui H, Shimizu K, Urakawa N, Nakajyo S. (2005). Inhibitory mechanism of papaverine on carbachol-induced contraction in bovine trachea. J Pharmacol Sci, 98, 275–282.
- Khan A, Gilani AH. (2008a). Cardiovascular inhibitory effects of Hyoscyamus niger. Methods Find Exp Clin Pharmacol, 30, 295–300.
- Khan A, Gilani AH. (2008b). Pharmacodynamic evaluation of Terminalia bellerica for its antihypertensive effect. J Food Drug Anal, 16, 6–14.
- Khan A, Gilani AH. (2009a). Antidiarrheal, antisecretory and bronchodilatory activities of Hypericum perforatum. Pharm Biol, 47, 962–967.
- Khan A, Gilani AH. (2009b). Antispasmodic and bronchodilator activities of Artemisia vulgaris are mediated through dual blockade of muscarinic receptors and calcium influx. J Ethnopharmacol, 126, 480–486.
- Musha S, Watanabe M, Ishida Y, Kato S, Konishi M, Tomoda A.(2005). A phenoxazine compound, 2-amino-4,4α-dihydro-4α-7-dimethyl-3H-phenoxazine-3-one reverses the phenylephrine or high-K+ induced contractions of smooth muscles in rat aorta and guinea-pig tenia cecum. Biol Pharm Bull, 28, 1521–1523.
- National Research Council (1996). Guide for the Care and Use of Laboratory Animals. Washington DC: National Academy Press, 1–7.
- Saegusa Y, Sugiyama A, Takahara A, Nagasawa Y, Hashimoto K. (2003). Relationship between phosphodiesterase inhibition induced by several Kampo medicines and smooth muscle relaxation of gastrointestinal tract tissues of rats. J Pharmacol Sci, 93, 62–68.