Abstract
Context: Phytochemical investigation of Clerodendrum chinense (Osbeck) Mabberley (Lamiaceae) cultivated in Egypt and evaluation for anti-inflammatory, analgesic, and antipyretic effects of the methanol and chloroform extracts of Clerodendrum chinense, Clerodendrum indicum (L.) Kuntze, Clerodendrum glabrum E. Meyer.
Objective: The objective of this study was to investigate the anti-inflammatory, analgesic, and antipyretic effects of the methanol and chloroform extracts of Clerodendrum species under investigation.
Materials and methods: Air-dried powdered leaves of C. chinense were extracted with MeOH 80%. This extract was fractionated with successive portions of chloroform, ethyl acetate and n-butanol. By further fractionation through silica gel, polyamide and reversed phase column chromatography several compounds were isolated which were elucidated by nuclear magnetic resonance (NMR) and mass spectroscopy. For biological study, the powdered leaves of C. chinense, C. indicum and C. glabrum were extracted by chloroform and then extracted with methanol. The acute anti-inflammatory effect of tested extracts of the leaves of Clerodendrum species under investigation was estimated by carrageenan-induced rat paw edema. Antipyretic effect was evaluated and compared with that of paracetamol as standard using the yeast-induced hyperthermia method on female albino rats. Analgesic effect was evaluated and compared with that of Novalgin (metamizol sodium) as standard using an electric current anxious stimulus.
Results: Verbascoside, isoverbascoside, decaffeoylverbascoside, hispidulin, lupeol and icariside B5 were isolated from the leaves of C. chinense for the first time. Cornoside and rengyolone were also isolated. The methanol extract of the leaves of C. chinense and verbascoside showed significant analgesic, anti-inflammatory and antipyretic effects.
Conclusion: The present study provided a scientific validation of the traditional claims suggested for C. chinense.
Introduction
The genus Clerodendrum (Lamiaceae) is widely distributed and has been used for ages in Indian and Chinese traditional medicine (CitationRajasekaran & Ponnusamy, 2006). In Nepal, the fresh juice of C. indicum (L.) Kuntze is used for the treatment of fever (CitationTaylor et al., 1996). In Thailand, C. chinense (Osbeck) Mabberley is used for its anti-pyretic and anti-inflammatory effects (CitationKanchanapoom et al., 2005). Unfortunately, there is no scientific study to support these traditional claims. Therefore, we conducted this research work to investigate the anti-inflammatory, antipyretic, analgesic effects of C. chinense, C. indicum and C. glabrum E. Meyer. Phytochemical investigation of the leaves of C. chinense was carried out. Three phenylpropanoid glycosides (verbascoside, isoverbascoside and decaffeoylverbascoside), one flavonoid (hispidulin), two cyclohexylethanoids (cornoside and rengyolone) and icariside B5 were isolated. The structures of isolated compounds were elucidated on the basis of spectroscopic data.
Material and methods
General
NMR spectra were recorded on a Bruker DRX-400 instrument operating at 400 MHz for 1H-NMR at 30°C in CD3OD (99.5% Deutrated (D)), CDCl3 (99.8% D) or DMSO-d6 (99.5% D). Chemical shifts were recorded in δ (ppm) relative to TMS (0 ppm). Mass spectra were recorded on a Bruker Esquire 3000 Plus Ion trap Mass Spectrometer with ESI and Esquire Control software. UV-visible spectra were recorded on a Shimadzu UV 240 spectrophotometer.
Chromatographic materials included silica gel 60 for CC (Davisil), reversed phase Lichroprep® RP-18 (40-63 µm) for CC (Merck), Polyamide CC 6 (0.05-0.16 mm) for CC (Macherey-Nagel), and TLC plates (silica gel 60 F254-Merck). p-Anisaldehyde-sulfuric acid spray reagent (CitationStahl, 1969), sodium methoxide solution, aluminum chloride solution, hydrochloric acid, sodium acetate powder and boric acid powder were used as reagents for UV-spectroscopic analysis of flavonoids (CitationMabry et al., 1970). All of the solvents used were of HPLC grade.
Collection of plant material
The leaves of C. chinense were collected from plants cultivated in Orman Botanical Garden, Giza, Egypt. The leaves of C. indicum were collected from plants cultivated in Giza Zoo, Giza, Egypt. The leaves of C. glabrum were collected from plants cultivated in Al-Zohrya Botanical Garden, Giza, Egypt. Collection of the plant materials was carried out in September 2006. The plants were identified by Mohamed Elgebaly, Department of Chemotaxonomy, National Research Centre, Cairo, Egypt. Voucher specimens were prepared and deposited at the Department of Pharmacognosy, Faculty of Pharmacy, Beni-Sueif University, Egypt.
Preparation of extracts for biological study
The leaves of the plants under investigation were separately air-dried, powdered and kept in amber-colored, well-closed containers at low temperature. The following procedure was followed for each plant. The leaves (100 g) were extracted with chloroform then the residual plant materials were allowed to dry, then extracted with methanol. The yields from chloroform and methanol extraction were 4.4, 4.8, 3.3 g and 10, 8.8, 11 g for C. chinense, C. indicum, and C. glabrum, respectively.
Extraction and isolation
Air-dried powdered leaves of C. chinense (900 g) were extracted with aqueous MeOH 80% (2 × 2000 mL, 72 h each). The solvent was evaporated under reduced pressure. The extract was dissolved in water and left overnight in a refrigerator, then filtered. The filtrate was extracted with successive portions of chloroform, ethyl acetate and n-butanol. The organic phases were evaporated to dryness under reduced pressure. The chloroform, ethyl acetate and n-butanol extracts yielded a residue of 1.5, 9 and 24 g, respectively. About 5 g of the dried ethyl acetate extract was applied on Silica gel column using n-hexane:ethyl acetate (50:50), gradually increasing the polarity untill 100% ethyl acetate; then the polarity was further increased using methanol (100 mL fractions). Forty fractions were collected and examined by TLC using ethyl acetate: hexane 7.5:2.5 v/v and/or ethyl acetate:methanol:water 9:1:1 v/v as the mobile phase. Fractions 5–6 (216 mg) were subjected to silica gel column to afford compound f1 (20 mg yellow crystals). Fractions 19–23 (3 g) were combined and subjected to polyamide column chromatography using a water/methanol gradient; 76 fractions were collected. The fractions were examined by TLC using the solvent system ethyl acetate:formic acid:acetic acid:water (30:1.2:0.8:8). Fractions 14–17 (330 mg) were combined and subjected to RP C18 column chromatography using water and increasing amounts of acetonitrile (50 mL fractions) to afford compound f2 (100 mg orange powder). Fractions 18–22 (350 mg) were combined and subjected to RP C18 column chromatography using water and increasing amounts of acetonitrile (52 mL fractions) to afford compound f3 (10 mg greenish powder). The n-butanol fraction (15 g) was subjected to polyamide column chromatography using water and increasing amounts of methanol; 80 fractions were collected and examined by TLC using the solvent system ethyl acetate:formic acid:acetic acid:water (30:1.2:0.8:8) and vanillin/sulfuric acid as spray reagent. Fractions 12–23 (5 g) were combined and subjected to RP C18 column chromatography using water and acetonitrile; 180 fractions were collected. Fractions 90–98 (100 mg), fractions 159–161 (50 mg) and fractions 173–175 (25 mg) were separately combined and further fractionated on a RP C18 column using water and acetonitrile to afford b1 (8 mg colorless oil), compound b2 (10 mg orange powder) and compound b3 (8 mg colorless oil), respectively. About 450 g air-dried powdered leaves of C. chinense were extracted with chloroform. The solvent was evaporated to dryness under reduced pressure (10 g). The residue was subjected to silica gel column chromatography using n-hexane and increasing amounts of ethyl acetate (50 ml fractions); 50 fractions were collected and examined by TLC using ethyl acetate:n-hexane (6:4). Fraction 24 afforded compound d1 (5 mg white powder). Repeated silica gel column chromatography of combined fractions 33–40 (250 mg) afforded compound d2 (70 mg colorless oil).
Biological study
Animals
Adult male albino rats of Sprague-Dawley strain (130–150 g body weight) and adult female albino rats of Sprague-Dawley strain (100 g body weight) were obtained from the animal house, National Research Center, Giza, Egypt. All the animals were kept under the same hygienic conditions of 25° ± 1°C, 12 h light-dark cycle and 55% ± 5% humidity. The animals were given water and a standard laboratory diet consisting of vitamin mixture (1%), mineral mixture (4%), corn oil (10%), sucrose (20%), cellulose (0.2%), casein 95% pure (10.5%) and starch (54.3%) ad libitum. All of the methods used in the present study were approved by the ethics committee of National Research Center.
Anti-inflammatory activity
This activity was determined according to a reported procedure (CitationWinter et al., 1962). Fifty-four male albino rats, weighing 130–150 g were divided into nine groups of six rats each. The first group received 1 mL of saline serving as control, the second group received 20 mg/ kg body weight indomethacin (Epico, Cairo, Egypt) (orally) serving as standard anti-inflammatory drug. The other groups received the test extracts of different Clerodendrum species under investigation using a dose of 100 mg/kg body weight (orally) and a dose of 25 mg/ kg body weight (orally) for the isolated verbascoside. After 1 h all animals received a subplantar injection of 0.1 mL of 1% carrageenan solution (Sigma, USA) in saline in the right hind paw, and 0.1 mL saline in the left hind paw. Four hours after drug administration the rats were sacrificed; both hind paws excised and weighed separately. The percentage of edema and inhibition was calculated.
Analgesic activity
This activity was evaluated according to a reported procedure (CitationCharlier et al., 1961). Male albino rats (54, weighing 130–150 g) were divided into nine groups of six rats each. The first group received 1 mL saline serving as control, the second group received 50 mg/kg body weight Novalgin (metamizol sodium) (Hoechst Orient, Cairo, Egypt) (orally) serving as standard analgesic drug. The other groups received extracts of different Clerodendrum species under investigation using a dose of 100 mg/ kg body weight (orally), and a dose of 25 mg/kg body weight (orally) for the isolated verbascoside. Electrical stimulation was applied to the rats’ tail by means of 515 Master Shocker (Lafayette, USA), using AC (50 Hz) for 0.2 s. The minimum voltage required for the animal to emit a cry was recorded before treatment at zero time (V0), after 1 h, and after 2 h intervals for the treated group (Vt), in order to calculate percentage change.
Antipyretic activity
Antipyretic activity was tested using the yeast-induced hyperthermia method according to a reported procedure (CitationBush & Alexander, 1960). The results were compared with that of paracetamol as standard drug. The initial vaginal temperature of female albino rats of average weight 100 g were recorded with a digital thermometer (SK-1250MC, Sato Keiryoki, Tokyo, Japan). The probe was attached to a digital display and was inserted 1 cm into the vagina. Pyrexia was induced by subcutaneous injection of 1 mg/100 g body weight of 44% (w/v) brewer’s yeast suspension in physiological saline into the animal’s dorsum region. When the temperature was at a peak, 18 h after yeast injection, only rats which developed satisfactory pyrexia (1°C or more increase in vaginal temperature) were divided into nine groups of six rats each. At this point the vaginal temperature was recorded for all groups to serve as the base line of elevated body temperature, to which the antipyretic effect will be compared. The first group received 1 mL of saline serving as control, the second group received 20 mg/kg body weight paracetamol (Misr, Cairo, Egypt) as standard antipyretic drug. The other groups received the test extracts of different species under investigation using a dose of 100 mg/kg body weight (orally), and a dose of 25 mg/kg body weight (orally) for the isolated verbascoside. Vaginal temperatures were recorded immediately before test extracts, paracetamol and saline administration and at 19 and 20 h after yeast injection, and the percentage change was calculated.
Statistics
The data are the mean of triplicate measurements. The results are expressed as mean ± SE, (n = 6). Statistical significance was determined by Student’s t-test with P <0.01 considered significant (CitationSnedecor & Cochran, 1971).
Results and discussion
Phytochemical study
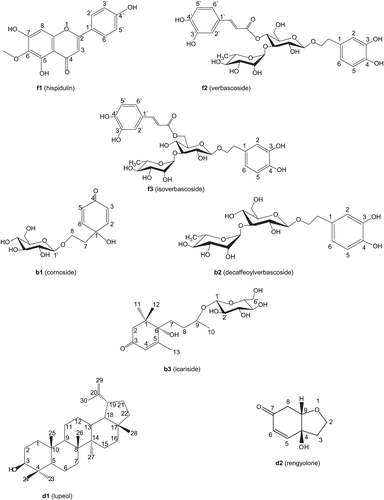
Compounds f1-f3 () were identified as verbascoside and isoverbascoside by comparing their obtained physicochemical and spectral data with published data (CitationCooper et al., 1980; CitationAndary et al., 1982; CitationMiyase et al., 1982, Citation1991; CitationCalis et al., 1984; CitationGrande et al., 1985; CitationSasaki, et al. 1989; CitationLiu et al., 1992; CitationHazekamp et al., 2001; CitationKim et al., 2001; CitationKanchanapoom et al., 2002). Compounds b1-b3 () were identified as cornoside, decaffeoylverbascoside and icariside B5 (CitationJensen et al., 1973; CitationAasen et al., 1974; CitationEndo & Hikino, 1984; CitationMiyase et al., 1988; CitationKuwajima et al., 1993; CitationHase et al., 1995; CitationKanchanapoom et al., 2002). Compounds d1 and d2 () were identified as lupeol and rengyolone (CitationEndo & Hikino, 1984; CitationNishino, et al., 1988; CitationBianco et al., 1993; CitationHase et al., 1995; CitationTian et al., 1997; CitationGauthier et al., 2006; CitationAhiahonu & Goodenowe, 2007; CitationSiddiqui et al., 2007). Except for cornoside and rengyolone, all other compounds were isolated from C. chinense for the first time.
Biological study
Anti-inflammatory activity
It is well known that the carrageenan paw edema test produces an acute inflammation that results from the sequential action of several mediators through two phases (CitationVinegar et al., 1961). The early phase (1.5 h) is attributed to the release of histamine and serotonin (CitationCrunkhon & Meacock, 1961), while the delayed phase (4 h) of inflammatory response has been linked to release of cyclooxygenase and prostaglandins (CitationPanthong et al., 2004). Since the second phase (4 h) of inflammatory edema induced by carrageenan is sensitive to most clinically effective anti-inflammatory drugs, this assay is useful for studying the anti-edematous effect of natural products (CitationCalixto et al., 2004).
Results () revealed that the rat groups that received the methanol extract of the leaves of C. chinense and C. indicum at an oral dose of 100 mg/g body weight and verbascoside at an oral dose of 25 mg/kg body weight showed highly significant anti-inflammatory activity (about 77–89% of the activity of indomethacin after 4 h). The rat groups that received the chloroform extract of the leaves of C. chinense and the methanol extract of leaves of C. glabrum at an oral dose of 100 mg/kg body weight showed significant anti-inflammatory activity (about 56–63% of the activity of indomethacin after 4 h). Chloroform extracts of the leaves of both C. indicum and C. glabrum showed less anti-inflammatory activity at an oral dose of 100 mg/kg body weight.
Table 1. Anti-inflammatory activity of different extracts of different Clerodendrum species and verbascoside in male albino rats
Our results demonstrated that methanol extracts of C. chinense and C. indicum have an acute anti-inflammatory effect. The effects of these extracts may correlate to the inhibition of the enzyme cyclooxygenase, leading to the inhibition of prostaglandin synthesis. Verbascoside, present in C. chinense and C. indicum, may be responsible for this activity.
Analgesic activity
A significant increase in voltage required for the animal to emit a cry indicated the potent analgesic activity of the methanol extract of leaves of C. chinense and purified verbascoside.
Results () revealed that the rat groups that received the methanol extract of the leaves of C. chinense at an oral dose of 100 mg/kg body weight and verbascoside at an oral dose of 25 mg/kg body weight showed highly significant analgesic activity (about 74–89% of the effect of Novalgin after 1 h and about 61–73% of the Novalgin effect after 2 h). Other groups showed less analgesic activity at an oral dose of 100 mg/kg body weight.
Table 2. Analgesic activity of different extracts of different Clerodendrum species and verbascoside in male albino rats.
Antipyretic activity
Results () revealed that the rat groups that received methanol extract of the leaves of C. chinense at an oral dose of 100 mg/kg body weight and verbascoside at an oral dose of 25 mg/kg body weight showed highly significant antipyretic activity (about 58-73% of the activity of paracetamol after 1 h and about 67-81% of paracetamol activity after 2 h). The rat group that received methanol extract of the leaves of C. glabrum at an oral dose of 100 mg/kg body weight showed significant antipyretic activity (about 57% of the activity of paracetamol after 1 h and about 55 % of paracetamol activity after 2 h).
Table 3. Antipyrectic activity of different extracts of different Clerodendrum species and verbascoside in female albino rats.
The rat group that received methanol extract of the leaves of C. indicum at an oral dose of 100 mg/kg body weight showed less antipyretic activity. The rat groups that received chloroform extracts of the leaves of C. indicum or C. glabrum showed no significant antipyretic activity at an oral dose of 100 mg/kg body weight.
The present study demonstrated that C. chinense has significant analgesic, anti-inflammatory and antipyretic properties, justifying the traditional use of this plant in the treatment of inflammation.
Acknowledgment
Haytham M. Wahba would like to express his gratitude to Sheila M. Maregesi, Department of Pharmacognosy, School of Pharmacy, Muhimbili University, College of Health Sciences, Dar El Salaam, Tanzania for her kind and unlimited help.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aasen AJ, Hlubucek JR, Enzell CR. (1974). Tobacco chemistry. 24(9R)-9-Hydroxy-4-megastigmen-8-one, a new tobacco constituent. Acta Chem Scand B, 28, 285–288.
- Ahiahonu PW, Goodenowe DB. (2007). Triterpenoids from leaves of Elaeophorbia drupifera. Fitoterapia, 78, 337–341.
- Andary C, Wylde R, Laffite C, Privat G, Winternitz F. (1982). Structures of verbascoside and orobanchoside caffeic acid sugar esters from Orobanche rapum-genistae. Phytochemistry, 21, 1123–1127.
- Bianco A, Scalzo RL, Scarpati ML. (1993). Isolation of cornoside from Olea europaea and its transformation into halleridone. Phytochemistry, 32, 455–457.
- Bush JE, Alexander RW. (1960). An improved method for the assay of anti-inflammatory substances in rats. Acta Endocrinol, 35, 268–276.
- Calis I, Lahloub MF, Rogenmoser E, Sticher O. (1984). Isomartynoside a phenylpropanoid glycoside from Galeopsis pubescens. Phytochemistry, 23, 2313–2315.
- Calixto JB, Campos MM, Otuki MF, Santos AR. (2004). Anti-inflammatory compounds of plant origin. Part II. Modulation of pro inflammatory cytokines, chemokines and adhesion molecules. Planta Med, 70, 93–103.
- Charlier R, Prost H, Binon F, Dellous G. (1961). Pharmacology of an antitussive, 1-phenethyl-4(2-propenyl)-4-propinoxypipridine acid fumarate. Arch Intern Pharmacodynam, 134, 306–327.
- Cooper R, Solomon PH, Kubo I, Nakanishi K, Shoolery JN, Occolowitz JN. (1980). Myricoside, an African armyworm antifeedant: Separation by droplet countercurrent chromatography. J Am Chem Soc, 102, 7953–7959.
- Crunkhon P, Meacock SER. (1961). Mediators of the inflammation induced in the rat paw by carrageenan. Brit J Pharmacol, 42, 392–402.
- Endo K, Hikino H. (1984). Structures of rengyol, rengyoxide, and rengyolone, new cyclohexylethane derivatives from Forsythia suspense fruits. Can J Chem, 62, 2011.
- Gauthier C, Legault J, Lebrun M, Dufour P, Pichette A. (2006). Glycosidation of lupane-type triterpenoids as potent in vitro cytotoxic agents. Bioorg Med Chem, 14, 6713–6725.
- Grande M, Piera F, Cuenca A, Torres P, Bellido IS. (1985). Flavonoids from Inula viscose. Planta Med, 5, 414–419.
- Hase T, Kawamoto Y, Ohtani K, Kasai R, Yamasaki K, Picheansoonthon C. (1995). Cyclohexyl ethanoids and related glucosides from Millingtonia hortensis. Phytochemistry, 39, 235–241.
- Hazekamp A, Verpoorte R, Panthong A. (2001). Isolation of a bronchodilator flavonoid from the Thai medicinal plant Clerodendrum petasites. J Ethnopharmacol, 78, 45–49.
- Jensen SR, Kujaer A, Nielsen BJ. (1973). A quinol glucoside isolated from Cornus species. Acta Chem Scand, 27, 367–369.
- Kanchanapoom T, Chumsri P, Kasai R, Otsuka H, Yamasaki K. (2005). A new iridoid diglycoside from Clerodendrum chinense. J. Asian Nat Prod Res, 7, 269–272.
- Kanchanapoom T, Kasai R, Yamasaki K. (2002). Phenolic glycosides from Markhamia stipulate. Phytochemistry, 59, 557–563.
- Kim HJ, Woo E, Shin C, Hwang DJ, Park H, lee YS. (2001). HIV-1 integrase inhibitory phenylpropanoid glycosides from Clerodendrum trichotomum. Arch Pharmacol Res, 24, 286–291.
- Kuwajima H, Takahashi M, Ito M, Wu H, Takaishi K, Inoue K. (1993). A quinol glucoside from Abeliophyllum distichum. Phytochemistry, 33, 137–139.
- Liu Y, Ho DK, Cassady JM. (1992). Isolation of potential cancer chemopreventive agents from Eriodictyon californicum. J Nat Prod, 55, 357–363.
- Mabry JT, Markham KR, Thomas MB. (1970). The Systematic Identification of Flavonoids. New York: Springer.
- Miyase T, Koizumi A, Ueno A, Noro T, Kuroyanagi M, Fukushima S, Akiyama Y, Takemoto T. (1982). Studies on the acyl glycosides from Leucoseptrum japonicum. Chem Pharm Bull, 30, 2732–2737.
- Miyase T, Ueno A, Takizawa N, Kobayashi H, Oguchi H. (1988). Studies on the glycosides of Epimedium grandiflorum. Chem Pharm Bull, 36, 2475–2484.
- Miyase T, Ishino M, Akahori C, Ueno A, Ohkawa Y, Tanizawa H. (1991). Phenylethanoid glycosides from Plantago asiatica. Phytochemistry, 30, 2015–2018.
- Nishino C, Kobayashi K, Fukushima M. (1988). Halleridone, a cytotoxic constituent from Cornus controversa. J Nat Prod, 51, 1281–1282.
- Panthong A, Kanjanapothi D, Taesotikul T, Phankummoon A, Panthong K, Reutrakul V. (2004). Anti-inflammatory activity of methanolic extracts from Ventilago harmandiana Pierre. J Ethnopharmacol, 91, 237–242.
- Rajasekaran A, Ponnusamy K. (2006). Antifungal activity of Clerodendrum inerme and Clerodendrum phlomidis. Turk J Biol, 30, 139–142.
- Sasaki H, Nishimura H, Chin M, Mitsuhashi H. (1989). Hydroxycinnamic acid esters of phenethyl alcohol glycosides from Rehmannia glutinosa var. purpurea. Phytochemistry, 28, 875–879.
- Siddiqui BS, Ahmad F, Sattar FA, Begum S. (2007). Chemical constituents from the aerial parts of Lippa nodiflora. Arch Pharmacol Res, 30, 1507–1510.
- Snedecor WG, Cochran GW. (1971). Statistical Methods, tenth edition. New York: Iowa State University.
- Stahl E. (1969). Thin Layer Chromatography, second edition. Berlin, Heidelberg, New York: Springer, 366–367.
- Taylor RSL, Edel F, Manandhar NP, Towers GHN. (1996). Antimicrobial activities of southern Nepalese medicinal plants. J Ethnopharmacol, 50, 97–102.
- Tian J, Zhao Q, Zhang H, Lin Z, Sun H. (1997). New cleroindicins from Clerodendrum indicum. J Nat Prod, 60, 766–769.
- Vinegar R, Schreiber W, Hugo R. (1961). Biphasic development of carrageenan edema in rats. J Pharmacol Exp Ther, 166, 96–103.
- Winter GA, Risley EA, Nuss GW. (1962). Carrageenan induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med, 11, 544–547.