Abstract
Context: The production of fish is limited by infectious diseases; when a fish grows in an immunosuppressed condition, it becomes highly susceptible to disease.
Objective: The present research work investigates immunomodulatory action of Aegle marmelos (Linn.) Corr. Serr. (Rutaceae) in Catla catla Hamilton (Cyprinidae) for enhancing immune protection of the fish against bacterial infections.
Material and methods: Doses of 0, 5, 10, 15, 20, 25 and 30 g aqueous plant leaf extract/kg feed were administered orally to the freshwater fish, Catla catla for a period of 30 days to investigate its efficiency to enhance the non-specific immune responses against the fish pathogen Pseudomonas aeruginosa (Schröter) Migula (Pseudomonadaceae).
Results and discussion: The fish were challenged with pathogens through water medium for 30 days and the immunomodulatory effect of the Aegle marmelos was evaluated on the blood samples every 5 days untill 15 days after infection. The results obtained from the study shows that the 25 g leaf extract/kg of feed was found to be competent to enhance optimum immune response. The effectiveness of the immunostimulant action was found to be best for the first 5 days after challenging with pathogen and subsequently, the immune response was found to decline in all the concentrations of plant extract.
Conclusion: The results of the study will be helpful for further investigation in the field to improve the immunocompetence of fish against bacterial pathogens.
Introduction
Aquaculture gains momentum at the advent of modern technologies in farming of aquatic organisms. Roughly 40% of all fish directly consumed by humans worldwide are now farmed (CitationWhyte, 2007). The accelerated spread of disease, especially infectious diseases, in waters, is a major factor limiting the production of fish, causing heavy loss to fish farmers. Bacterial fish diseases have been studied extensively (CitationLogambal et al., 2000), and to nurture healthy fish it requires potent defense mechanisms against pathogen invasion. Like humans, fish rely on both specific and non-specific mechanisms to protect themselves against invading pathogens. Phagocytosis, a non-specific defense mechanism, is the most common of the cellular defense reactions. This non-specific immune system in fish can be enhanced through feed additives (CitationSiwicki et al., 1994).
The literature shows that the immunostimulants which have been effective in enhancing immune responses in salmonids, carp Cyprinus carpio Linn. (Cyprinidae), channel catfish Ictalurus punctatus Rafinesque (Ictaluridae), and giant freshwater prawn Macrobrachium rosenbergii De Man (Palaemonidae) include levamisole (CitationSiwicki et al., 1990), glucans (CitationYano et al., 1989; CitationRobertsen et al., 1990; CitationNikl et al., 1991; CitationMishra et al., 2004) and chitin (CitationSakai et al., 1992; CitationAnderson & Siwicki, 1994). Traditional Chinese Medicine (TCM) [Astragalus radix Linn. (Fabaceae) and Ganoderma lucidum (Curtis) P. Kars (Ganodermataceae)] has also received attention with regard to the immune stimulating function in aquaculture recently (CitationYoshida et al., 1993; CitationSiwicki et al., 1994; CitationZhang et al., 2009). On perusal of the literature it was evident that many exogenous factors (dietary components, stress, pathogens, xenobiotics, etc.) enhance or modulate the fish phagocytic activity (CitationSecomber, 1994).
In conjunction with the ongoing research in our laboratory, the present research work investigates the immunostimulant action of Aegle marmelos (Linn.) Corr. Serr. (Rutaceae) in Catla catla Hamilton (Cyprinidae), for enhancing immune protection of the fish against bacterial infections (Pseudomonas aeruginosa ). The Catla catla is one of the Indian major commercial carp other than Labeo rohita (CitationRao & Chakrabarti, 2005). The Aegle marmelos leaves, roots, bark, seeds and fruits are edible and have medicinal value. The medicinal properties of this plant have been described in the Ayurveda (CitationSinganan et al. 2007). Hence, this plant was considered for the present study. Pseudomonas aeruginosa is a bacterial pathogen and like Aeromonas hydrophila Chester, it also causes septicemia in fish resulting in petechial hemorrhage on fins and tail and ulceration of the skin.
Earlier studies on this fish were carried out with different plant extracts and infected with Aeromonas species (CitationGuojun et al., 2009; CitationXue et al., 2008; CitationArdo et al., 2008; CitationXie et al., 2008; CitationYin et al., 2009). So in this present study, the extract of Aegle marmelos was administered to monitor the phagocytic activity on the fish infected with Pseudomonas aeruginosa.
Material and methods
The freshwater fish, Catla catla (16 ± 5 g), were procured from State Fisheries fish seed farm, Kadana, Tamil Nadu, India, and was authenticated by aquaculturists in the university. The oxygen content of the ambient fresh water (30 ± 1°C) was kept above 50% air saturation throughout the period of acclimation and during experiments. The experimental fish were stocked in six troughs with ten fish each in triplicate (including control). Fish were fed daily with normal balanced fish feed during the period of acclimation. The basal diet was nutritionally balanced with 40% protein and also formulated with the powdered form of the ingredients as given in and . The ingredients were made into dough, steamed and mixed with vitamins and minerals (the extract was added in the medicated feed). Then the feed mix was pelletized and dried to reduce the moisture content to 10%.
Table 1. Basal feed ingredients and their protein content.
Table 2. Composition of vitamins in the Beplex mix.
A virulent strain of Pseudomonas aeruginosa was isolated from diseased fish with symptoms of hemorrhagic septicemia. After isolation of the strain, it was identified using standard microbial identification tests and the culture was maintained on tryptose soya agar slopes at 4°C for long-term preservation, which was used for infecting the healthy fish.
Preparation of leaf extract
Aegle marmelos leaves were collected from Sri Paramakalyani temple, Alwarkurichi, Tamil Nadu, India. The plant material of Aegle marmelos used in this study was authenticated by the professors in the Botany Department, MS University, Tirunelveli, Tamil Nadu, India (file/fish/2009/5). The plant leaves were washed thoroughly with distilled water to remove the adhered soils. The water extract of the fresh Aegle marmelos leaves was prepared by crushing and soaking the leaves in distilled water for 48 h. Then the extract was centrifuged at 100 rpm for 5 min and filtered to get an extract free of debris. The water in the extract solution was removed under reduced pressure, which yielded the crude extract which was used for further study.
Determination of antimicrobial activity (minimum inhibitory concentration)
The isolated bacterial culture was tested for its sensitivity to crude leaf aqueous extract of Aegle marmelos by disc diffusion test (agar medium). The minimum inhibitory concentration (MIC) of the Aegle marmelos against the Pseudomonas aeruginosa was determined by microdilution techniques on agar plates (CitationBergan et al., 1997; CitationNCCLS, 2008). The concentration of the extract was prepared using 0 (control), 50, 100, 150, 200, and 250 µg/mL concentrations. A stock solution of 10 µg/mL reference standard of cephalosporin was prepared with the help of sterile distilled water. The pH of the media was adjusted to 7.2–7.4 and the Petri plates were refrigerated overnight (the area of the extract placed was marked). The average size of the inoculum was about 105 cells contained in a 2 mm diameter standard loop. When the nutrient agar plates containing the extract and also the control plates having equal volumes of solvent were made ready, the broth culture of test organism grown overnight was spot inoculated by checkerboard technique on the marked area of the plates. These were then incubated for 72 h at 37°C. No growth of the organism on the test plate along with growth on the control plate was taken as an indication of antimicrobial activity of the extract. Minimum inhibitory concentration (MIC) was indicated by the lowest concentration of the extract, which inhibited the bacterial growth (CitationIslam et al., 2008).
Experimental design
The antibacterial (Pseudomonas aeruginosa) sensitivity test was carried out by disc diffusion test (agar medium) using 100, 200, 300, 400 and 500 µg/mL concentrations of the crude extract of Aegle marmelos leaves. In consequence of this, Aegle marmelos incorporated artificial feed at different concentrations: 0, 5, 10, 15, 20, 25 and 30 g plant leaf extract/kg feed was given as an immunostimulant to the freshwater fish, Catla catla for a period of 30 days to check its efficacy in enhancing the non-specific immune response. On day 30 of immunomodulation, the experimental fish were subjected to infection with the fish pathogen, P. aeruginosa, with a cell density of about 19.5 × 104 cells/mL through water medium and the pathogen level was also maintained in the water for 3 days.
Collection of blood and antiserum
After day 5 of infection, blood samples were drawn and consequently every 5 days untill day 15. The fish were bled serially using a tuberculin syringe with 24 gauge needle from the caudal vein and the blood was collected in EDTA-rinsed small serological tubes. The blood (without anticoagulant) collected from fish was kept overnight at 4°C for serum separation. The serum was separated by spinning down at 3000 rpm for 15-20 min in a centrifuge. The supernatant was collected and stored in sterile vials. The serum was kept at 57°C in a water bath for 30 min to inactivate the complement system and was stored at −20°C for further analysis.
Assay of phagocytic activity
The phagocytic activity assay was performed by the following modified method of CitationSahoo and Mukherjee (2002). The collected blood (100 µL) was mixed with an equal quantity of bacterial suspension (1:1) in Eppendorf tubes. The density of the bacterial culture was maintained throughout the experiment at 104 cells/mL in PBS. The mixture was incubated for 20 min at room temperature. After incubation, a thin smear was prepared and fixed with absolute alcohol for 5 min. The smear was later stained with Giemsa stain for 5 min and the phagocytic cells that had engulfed the bacteria were counted (under a microscope) as positive (CitationSeeley et al., 1990).
The percentage of bacteria ingested by phagocytes (phagocytic ratio) was calculated by Equation 1.
Statistical analysis
The data collected were statistically analyzed using two-way analysis of variance (ANOVA) to test the effects of experimental diets for all parameters. Student’s t-test was used to test differences among individual means and the control. The difference was recorded as significant when P <0.01 and P <0.05. This statistical work was performed manually with the help of Microsoft Excel software.
Results and discussion
The immunomodulatory effect of the aqueous leaf extract of A. marmelos on P. aeruginosa infected C. catla by improving the phagocytic activity was found by feeding different concentrations of the extract to the fish.
Initially, the sensitivity of the Aegle marmelos leaf extracts against the pathogen Pseudomonas aeruginosa was performed by the agar plate diffusion method. The result obtained from the study describes that among the various concentrations tested, the extracts have sensitivity at 150 µg/mL concentration (minimum inhibitory concentration) against the bacterial pathogen P. aeruginosa. Earlier literature has also shown that the A. marmelos extracts possess antimicrobial activity against bacterial species (CitationMadasamy, 2003).
The results (percentage phagocytic ratio) obtained from the study are provided in . The results reveal that the aqueous extract of A. marmelos enhances the percentage of phagocytic ratio in experimental fish compared to control fish. Among the experimental groups, the highest immunostimulation (phagocytic ratio) was observed in fish fed with 25 g of plant extract/kg of feed incorporated diet. However, other concentrations were also able to stimulate the phagocytic activity at a lower rate.
Table 3. Efficiency of the plant extract, Aegle marmelos, on phagocytic ratio (%) of the freshwater fish Catla catla.
It is evident from that the immunostimulant action of A. marmelos was found to be at its best for the first 5 days. Similarly, CitationLogambal et al. (2000) observed that 20 mg of Ocimum sanctum Linn. (Lamiaceae) extract enhanced the neutrophil activity maximally for 6 days. CitationMulero et al. (1998) observed that the dietary intake of levamisole enhances the phagocytic ratio and the peak response was noted in week 5 of treatment. CitationOrtuno et al. (1999) observed that phagocytic activity was at its peak after 2 weeks of administration of vitamin C.
Figure 1. Efficiency of plant extract, Aegle marmelos, on phagocytic ratio of the freshwater fish Catla catla.
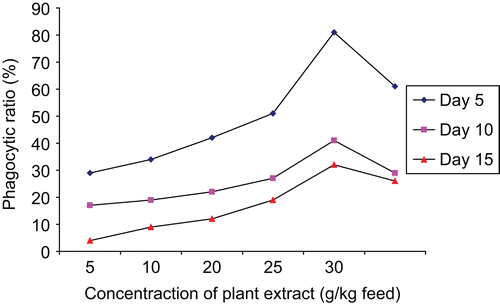
It is interesting to note from that the phagocytic ratio or phagotic activity decreased on days 10 and 15 of analysis. CitationSakai (1999) explained in his review that the immunostimulants increased resistance to infectious disease, not by enhancing specific immune responses, but by enhancing non-specific defense mechanisms. Therefore, there is no memory component and the response is likely to be of short duration, as observed in the present study.
From it is also apparent that the concentration of 25 g plant extract/kg of feed was the optimum, and overdose or high concentration (50 g plant extract/kg of feed) induced immunosuppression to the fish, as observed by CitationSakai (1999). The fish fed with leaf extract of A. marmelos were significantly enhanced (P <0.05) in the phagocytic ratio when compared with the control fish and the ratios were maximally significant (P <0.05) on day 5 of infection. The results also reveal that macrophage migration in the presence of exoantigen was enhanced with the incorporation of different levels of leaf extract of Aegle marmelos in fish feed. The increase in phagocytic ratio indicates that the leaf extract stimulates the synthesis of chemotactic factors as observed in the study of CitationFujiki K, Yano, T. (1997).
In conclusion, these findings suggest that plant extract Aegle marmelos can be incorporated in the diet in order to increase immune function and protection against infectious disease in the freshwater fish Catla catla. The optimum dose of dietary plant extract required to increase the immune response in Catla catla was found to be 25 g of plant extract/kg of feed administered for 30 days at a feeding rate of 5% body weight. The study also indicates that lower or higher plant extract concentrations may not be as effective at eliciting complete protection against bacterial infection.
In fish farms and hatcheries containing millions of young fish, individual injections are very expensive; immersion, bathing and shower techniques are also costly and involve handling stress. Incorporating an immunostimulant into the feed may be the best way of delivering it to fish (CitationSiwicki et al., 1994). Further studies are needed to understand the exact mechanism of action of the immunostimulant, which would help to identify a variety of cheap and efficient immunostimulants and this in turn could be applied in aqua farming to control infectious bacterial diseases.
Declaration of interest
The authors report no conflicts of interests. The authors alone are responsible for the content and writing of the paper.
References
- Anderson DP, Siwicki AK. (1994). Duration of protection against Aeromonas salmonicida in brook trout immunostimulated with glucan or chitosan for injection or immersion. Prog Fish Cult, 56, 258–261.
- Ardo L, Yin G, Xu P, Varadi L, Szigeti G, Jeney Z, Galina J. (2008). Chinese herbs (Astragalus membranaceus and Lonicera japonica) and boron enhance the non-specific immune response of Nile tilapia (Oreochromis niloticus) and resistance against Aeromonas hydrophila. Aquaculture, 275, 26–33.
- Bergan T, Bruun JN, Digranes A, Lingaas E, Melby KK, Sander J. (1997). Susceptibility testing of bacteria and fungi. Report from the Norwegian Working Group on Antibiotics. Scand J Infect Dis Suppl, 103, 1–36.
- Fujiki K, Tomoki Y. (1997). Effects of sodium alginate on the non-specific defence system of the common carp (Cyprinus carpio L.). Fish Shellfish Immunol, 7, 417–427.
- Guojun Y, Ardo L, Thompson KD, Adams A, Jeney Z, Jeney G. (2009). Chinese herbs (Astragalus radix and Ganoderma lucidum) enhance immune response of carp, Cyprinus carpio and protection against Aeromonas hydrophila. Fish Shellfish Immunol, 26, 140–145.
- Islam MA, Alam MM, Choudhary ME, Kobayashi N, Ahmed MU. (2008). Determination of minimum inhibitory concentration (MIC) of cloxacilin for selected isolates of methicilin-resistant Staphylococcus aureus (MRSA) with their antibiogram. Bangl J Vet Med, 6, 121–126.
- Logambal SM, Venkatalakshmi S, Dinakaran MR. (2000). Immuno stimulatory effect of leaf extract of Ocimum sanctum Linn. in: Oreochromis mossambicus (Peters). Hydrobiologia, 430, 113–120.
- Madasamy D. (2003). Influence of plant extract Aegle marmelos on growth and immune responses in freshwater fish, Catla catla infected with bacterial pathogen, Pseudomonas aeruginosa. M.Sc. thesis, SPKCES, M.S. University, Tirunelveli, India.
- Mishra CK, Das BK, Pradhan J, Pattanaik P, Sethi S, Mukherjee SC. (2004). Changes in lysosomal enzyme activity and protection against Vibrio infection in Macrobrachium rosenbergii (De Man) post larvae after bath immunostimulation with β-glucan. Fish Shellfish Immunol, 17, 389–395.
- Mulero V, Esteban MA, Munoz J, Meseguer J. (1998). Dietary intake of levamisole enhances the immune response and disease resistance of the marine teleost gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol, 8, 49–62.
- NCCLS. (2008). Performance standards for antimicrobial susceptibility testing. Ninth informational supplement NCCLS.document M100-S9. New York: Informa Healthcare.
- Nikl LLJ, Albright Evelyn, TPT. (1991). Influence of seven immunostimulants on the immune response of coho salmon to Aeromonas salmonicida. Dis Aquat Org, 12, 7–12.
- Ortuno J, Esteban MA, Mesguer J. (1999). Effect of high dietary intake of vitamin C on non-specific immune response of gilt head seabream (Sparus aurata L.). Fish Shellfish Immunol, 9, 429–443.
- Rao VY, Chakrabarti R. (2005). Stimulation of immunity in Indian major carp Catla catla with herbal feed ingredients. Fish Shellfish Immunol, 18, 327–334.
- Robertsen B, Rorstad G, Engstad R, Ras J. (1990). Enhancement of nonspecific disease resistance in Atlantic salmon, Salmo salar L., by a glucan from Saccharomyces cerevisiae cell walls. J Fish Dis, 13, 391–400.
- Sahoo PK, Mukherjee SC. (2002). Influence of high dietary α-tocopherol intakes on specific immune responses, non-specific resistance factors and disease resistance of healthy and aflatoxin B1-induced immunocompromised Indian major carp Labeo rohita (Hamilton). Aquaculture Nutrition, 8, 159–167.
- Sakai M, Kamiya H, Ishii S, Atsuta S, Koybayashi M. (1992). The immunostimulating effects of chitin in rainbow trout. Oncorhynchus mykiss. In: Shariff M, Subasinghe RP, Arthur JR, eds. Diseases in Asian Aquaculture. Proceedings of the first symposium on diseases in Asian Aquaculture. Manila: Asian Fisheries Society, 413–417.
- Sakai M. (1999). Current research status of fish immunostimulants. Aquaculture, 72, 63–92.
- Secomber CJ. (1994). Enhancement of fish phagocyte activity. Fish Shellfish Immunol, 4, 421–436.
- Seeley KR, Gillespie PD, Weeks BA. (1990). A simple technique for the rapid spectrophotometric determination of phagocytosis by fish macrophages. Mar Environ Res, 30, 123–128.
- Singanan V, Singanan M, Begum H. (2007). The hepatoprotective effect of bael leaves (Aegle marmelos) in alcohol induced liver injury in albino rats. Int J Sci Technol, 2, 83–92.
- Siwicki AK, Anderson DP, Dixon OW. (1990). In vitro immunostimulation of rainbow trout (Oncorhynchus mykiss) spleen cells with levamisole. Dev Comp Immunol, 14, 231–237.
- Siwicki AK, Anderson DP, Rumsey GL. (1994). Dietary intake of immunostimulants by rainbow trout affects nonspecific immunity and protection against furunculosis. Vet Immunol Immunopathol, 41, 125–139.
- Whyte SK. (2007). The innate immune response of finfish: A review of current knowledge. Fish Shellfish Immunol, 23, 1127–1151.
- Xie J, Liu B, Zhou Q, Su Y, He Y, Pan L, Ge X, Xu P. (2008). Effects of anthraquinone extract from rhubarb Rheum officinale Bail. on the crowding stress response and growth of common carp Cyprinus carpio var. jian. Aquaculture, 281, 5–11.
- Xue J, Xu Y, Jin L, Liu G, Sun Y, Li S, Zhang J. (2008). Effects of traditional Chinese medicine on immune responses in abalone, Haliotis discus hannai Ino. Fish Shellfish Immunol, 24, 752–758.
- Yano T, Mangidaar REP, Matsuyama H. (1989). Enhancement of the resistance of carp Cyprinus carpio to experimental Edwardsiella tarda infection, by some β-1.3-glucans. Nippon Suisan Gakkaishi, 55, 1815–1819.
- Yin G, Ardo L, Thompson KD, Adams A, Jeney Z, Jeney G. (2009). Chinese herbs (Astragalus radix and Ganoderma lucidum) enhance immune response of carp, Cyprinus carpio, and protection against Aeromonas hydrophila. Fish Shellfish Immunol, 26(1), 140–145.
- Yoshida T, Sakai M, Kitao T, Khlil SM, Araki S, Saitoh R, Ineno T, Inglis V. (1993). Immunomodulatory effects of the fermented products of chicken egg, EF203, on rainbow trout, Oncorhynchus mykiss. Aquaculture, 109, 207–214.
- Zhang G, Gong S, Yu D, Yuan H. (2009). Propolis and Herba epimedii extracts enhance the non-specific immune response and disease resistance of Chinese sucker, Myxocyprinus asiaticus. Fish Shellfish Immunol, 26, 467–472.