Abstract
Context: Allanblackia floribunda Oliver (Guttiferae) is an African medicinal plant used traditionally to treat a variety of ailments.
Objective: We investigated the antitumor, radical scavenging, antimycobacterial, antibacterial and antifungal activities of the root bark extract of A. floribunda and three isolated phenolics, namely 1,7-dihydroxyxanthone (1), morelloflavone (2) and 7′-O-glucoside of morelloflavone (3).
Materials and methods: The 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) radical scavenging assay was used for antioxidant tests, while crown gall tumor assay was used for assay of antitumor activity. The p-iodonitrotetrazolium chloride (INT) colorimetry and Microplate Alamar Blue Assay (MABA) were used for antimicrobial investigations.
Results: Moderate tumor reducing activity was observed with the extract, while better activities were recorded with compounds 2 and 3. The antimycobacterial and antitumor activities of the extract are being reported for the first time. The DPPH radical scavenging test showed that all the studied samples were able to scavenge more than 50% of the free radical, with compound 3 showing the best inhibitory activity (IC50 of 49.08 µg/mL). Compounds 1 to 3 prevented the growth of Mycobacterium smegmatis and both extract and compound 2 were active on M. tuberculosis. The lowest MIC value for the extract (9.76 μg/mL) was recorded against Enterobacter aerogenes while the corresponding value for the compounds (4.88 µg/mL) was obtained with compound 2 on Trichophyton rubrum.
Discussion and conclusion: The overall results of the present work provide baseline information for the potential use of the root bark extract of A. floribunda as an antimicrobial, antitumor and antioxidant phytomedicine.
Introduction
A large part of the world’s population today relies on natural product remedies to treat a variety of ailments. The World Health Organization (WHO) estimates that 80% of the population in some Asian and African countries depend on traditional medicine for primary health care (CitationWHO, 2002). Medicinal plants and their components are widely used in traditional medicine and have led to the development of new pharmaceutical drugs (CitationLewis & Elvin-Lewis, 1977). Approximately 25% of the active substances prescribed in the United States come from plant materials (CitationCéspedes et al., 2006). It is estimated that nearly 20,000 species from several families are useful for this purpose (CitationPenso, 1982). Our research on herbal medicine includes plants of the Guttiferae family. Most of these plants and their metabolites have been found to possess significant biological properties (CitationVivien & Faure, 1979; CitationNkengfack et al., 2002a, Citationb; CitationOuahouo et al., 2004; CitationMbaveng et al., 2008a). In this study we targeted another plant of this family, Allanblackia floribunda Oliver. Different parts of A. floribunda are used traditionally to treat many ailments. In Cameroon the decoction of the stem bark is used to treat dysentery or as a gargle against toothache. The seeds are used in the manufacture of ointment against itching (CitationVivien & Faure, 1979). Extracts from leaves, stem bark, and roots are used alone or combined with other plants in several African countries such as Gabon, Congo, and Cameroon to treat respiratory infections, dysentery, diarrhea, and toothache (CitationVivien & Faure, 1979). The present work was therefore undertaken to evaluate the antitumor, antioxidant, antimycobacterial, antibacterial and antifungal activities of the root bark extract of A. floribunda and three phenolic compounds purified from this extract.
Materials and methods
Plant material
The root bark of Allanblackia floribunda was collected at Mont Kala, Central Region of Cameroon, in October 2006. The plant was identified by Louis Zapfack of the Botany Department, University of Yaoundé I, where a voucher specimen was deposited.
Extraction and purification
The air-dried and powdered root bark (5 kg) of A. floribunda were successively macerated in CH2Cl2-MeOH (1:1) for 24 h and MeOH (20 l) for 4 h. Each filtrate was then concentrated in a vacuum under reduced pressure and the two concentrated filtrates were then combined after TLC analysis to give the crude extract (AFR; 182 g).
AFR (150 g) was subjected to vacuum flash chromatography using silica gel (70–230 mesh; 900 g), and eluted sequentially with hexane (2000 mL), hexane-ethyl acetate 50:50 v/v (1600 mL), ethyl acetate (1600 mL) and ethyl acetate-methanol 90:10 v/v (EtOAc-MeOH; 2800 mL). Twenty fractions of 400 mL each were collected and pooled on the basis of their TLC profiles in three fractions named A (fractions 1–5), B (6–13) and C (14–20).
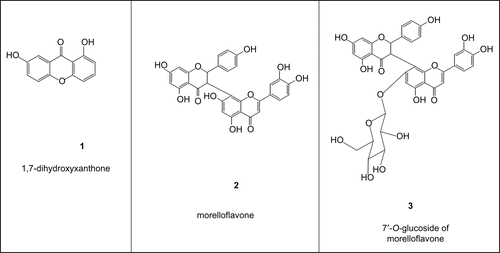
Fraction A (15 g) was column chromatographed using silica gel 60 (100 g) and eluted with hexane and hexane-ethyl acetate gradient (97.5:2.5; 95:5; and 90:10 v/v); 144 fractions of 150 mL each were collected and pooled on the basis of their TLC profiles. Fractions 14 and 15 crystallized after 24 h to yield a yellow powder, 1,7-dihydroxyxanthone C13H8O4 [1; 50 mg; MW (Molecular Weight): 228; melting point (m.p.): 240°C] (CitationMonache et al., 1983). Fraction B (40 g) was column chromatographed using silica gel 60 (300 g), with CH2Cl2 (1250 mL) and CH2Cl2/MeOH gradients [97.5:2.5 v/v (2000 mL); 95:5 v/v (950 mL); 90:10 v/v (1250 mL); 85:15 v/v (1250 mL); and 75:25 v/v (550 mL)] as eluents. 145 fractions of 50 mL each were collected and fractions 50–62 eluted with CH2Cl2/MeOH 97.5: 2.5 v/v yielded a yellow powder, morelloflavone C30H20O10 (2; 300 mg; MW: 556; m.p.: 244–245°C) (CitationLocksley & Murray, 1971). Fractions 87–102 (4 g) eluted with CH2Cl2/MeOH (90:10 v/v) on silca gel 60 (35 g) column yielded another yellow powder, 7′-O-glucoside of morelloflavone C36H30016 (3; 150 mg; MW: 760; amorphous powder) (CitationMonache et al., 1983). The chemical structures of the isolated compounds are shown in .
General experimental procedure
IR spectra were recorded on an ATI Mattson Genesis Series FTIR spectrometer as KBr disc. 1H-NMR, 13C-NMR, two-dimensional COSY, ROESY, HSQC and HMBC analysis were performed on a Bruker Avance DPX instrument (Muenster, Germany) (300.13 MHz for 1H and 75.47 MHz for 13C). The 2.5 and 40.0 ppm resonances of residual CD3SOCD3 were used as internal references for 1H and 13C-NMR spectra, respectively. Mass spectra were recorded on a Bruker micrOTOF instrument. All melting points were determined on a micro-melting point apparatus and are uncorrected. The structures of the compounds were confirmed by comparing with reference data from available literature.
Antioxidant investigation: DPPH assay
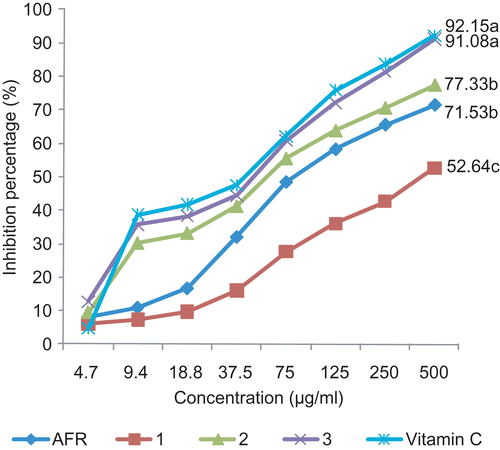
The free radical scavenging activity of the extract and compounds was evaluated as described by CitationMensor et al. (2001). Briefly, the test samples were dissolved in pure dimethylsulfoxide (DMSO, Sigma-Aldrich, St Quentin Fallavier, France) and mixed with a 0.3 mM 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH; Sigma) solution in ethanol. After 30 min at room temperature, the absorbance was measured at 517 nm and converted into percentage of antioxidant activity. Ascorbic acid was used as a standard control. Each assay was repeated thrice and the results recorded as mean of the triplicate experiments (). The inhibition ratio (%) was calculated as follows: % inhibition = [(Absorbance of control−Absorbance of test sample)/Absorbance of control] × 100. IC50 value is the concentration of sample required to scavenge 50% DPPH free radical and was calculated from a calibration curve by linear regression (CitationJoshi et al., 2010).
Figure 2. Antioxidant activity of the crude extract and compounds isolated from A. floribunda (AFR: crude extract from the root bark of Allanblackia floribunda), 1: 1,7-dihydroxyxanthone, 2: morelloflavone, 3: 7′-O-glucoside of morelloflavone; values with the same letter are not significantly different, P <0.05; ANOVA).
Preliminary antitumor test: Potato disc tumor induction (Crown gall) assay
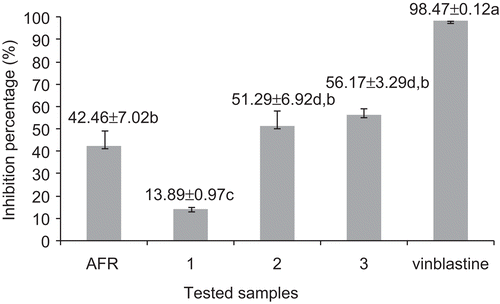
The antitumor assay was carried out as described by CitationCoker et al. (2003) and CitationMcLaughlin and Roger (1998). Briefly, Agrobacterium tumefaciens LMG 184 (from the Laboratory of Microbiology, University of Gent, Belgium) was grown on yeast extract medium (YEM) for 48 h at 28°C. Red potato discs (Solanum tuberosum L.) were impregnated with A. tumefaciens suspension [109 colony forming units (CFU) in phosphate-buffered saline (PBS)] and extract or compounds dissolved in pure DMSO at a final concentration of 100 µg/disc. Vinblastine (Sigma) at 5 µg/disc was used as positive control. Negative controls included pure DMSO with PBS; pure DMSO without the bacterium and pure DMSO with the bacterium. At day 12 of incubation at 28°C, the discs were stained with Lugol’s reagent, and the tumors were counted under a dissecting microscope. Twelve replicates were analyzed for each sample, and the final results were graphically reported ().
Figure 3. Antitumor activity of the crude extract and compounds isolated from A. floribunda (AFR: crude extract from the root bark of Allanblackia floribunda), 1: 1,7-dihydroxyxanthone, 2: morelloflavone, 3: 7′-O-glucoside of morelloflavone; values with the same letter are not significantly different, P< 0.05; ANOVA).
Antimicrobial assays
Microbial strains
The test organisms included mycobacteria, fungi, Gram-negative and Gram-positive bacteria. Mycobacteria were obtained from the American Type Culture Collection. Other microbial species were clinical isolates from Yaoundé General Hospital (Cameroon). Their identification was confirmed before use at the Laboratory of Applied Microbiology and Molecular Pharmacology (LMP) (Faculty of Science, University of Yaoundé I). This was followed by culturing on the specific media and biochemical test using the API system (CitationMbaveng et al., 2008b).
Culture media
M. smegmatis was cultured on Middlebrook 7H11 agar (7H11) and allowed to grow for 24 h. M. tuberculosis was plated onto Löwenstein-Jensen medium and allowed to grow for 3–4 weeks at 37°C. Middlebrook 7H9 broth (Becton Dickinson, Sparks, MD), supplemented with 1% casamino acids (Fisher, Pittsburgh, PA), 0.2% glycerol (Fisher), 0.2% glucose, and 0.05% Tween-80 for M. smegmatis or supplemented with 0.2% glycerol (Sigma, St. Louis, MO) and 10% oleic acid-albumin-dextrose-catalase (OADC, Becton Dickinson), 0.5% glycerol, and 0.05% Tween 80 for M. tuberculosis, was used to determine the minimal inhibitory concentration (MIC) and the minimal bactericidal concentration (MBC) of the test samples on M. smegmatis and M. tuberculosis. Nutrient Agar (NA) containing bromocresol purple was used for the activation of Bacillus cereus, while NA was used for other bacteria. Sabouraud glucose agar was used for the activation of the fungi. Mueller Hinton agar (MHA) was also used for the determination of the minimal microbicidal concentration (MMC) of the test samples.
Chemicals for antimicrobial assay
Ciprofloxacin and isoniazid (INH) (Sigma) were used as positive control for M. smegmatis and M. tuberculosis, respectively. Nystatin (Maneesh Pharmaceuticals, Mumbai) and gentamycin (Jinling Pharmaceutical, Nanjing) were used as reference antibiotics (RA), respectively, against fungi and bacteria other than M. smegmatis and M. tuberculosis.
Antimycobacterial assays
Microplate susceptibility testing against M. smegmatis
All samples were tested against M. smegmatis using the p-iodonitrotetrazolium chloride (INT) microplate dilution method. The MIC, MBC and bacterial preparation were performed in 96-well microplates according to CitationSalie et al. (1996) and CitationNewton et al. (2002). The extract and compounds (both in 10% DMSO/7H9) were tested in concentrations ranging from 1.22–625 μg/mL. Ciprofloxacin served as the positive drug control. DMSO at 2.5% served as solvent control. Tests were done in triplicates. The cultured microplates were incubated at 37°C for 24 h. The MIC of samples was detected following addition (40 µL) of 0.2 mg/mL of INT (Sigma-Aldrich, Johannesburg, South Africa) and incubated at 37°C for 30 min (CitationEloff, 1998). MIC was defined as the lowest sample concentration that prevented the color shift (yellow to pink). The MBC was determined by adding 50 µL aliquots of the preparations (without INT), which did not show any growth after incubation during MIC assays, to 150 µL of 7H9 broth. These preparations were incubated at 37°C for 48 h. The MBC was regarded as the lowest concentration of extract which did not produce a color change after addition of INT as mentioned above.
Antituberculosis assay using M. tuberculosis: MABA susceptibility testing
The activities of all test samples against M. tuberculosis were evaluated using the Microplate Alamar Blue Assay (MABA) according to CitationCollins and Franzblau (1997) as modified by CitationJimenez-Arellanes et al. (2003). M. tuberculosis was cultured at 37°C in Middlebrook 7H9 broth. The extract, compounds and INH were dissolved in 10% DMSO/7H9 broth to final concentrations ranging from 0.31 to 625 μg/mL. The final concentration of DMSO in all assays was 2.5% or less. The samples were assayed twice in duplicate. Test inoculum was 6 × 106 CFU/mL. Microplates were incubated for 5 days at 37°C in a 5% CO2 atmosphere and growth was detected by observing color shift (blue to pink) following addition of Alamar blue solution (Sigma) and 20% sterile Tween 80 (Sigma) 1:1 v/v. The MIC corresponded to the greatest dilution of the sample in which the color shift from blue to pink was not observed.
Determination of mycobactericidal effect (MBC)
Samples with detected MIC values following MABA (CitationCollins & Franzblau, 1997; CitationJimenez-Arellanes et al., 2003) were assayed for their mycobactericidal effect as follows. The mycobacterial suspensions showing no growth (5 μL) were transferred from the former to a new microplate that contained 195 μL of fresh culture medium. The microplates were incubated and developed with Alamar blue solution as for MABA. The MMC corresponded to the minimum sample concentration that did not cause a color shift in cultures re-incubated in fresh medium.
Antimicrobial assay on Gram-positive and Gram-negative bacteria and fungi
Sensitivity test: Agar disc diffusion assay
Preparation of discs. Whatman filter paper (No.1) discs of 6 mm diameter impregnated with extract at 200 µg/disc, isolated compounds at 80 µg/disc or RA at 40 µg/disc, were prepared using 100% DMSO as solvent. Three discs were prepared as part of western medicine for each sample. Negative control discs were also prepared as above with 10 µL of the 100% DMSO solution.
Diffusion test. The antimicrobial disc diffusion test was carried out as described by CitationKuete et al. (2007a; Citation2008a,Citationb) using a cell suspension of about 1.5 × 106 CFU/mL obtained from a McFarland turbidity standard No. 0.5. The suspension was standardized by adjusting the absorbance to 0.1 at 600 nm (Shimadzu UV-120-01 spectrophotometer, Kyoto, Japan). A disc prepared with 100% DMSO was used as negative control. The plates were incubated at 30°C for 48 h (Microsporum audouinii) or 37°C for 24 h (other organisms). Antimicrobial activity was evaluated by measuring the diameter of the inhibition zone (IZ) around the disc. The assay was repeated three times and results were recorded as mean ± SD of the three experiments.
MIC and MMC determinations. The MICs of the extract and compounds were determined in a microdilution assay as previously described (CitationKuete et al., 2007a, Citationb, Citationc, Citation2008a, Citationb). The test samples were dissolved in 10% DMSO/MHB (Mueller Hinton Broth) to a final concentration range of 1.22 to 625 µg/mL. Inoculum concentration was standardized at 1.5 × 106 cfu/mL. The final concentration of DMSO in each well was less than 1%. The microplates were incubated at 30°C for 48 h (M. audouinii) or 37°C for 24 h (other organisms). The assay was repeated thrice. The MICs of samples were detected following addition (40 µL) of 0.2 mg/mL p-iodonitrotetrazolium chloride and incubated at 37°C for 30 min (Kuete et al., Citation2009a, Citationb). The MIC corresponded to the greatest dilution of sample in which the color shift from yellow to pink was not observed
For the determination of MMC, a portion of liquid (5 µL) from each well that showed no change in color was plated on MHA and incubated at 30°C for 48 h (M. audouinii) or 37°C for 24 h (other organisms). The lowest concentration that yielded no growth after this sub-culturing was taken as the MMC (CitationKuete et al., 2007a, Citationb, Citationc, Citation2008a, Citationb)
Results
The purification of A. floribunda extract led to the isolation of three major compounds, 1,7-dihydroxyxanthone (1) (MW: 228; m.p.: 240°C) (CitationMonache et al., 1983), morelloflavone (2) (MW 556; m.p.: 244–245°C) (CitationLocksley & Murray, 1971) and 7′-O-glucoside of morelloflavone (3) (MW: 760; amorphous powder) (CitationMonache et al., 1983). The three phenolic compounds belong to the classes of xanthone (1) and biflavonoids (2 and 3) (). In the present report we evaluated the antitumor, antioxidant, antimycobacterial, antibacterial and antifungal activities of the extract and compounds from A. floribunda.
In the antitumor experiment, it appeared from the results of the bacterial viability test that the tested concentrations of the plant extract do not alter A. tumefaciens growth at 10, 20, 30 min and 1 h of treatment. The three controls used in this assay included DMSO with PBS, DMSO without the bacteria and DMSO with the bacteria. The two controls did not induce tumor, showing that neither DMSO nor PBS interfere with the activity of A. tumefaciens or induce tumor themselves. DMSO with A. tumefaciens induced an average of 33 tumors. The antitumor activity of the tested samples is summarized in . Moderate tumor reducing activity was observed with the extract (42.46% at 100 µg/disc). Better activity was recorded with compounds 2 and 3, their tumor inhibition percentages being 51.29% and 56.17%, respectively. Compound 1, with 13.89% activity, was less active compared to 2 and 3. However, the reference drug, vinblastine, at 5 µg/disc, was still more active (98.47%) than compounds 2 (51.29%) and 3 (56.17%) at 100 µg/disc.
summarizes the DPPH• scavenging activity of the extract and compounds isolated from the root bark of A. floribunda. It appeared that at the concentration of 500 µg/mL all the studied samples were able to scavenge more than 50% of the free DPPH radical. Compound 3 showed the best activity, exhibiting 91.08% inhibition. This activity was not significantly different (P < 0.05) from that of ascorbic acid used as reference antioxidant compound. The IC50 as determined by graphic extrapolation were 45.7, 49.08, 62.8, 76.3, and 488.53 µg/mL, respectively, for vitamin C, compounds 3, 2, the crude extract and compound 1.
The results of the antimycobacterial assays () show that the extract as well as compounds 1 to 3 were able to prevent the growth of M. smegmatis in the tested concentration range. Only the extract and compound 2 were active on M. tuberculosis. MIC values of 39.06 μg/mL for the extract and 19.53 µg/mL for compound 2 were recorded for M. smegmatis. Results of the MMC determination () showed detectable values for the samples on several organisms.
Table 1. Antimycobacterial activity of the crude extract, compounds isolated from Allanblackia floribunda and reference antibiotics.
and also summarize the results of the antimicrobial assays against fungi, Gram-positive and -negative bacteria. Results of the diffusion test () demonstrated that the extract and compound 2 prevented the growth of all the tested organisms. The IZ obtained ranged from 7–22 mm and 7–22.5 mm, respectively, for the extract and compound 2. Compound 3 was active on 11 of the 18 (61.1%) studied organisms, including Gram-positive and Gram-negative bacteria, and fungi. The results of MIC determinations () indicated values for the extract range from 19.53 to 312.5 μg/mL for most of the tested microorganisms. As previously observed, compound 3 was selectively active. A MIC value of 9.76 µg/mL for the extract was recorded against E. aerogenes. A MIC value of 4.88 µg/mL was noted with compound 2 on Trichophyton rubrum. The reference antibiotics exhibited MICs ranging from 2.44 to 19.53 μg/mL. The inhibitor potential of the extract and compound 2 can be considered important in regard to the antibacterial and antifungal activities of the RA. This was as active as nystatin on T. rubrum. The results of the MMC determinations () showed microbicidal activity on 83.3% (15/18), 77.8% (14/18) and 27.7% (5/18) of the tested organisms for the extract, compounds 2 and 3, respectively. The MMC values (ranging from 4.88–39.06 µg/mL) obtained with reference antibiotics were generally lower than those of the extract and compounds in the corresponding microbial species. Nevertheless, the value obtained once with compound 2 on T. rubrum was lower than that of nystatin, highlighting its good antimicrobial potency.
Table 2. Inhibition zone diameters (mm) of the extract and compounds isolated from Allanblackia floribunda and reference antibiotics as determined by diffusion testa.
Table 3. Minimal inhibition concentration (MIC) and minimal microbicidal concentration (MMC in parenthesis) of the extract and compounds isolated from Allanblackia floribunda and reference antibiotics as determined by microdilution assay.
Discussion
The role of plant secondary metabolites as antitumor compounds is well known, with flavonoids shown to possess antimutagenic and anticarcinogenic activity (CitationBrown, 1980; CitationHirano et al., 1989). The inhibition of Agrobacterium tumefaciens-induced tumors (or crown gall) in potato disc tissue is an assay based on antimitotic activity and has been used to detect a broad range of known and novel antitumor agents (CitationCoker et al., 2003). Crown gall is a neoplastic plant disease caused by A. tumefaciens. The validity of this bioassay is based on the observation that certain tumorigenic mechanisms are similar in plants and animals (CitationLiu et al., 2007). It has been shown that the inhibition of crown gall tumor initiation on potato discs and subsequent growth showed good correlation with compounds and extracts active in the 3PS leukemic mouse assay (CitationGalsky et al., 1980). A number of well-known antineoplastic agents such as podophyllin, taxol, camptothecin, vincristine and vinblastine have all shown significant tumor inhibition of crown gall (CitationCoker et al., 2003). This experiment therefore indicates the possible use of this plant for anticancer treatment, and shows that some of its components could be more useful from the perspective of the development of antitumor medicine. However, further studies on more specific tumor cell lines will be necessary to confirm this hypothesis.
In the DPPH radical scavenging assay, all the three studied phenolic compounds were active with IC50 closer to that of ascorbic acid. This result is in conformity with the role of phenolics as antioxidant compounds (CitationLiu et al., 2007) and also consistent with the role of ascorbic acid as DPPH scavenging agent, as the IC50 (45.7 µg/mL) obtained is closer (40.2 µg/mL) to that obtained by CitationBhandari et al. (2010).
Observation of MBC values of samples against the mycobacteria indicated that they were not more than four times their corresponding MICs. This suggests that bactericidal effect of studied samples could be expected (CitationMims et al., 1993). The data obtained when samples were tested against fungi, Gram-positive, and Gram-negative bacteria also confirmed that they could have killing effect on most of the tested organisms (CitationKuete et al., 2007a, Citation2007b).
The use of M. smegmatis in this assay was a preliminary step to select the concentration range to be tested on M. tuberculosis species. The results obtained validated the necessity of such experiments. However, it is well known that the sensitivity of M. smegmatis is closer to that of M. tuberculosis and that this non-pathogenic mycobacterial species can be used in selecting samples for M. tuberculosis studies (CitationNewton et al., 2002).
In regard to the structure–activity relationship, it appeared that transformation of morelloflavone (2) to 7′-O-glucoside of morelloflavone (3) significantly reduced the antimicrobial activity of the latter compound. This could be due to the ability of the microorganisms to break the 7-O-glucoside bond of compound 3 to yield glucose and compound 2. The release glucose can therefore be used as source of energy for their growth (CitationBacq-Calberg et al., 1999). In the antitumor assay, such structure-related activity is not pronounced. This confirms the fact that the two compounds do not prevent the growth of A. tumefaciens, but act directly on the tumor-inducing mechanism. The glucose moiety also increases the antioxidant potency of compound 3, explaining why the resultant activity is better than that of compound 2.
To the best of our knowledge, the antimycobacterial and antitumor activities of the extract of A. floribunda is being reported for the first time. However, the ability of this crude extract to prevent in vitro the growth of Candida albicans, and that of some Gram-positive and Gram-negative bacteria has been demonstrated (CitationAjibesin et al., 2008). The results obtained in the present work corroborate the earlier report and confirm also the activity of A. floribunda on filamentous fungi such as Trichophyton rubrum and M. audouinii. In the present study, compound 1 was not tested against fungi, Gram-positive, and Gram-negative bacteria. However, this compound from the stem bark of Vismia rubescens has been found to exhibit both antibacterial and antifungal activities (CitationTamokou et al., 2009). The antitumor activity could essentially be due to the presence of anticancer compounds such as morelloflavone or 7′-O-glucoside of morelloflavone. Morelloflavone is known to inhibit tumor growth and tumor angiogenesis of prostate cancer in mouse tumor model in vivo, suggesting that the inhibition of tumorigenesis by targeting angiogenesis could be its mode of action (CitationXiufeng et al., 2009).
Conclusions
The overall results of this study provide baseline information for the use of the extract of A. floribunda as well as some of its components as sources of antimycobacterial, antibacterial, antifungal, antitumor and antioxidant drug. However, further toxicological studies need to be done to confirm this hypothesis.
Acknowledgments
The authors are grateful for the technical support of Paul Lunga of the University of Dschang.
Declaration of interest
The authors acknowledge the financial support of the International Foundation for Science (IFS) (Grant no. F /4579-1 to V.K.) for the antimycobacterial assays, and both the IFS and the Organization for the Prohibition of Chemical Weapons, The Hague, Netherlands, grant F/3969 to A.G.B.A.
References
- Ajibesin KK, Rene N, Bala DN, Essiett UA. (2008). Antimicrobial activities of the extracts and fractions of Allanblackia floribunda. Biotechnology, 7, 129–133.
- Bacq-Calberg CM, Coyotte J, Hoet P, Nguyem-Disteche M. (1999). Microbiologie. Brussells: De Boeck & Larcier, 338–339.
- Bhandari P, Kumar N, Singh B, Ahuja PS. (2010). On-line HPLC-DPPH method for antioxidant activity of Picrorhiza kurroa Royle ex Benth. and characterization of kutkoside by ultra-performance LC-electrospray ionization quadrupole time-of-flight mass spectrometry. Indian J Exp Biol, 48, 323–328.
- Brown JP. (1980). A review of the genetic effect of naturally occuring flavonoids, anthraquinones and related compounds. Mutat Res,75, 243–277.
- Céspedes CL, Avila JG, Martınez A, Serrato B, Calderon-Mugica JC, Salgado-Garciglia R. (2006). Antifungal and antibacterial activities of Mexican tarragon (Tagetes lucida). J Agric Food Chem, 54, 3521–3527.
- Coker PS, Radecke J, Guy C, Camper ND. (2003). Potato disc tumor induction assay: A multiple mode of drug action assay. Phytomedicine, 10, 133–138.
- Collins L, Franzblau SG. (1997). Microplate Alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother, 41, 1004–1009.
- Eloff JN. (1998). A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med, 64, 711–713.
- Galsky AG,Wilsey JP, Powell RG. (1980). Crown gall tumor disc bioassay: A possible aid in the detection of compounds with antitumor activity. Plant Physiol, 65,184–185.
- Hirano T, Oka K, Akiba M. (1989). Antiproliferative effect of synthetic and natural occurring flavonoids on tumor cells of the human breast carcinoma cells lines ZR-75-1. Res Commun Chem Pathol Pharmacol, 64, 69–78.
- Jimenez-Arellanes A, Meckes M, Ramirez R, Torres J, Luna-Herrera J. (2003). Activity against multidrug-resistant Mycobacterium tuberculosis in Mexican plants used to treat respiratory diseases. Phytother Res, 17, 903–908.
- Joshi SC, Verma AR, Mathela CS. (2010). Antioxidant and antibacterial activities of the leaf essential oils of Himalayan Lauraceae species. Food Chem Toxicol, 48, 37–40.
- Kuete V, Ngameni B, Mbaveng TA, Ambassa P, Konga SI, Bezabih M, Etoa FX, Ngadjui TB, Abegaz BM, Penlap BV. (2007a). Antimicrobial activity of the extract from the twigs of Dorstenia elliptica (Moraceae). Pharmacology Online, 1, 573–580.
- Kuete V, Konga SI, Ngameni B, Bigoga DJ, Watchueng J, Nzesse KR, Etoa FX, Ngadjui TB, Penlap BV. (2007b). Antimicrobial activity of the methanolic extract, fractions and four flavonoids from the twigs of Dorstenia angusticornis Engl. (Moraceae). J Ethnopharmacol, 112, 271–277.
- Kuete V, Metuno R, Ngameni B, Mbaveng TA, Ngandeu F, Fotso WG, Bezabih M, Etoa FX, Ngadjui TB, Abegaz BM, Penlap BV. (2007c). Antimicrobial activity of the methanolic extracts and compounds from Treculia obovoidea (Moraceae). J Ethnopharmacol, 112, 531–536.
- Kuete V, Eyong KO, Folefoc GN, Beng VP, Hussain H, Krohn K, Nkengfack AE. (2007d). Antimicrobial activity of the methanolic extract and of chemical constituents isolated from Newbouldia laevis. Pharmazie, 62, 552–556.
- Kuete V, Mbaveng TA, Tsafack M, Beng PV, Etoa FX, Nkengfack AE, Meyer JJM, Lall N. (2008a). Antitumor, antioxidant and antimicrobial activities of Bersama engleriana (Melianthaceae). J Ethnopharmacol, 115, 494–501.
- Kuete V, Ngameni B, Fotso Simo CC, Kengap TR, Ngadjui BT, Meyer JJM, Lall N, Kuiate JR. (2008b). Antimicrobial activity of the crude extracts and compounds from Ficus chlamydocarpa and Ficus cordata (Moraceae). J Ethnopharmacol, 120, 17–24.
- Kuete V, Nana F, Ngameni B, Mbaveng AT, Keumedjio F, Ngadjui BT. (2009a). Antimicrobial activity of the crude extract, fractions and compounds from stem bark of Ficus ovata (Moraceae). J Ethnopharmacol, 124,556–561.
- Kuete V, Fozing DC, Kapche WFGD, Mbaveng AT, Kuiate JR, Ngadjui BT, Abegaz BM. (2009b). Antimicrobial activity of the methanolic extract and compounds from Morus mesozygia stem bark. J Ethnopharmacol, 124,551–555.
- Lewis WH, Elvin-Lewis MPF. (1977). Medical Botany: Plants Affecting Man’s Health. New York: Wiley.
- Liu X, Ardo S, Bunning M, Parry J, Zhou K, Stushnoff C, Stoniker F, Yu L, Kendall P. (2007). Total phenolic content and DPPH radical scavenging activity of lettuce (Lactuca sativa L.) grown in Colorado. LWT - Food Sci Technol, 40, 552–557.
- Locksley HD, Murray IG. (1971). Extractives from Guttiferae. Part XIX. The isolation of two benzophenones, six xanthones and two biflavonoids from the heartwood of Allanblackia floribunda Oliver. J Chem Soc (C), 1332–1340.
- Mbaveng AT, Ngameni B, Kuete V, Konga Simo I, Ambassa T, Roy R, Bezabih M, Etoa FX, Ngadjui BT, Abegaz BM, Meyer JJM, Lall N, Penlap BV. (2008b). Antimicrobial activity of the crude extracts and five flavonoids from the twigs of Dorstenia barteri (Moraceae). J Ethnopharmacol, 116, 483–489.
- Mbaveng TA, Kuete V, Nguemeving JR, Penlap BV, Nkengfack AE, Meyer JJM, Lall N, krohn K. (2008a). Antimicrobial activity of the extracts and compounds obtained from Vismia guineensis (Guttiferae). Asian J Trad Med, 3, 211–223.
- McLaughlin JL, Roger LL. (1998). The use of biological assays to evaluate botanicals. Drug Inf J, 32, 513–524.
- Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC, Coube CS, Leitao SG. (2001). Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res, 15, 127–130.
- Mims CA, Playfair JHL, Roitt IM, Wakelin D, Williams R. (1993). Antimicrobials and chemotherapy. In: Mims CA, Playfair JHL, Williams R, Roitt IM, Wakelin D (Eds.), Medical Microbiology Review Vol. 35, pp. 1–34.
- Monache FD, Mac-Quhae MM, Monache GD, Bettolo GBM, De Lima RA. (1983). Xanthones, xanthonolignoids and other constituents of the roots of Vismia guaramirangae. Phytochemistry, 22, 227–232.
- Newton SM, Lau C, Gurcha SS, Besra GS, Wright CW. (2002). The evaluation of forty-three plant species for in vitro antimycobacterial activities: Isolation of active constituents from Psoralea corylifolia and Sanguinaria canadensis. J Ethnopharmacol, 79, 57–67.
- Nkengfack AE, Mkounga P, Meyer M, Fomun ZT, Bodo B. (2002a). Globulixanthone C, D and E. Three prenylated xanthones with antimicrobial properties from the roots of Symphonia globulifera. Phytochemistry, 61, 181–187.
- Nkengfack AE, Azebaze GB, Vardamides JC, Fomum ZT, Van Heerden FR. (2002b). A prenylated xanthone from Allanblackia floribunda. Phytochemistry, 60, 381–384.
- Ouahouo BMW, Azebaze AGB, Meyer M, Bodo B, Fomun ZT, Nkengfack E. (2004). Cytotoxic and antimicrobial coumarins from Mammea africana. Ann Trop Med Parasitol, 98, 733–739.
- Penso G. (1982). Index Plantarum Medicinalium Totius Mundicorunque Synoni Morum. Milan: Organizacione Editoriale Medico Farmacéutica.
- Salie F, Eagles PFK, Leng HMJ. (1996). Preliminary antimicrobial screening of four South African Asteraceae species. J Ethnopharmacol, 52, 27–33.
- Tamokou JDD, Tala MF, Wabo KH, Kuiate JR. (2009). Antimicrobial activities of methanol extract and compounds from stem bark of Vismia rubescens. J Ethnopharmacol, 124, 571–575.
- Vivien J, Faure JJ. (1979). Trees of Central Africa forests. Species of Cameroon. Paris: Agence de Coopération Culturelle et Technique, 212–213.
- WHO. (2002). WHO traditional medicine strategy 2002-2005. Geneva: World Health Organization. Available at: http://whqlibdoc.who.int/hq/2002/WHO.EDM_TRM_2002.1.pdf (accessed 13 January 2008).
- Xiufeng P, Tingfang Y, Zhengfang Y, Sung Gook C, Weijing Q, Decha P, Ken F, Mingyao L. (2009). Morelloflavone, a biflavonoid, inhibits tumor angiogenesis by targeting Rho GTPases and extracellular signal-regulated kinase signaling pathways. Cancer Res, 69, 518–525.