Abstract
Context: This study evaluated the in vitro and in vivo antidiarrheal activity, oral acute toxicological profile, and developed a chemical fingerprint of Berberis aristata Linn. (Berberidaceae).
Materials and methods: The ethanol (by maceration) and aqueous (by Soxhlet) extracts of Berberis aristata bark were used for the study. The study involved the antimicrobial (minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) by micro dilution) and antidiarrheal (castor oil induced diarrhea, charcoal motility) tests. The active principle, berberine was characterized by different spectroscopic and chromatographic techniques.
Results: The MIC and MBC (of both extracts) against all strains of Shigella were recorded between 125 to 500 µg/mL and 300 to 600 µg/mL, respectively. The MIC and MBC values of berberine are almost comparable to standard ciprofloxacin. UV and IR spectroscopy along with HPTLC and HPLC studies showed presence of berberine in the extracts. The onset of castor oil induced diarrhea was delayed and number of diarrheal episodes was reduced by both the extracts in a dose dependent manner. Similarly, the length of intestine traveled by the feed was also significantly reduced in the charcoal motility test by both the extracts. LD50 of >5000 mg/kg body weight was observed for both extracts in the acute oral toxicity studies with Swiss albino mice.
Conclusion: The results validate in vivo and in vitro antidiarrheal activity of Berberis aristata extracts and provide its chemical fingerprint.
Introduction
Gastrointestinal infections (GI) encompass a wide variety of symptom complexes and recognized infectious agents. Among the GI infections, diarrhea is a common symptom of intestinal disorder and has remained a global threat to human health. Diarrhea is one of the major health threats to populations in tropical and subtropical countries, responsible for about 5 million deaths annually, of which 2.5 million are children of less than 5 years (CitationHeinrich et al., 2005). Drug associated diarrheal diseases are mainly caused by a disruption of normal micro flora of the gut by broad spectrum antibiotics. Dysentery may be defined as diarrhea containing blood. Although several organisms cause dysentery, the Shigella species is the most important. Shigella dysenteriae type 1 is the only cause of epidemic dysentery (CitationWHO, 1979).
Medicinal plants used in the traditional Indian system of medicine are known to produce a variety of compounds with acknowledged therapeutic properties (CitationGerald et al., 2007). The use of medicinal herbs for curing diarrheal infections has been documented in all civilizations (CitationGerald et al., 2007). Increased side effects, high cost of new drugs, increasing microbial resistance and emerging new intestinal pathogens have led to renewed interest and focus in medinial plant research.
Berberis aristata Linn. (Berberidaceae), commonly known as tree turmeric or Indian berberry, occurs in the Himalayas. It is an erect, glabrous, spiny shrub with yellow flowers and oblong, ovoid, bright red berries and is known to contain the isoquinoline alkaloid berberine (CitationCSIR, 1985; CitationMukherji et al., 2006). Berberine has a long history of medicinal use in both Indian and Chinese systems of medicine. Pharmacological studies have reported alkaloid berberine to be effective in reducing mortality rate, volume and duration of diarrhea (CitationSabir et al., 1978). The extract of stem bark of Berberis aristata is reported to be useful in curing bacillary dysentery and diarrhea (CitationAyurvedic Pharmacopeia, 2001). Evidence indicates that it also has multiple pharmacological effects such as immunomodulatory, anti-inflammatory (CitationGacche & Dhole, 2006), antidysenteric (CitationMukherji, 2002), etc. In view of the above observations, the present study was undertaken to evaluate the antidiarrheal activity of bark extracts of Berberis aristata along with their chemical characterization and toxicity profile.
Methodology
Plant material and preparation of extracts
The dried stem bark of the plant was procured from a vendor in Ahmedabad, Gujarat, India. The bark samples were authenticated by S.J. Surana, Head of the Department of Pharmacognosy, R.C. Patel Institute of Pharmaceutical Education and Research, Shirpur, India. A voucher specimen (No. 16.7) of the bark is deposited in the department for future reference. Coarsely ground powder of the shade-dried plant bark pieces (100 g) were extracted using water and ethanol as solvents. Aqueous extraction was carried out by Soxhlet method and alcoholic extraction was carried out by cold maceration. The extracts obtained were filtered and concentrated under vacuum using a rotary vacuum evaporator (Buchi, Flawil, Switzerland) and stored in desiccators at 4°C until use. The marker standard berberine was a kind gift from Anchrom India, Mumbai.
Preliminary phytochemical analyses
Phytochemical analyses of the plant extracts for the major phytoconstituents were undertaken using standard qualitative methods as described in the literature (CitationHarborne, 1973; CitationEdeoga et al., 2005). The plant extracts were screened for the presence of biologically active compounds such as glycosides, phenolics, alkaloids, tannins, flavonoids, saponins and steroids.
Chromatographic and spectrophotometric analyses
Thin layer chromatography (TLC) and high performance thin layer chromatography (HPTLC) densitometric studies (CitationIHP, 1999; CitationMukherji, 2002) were performed with standard silica gel 60 F254 plates (Merck, Darmstadt, Germany). The sample was applied using a thin capillary for TLC whereas an automatic sample applicator Linomat 5 (CAMAG, Muttenz, Switzerland) was used for the band application in HPTLC. The samples (ethanol extract/aqueous extract) were prepared by refluxing 10 mg with 10 mL of chloroform for 10 min. The filtrate was evaporated to dryness and the residue dissolved in the 10 mL chloroform. A solvent system comprising propanol:formic acid:water (9.0:0.1:0.9, v/v) was found to give excellent resolution in our studies. The plates were developed in twin trough chamber (CAMAG) under saturating conditions. After drying, the plates were scanned densitometrically in a TLC scanner 3 (CAMAG) at 366 nm. Berberine was used as a marker standard. HPLC studies were pursued using a Proteo Jupiter reversed phase column (Shimadzu, Model LC20 AT, Kyoto, Japan) with acetonitrile:water (10:90, v/v) as mobile phase and the absorbance at 266 nm was recorded using a PDA detector (Shimadzu).
The UV visible and Fourier Transform-Infra Red (FTIR) spectra of the isolated compounds were recorded on an UV visible spectrophotometer (Shimadzu Model 2450) and IR spectrophotometer (Shimadzu model-8400S), respectively. The TLC purified fractions were dissolved in methanol for UV visible spectrophotometry (CitationJoshi et al., 1989) and a KBr pallete was used for FTIR scanning.
Antimicrobial activity
The antimicrobial activity was evaluated on the following intestinal pathogens. Shigella flexneri – MTCC 1457, Shigella sonnei – MTCC 2957, Shigella dysenteriae – LMP 0208U and Shigella boydii – ATCC 8700. The agar well diffusion method (Perez et al., 1990) was used to determine the antimicrobial activity. Diluted inoculum (0.1 mL) of test organism (optical density (OD) 0.22 at 600 nm, approximately 105 CFU/mL, prepared in isotonic saline) was spread on Muller-Hinton agar plates. Wells of 8 mm diameter were punched in to the agar medium with a sterile cork borer under aseptic conditions and filled with 100 µL of plant extracts, solvent blank, standard antibiotics; ciprofloxacin and tablet O2 (a combination of ofloxacin and ornidazole) at the concentrations of 0.5 to 4 mg/mL were used as standards. The plates were kept at 4°C for 15 min for diffusion and were then incubated for 18 h at 37°C. Antimicrobial activity was evaluated by measuring the zone of inhibition against the test organisms (CitationMukherji et al., 1995).
The micro dilution broth method (CitationNCCLS, 2008) was used to determine the minimum inhibitory concentration (MIC). The ethanol and aqueous extracts inhibiting growth of one or more microorganisms were tested for MIC. The dilutions were prepared using DMSO in the decreasing order of concentration of extracts. The least concentration of each extract showing inhibition of growth was selected for preparing the dilution. The wells were inoculated with 0.1 mL aliquot of test organisms having serial dilutions of extract. The micro plates were incubated at 37°C ± 1°C for 24 h. The highest dilution of each extract corresponding to respective test organism showing no visible growth was considered as MIC. Ciprofloxacin was used as a reference and appropriate control with no extract and solvent were used. The growth was compared with the reference as well as the control. Each experiment was repeated at least three times. The MBC minimum bactericidal concentration was determined by sub-culturing the negative samples. MBC was determined as the lowest concentration that yielded negative sub-cultures.
In vivo studies
Swiss albino mice were used in the present investigation. The animals were purchased from the National Toxicology Centre, Pune, India and maintained in polypropylene cages in the Institutional Animal House Facility with 12 h light: 12 h dark cycle. All the animals were kept under laboratory conditions (temperature 25° ± 2°C, relative humidity 75% ± 5%) for an acclimatization period of 7 days before carrying out the experiments. Normal pellet diet (Amrut laboratory animal feed, Maharashtra, India) and filtered water was provided ad libitum. All the experimental protocols were approved by the Institutional Animal Ethics Committee and complied with the National Institute of Health (USA) guidelines on handling of experimental animals.
Acute oral toxicity study
In the acute oral toxicity study of ethanol and aqueous extracts of B. aristata, a limit dose each of 2000 mg per kg body weight of the animal was administered on a single test animal orally by gavage. The limit test was repeated three times on a single test animal for both extracts as a part of an oral acute toxicity assay. As no mortality of experimental animals was observed at the limit dose for the LD50 study, a dose regime of more than the limit dose, i.e., 5000 mg per kg body weight was planned and performed on a single test animal at a time and repeated three times.
Castor oil-induced diarrhea
An in vivo antidiarrheal activity experiment was carried out on the following groups (control, positive control, and test with 5 subgroups, one for each test concentration for each extract, separately) containing six animals each (n = 6). All the animals were screened initially by giving 6.5 mL of castor oil and only those showing diarrhea were selected for final experiment. Each animal was placed in an individual cage, the floor of which was lined with blotting paper. The floor lining was changed every hour. Diarrhea was induced by oral administration of 0.5 mL of castor oil to each mouse. The control group was fed with 0.3 mL physiological saline 15 min before the administration of castor oil. The positive control group received a standard antidiarrheal drug, loperamide (25 mg/kg) and the test group was administered with aqueous and ethanol extracts in the dose range of 31.25 to 500 mg/kg body weight 15 min before the administration of the castor oil (CitationUddin et al., 2005). The mice were observed for a period of 5 h and the time of onset of diarrhea, stool mass and number of diarrheal episodes were recorded.
Gastrointestinal transit test
The effect of aqueous and ethanol extracts on the intestinal propulsion of feed in mice was tested using the charcoal method (CitationWilliamson et al., 1996). Mice were fasted for 16 h before commencing the experiment which was carried out on three groups containing six animals each (n = 6) as described above. The animals were administered with 0.4 mL of charcoal meal (5% aqueous suspension in 5% gum acacia). After 20 min of charcoal administration the mice were sacrificed by ether anesthesia. The intestine was rapidly dissected out from cardia to distal end. The distance traveled by the charcoal from pyloric end to ileocaecal junction was measured and expressed as a percentage.
Results and discussion
Preliminary phytochemical analysis of extracts
Results indicated the presence of biologically active compounds such as glycosides, phenolics, alkaloids, tannins, flavonoids, saponins and steroids in ethanol and aqueous extracts of Berberis aristata.
The yield of ethanol (maceration) and aqueous (Soxhlet) extracts of bark of Berberis aristata was 5.8 g % w/w and 9.98 g % w/w, respectively.
In vitro study
The antimicrobial profile of extracts of bark of Berberis aristata and its comparison with the standard drugs against four strains of Shigella (main causative agent of the disease) was evaluated and presented in . Both the extracts of Berberis aristata showed antibacterial activity against four strains of Shigella with zones of inhibition ranging between 8 and 23 mm. Of the four strains evaluated, S. dysenteriae demonstrated the minimum response to both extracts. The maximum inhibition zones (20–23 mm) were observed against clinical isolates of Shigella sonnei with both extracts. The extracts were found to be more effective than the standard drugs at the lower end of the concentrations tested (0.5 and 1 mg). However, the zones of inhibitions were bigger with the drugs, particularly tablet O2 at higher concentrations (2 and 4 mg) against all the strains (). The MIC and MBC of extracts required for different strains of Shigella were calculated to be in the range of 125–500 µg/mL and 300–600 µg/mL for ethanol and aqueous extracts, respectively ().The MIC and MBC values of berberine standard are almost comparable to standard ciprofloxacin (). CitationSingh et al. (2007) demonstrated the antimicrobial activity of hydro alcohol extract of four Berberis species including Berberis aristata against 11 bacterial and eight fungal strains excluding Shigella.
Table 1. Inhibition zones (mm) for ethanol and aqueous extract of Berberis aristata bark and reference antibiotics.
Table 2. The minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC) of bark extracts of Berberis aristata, Berberine standard and ciprofloxacin against species of Shigella.
Chromatographic and spectrophotometric analyses
The phytochemical analysis of the ethanol and aqueous extracts demonstrated the presence of alkaloids in both extracts. In HPTLC studies the extracts showed the presence of distinct yellow fluorescent bands with a Rf value of 0.25 matching with the band of standard berberine (). Of the various mobile phases tried, the best resolution was obtained with propanol:formic acid:water (9:0.1:0.9, v/v). The band at Rf 0.25 of both the extracts and that of standard berberine gave overlapping spectra when scanned between 200 to 800 nm with distinct peaks at 450 nm (). The FTIR spectrum of the standard berberine was consistent with berberine band obtained from ethanol and aqueous extract of the Berberis aristata stem bark, indicating presence of common bonds: 3358 (N-H) stretching, 2929 (C-H stretching sym. CH3), 1689 (C = O stretching), 1448-asym. CH3 bending 1529 (N-H bending).
Figure 1. (A) HPTLC profile of standard berberine (lanes 5 and 6), ethanolic extract (lanes 3 and 4) and aqueous extract (lanes 1 and 2) of Berberis aristata. (B) HPTLC densitogram of bands at Rf 0.25 (a) standard berberine, (b) aqueous extract and (c) ethanolic extract.
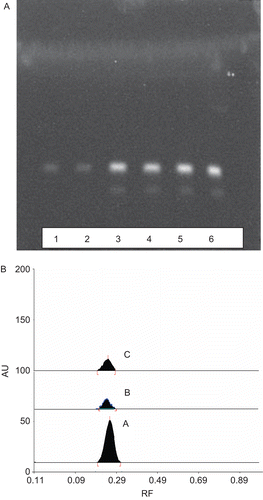
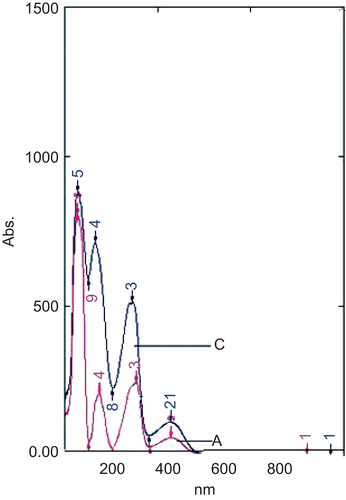
Figure 2. Absorption spectra of standard berberine (A) and at Rf 0.25 isolated from aqueous extract of B. aristata (C) showing nearly overlapping curves.
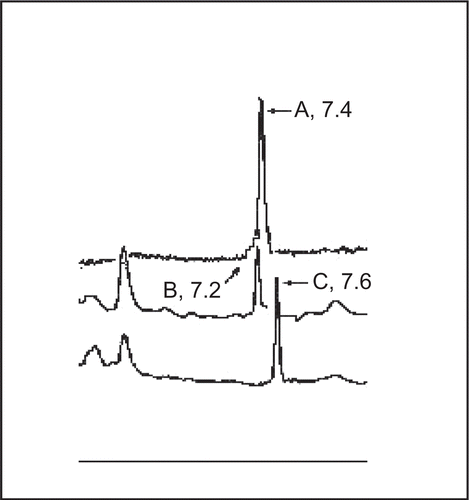
The presence of berberine in both the extracts was further confirmed by HPLC studies. The TLC eluted band at Rf 0.25 from both the extracts, when injected individually in HPLC column showed peaks at retention time 7.2 and 7.6 min. The standard berberine showed a single, sharp, and symmetrical peak at a retention time of 7.4 min (). Berberine is reported to be one of the active ingredients present in the bark of Berberis aristata (CitationCSIR, 1985; CitationMukherji et al., 2006) and our observations seem to confirm this. Berberine has been reported to markedly inhibit secretory response of heat stable enterotoxin (E. coli) in infant mouse model (Sack & Froehlich, 1982) and Cox-2 transcriptional activity in colon cancer cells (CitationFukuda et al., 1999). Moreover, the pharmacological studies have shown berberine to be effective in reducing mortality rate, volume and duration of diarrhea (CitationSabir et al., 1978). The amount of berberine found to be present in the extracts by HPLC and HPTLC was 5.58 mg% and 1.2 mg% for ethanol and aqueous extracts, respectively.
Acute oral toxicity study
The oral acute toxicity assays of ethanol and aqueous extracts were conducted in order to determine their biosafety as a therapeutic agent and to decide the dose of the extract for further antidysenteric experiments such as the charcoal motility test and castor oil-induced diarrhea. The acute oral toxicity studies provided information on health hazards likely to arise from short-term exposure to the test substance by the oral route. Since no mortality of experimental animals was observed at the limit dose of 2000 mg/kg body weight, for the LD50 study, a dose regimen of more than this limit dose value was planned and done. The results of the toxicological studies showed that administration of ethanol and aqueous extracts of Berberis aristata by oral route at doses up to 5000 mg/kg body weight did not produce any signs of toxicity or death in experimental animals. The results indicate no or minimal chance of toxicity of these extracts at the likely therapeutic doses in humans which are lower by several orders of magnitude than no observed adverse effect level.
Castor oil-induced diarrhea
The in vivo antidiarrheal activity was evaluated on mice with castor oil-induced diarrhea. Approximately 90% of animals developed diarrhea at the end of 4 h and 100% by the end of 5 h. Aqueous and ethanol extracts of Berberis aristata considerably reduced fecal output stimulated by castor oil at the given doses in a dose-dependent manner, with the ethanol found to be more effective than the aqueous in all the parameters studied (). The extracts could prolong the onset of castor oil-induced diarrhea and reduce the number of diarrheal episodes.
Table 3. Effect of Berberis aristata aqueous and alcoholic extracts on castor oil-induced diarrhea and gastrointestinal transit of charcoal meal in mice.
Gastrointestinal transit test
The mean length of intestine traveled by the diet was found to be reduced compared to the control in a dose-dependent manner by both extracts in the gastrointestinal feed transit study (). Similar to the previous experiment, the effect of ethanol extract was more pronounced than the aqueous extract, presumably due to a higher proportion of berberine in the former (in a 500 mg/kg body weight dose, the amount of berberine actually getting into the animal body is 27.9 mg in ethanol extract compared to 6 mg in aqueous extract). The percentage inhibition by the highest dose was however, lower than that of the standard drug, loperamide.
Conclusion
The results support the efficacy of ethanol and aqueous extracts of bark of Berberis aristata as an antidiarrheal agent. The antidiarrheal activity of any extract in vivo is expressed in terms of its ability to reduce the number of animals exhibiting the diarrhea and total number of diarrheal episodes in an animal (CitationWilliamson et al., 1996). The antidiarrheal activity of the bark extracts of Berberis aristata could be attributed to the presence of berberine in the extracts. Berberine, the isoquinoline alkaloid, has been used as a standard antidiarrheal drug in many pharmacological experiments (CitationJia et al., 2008).The present study provides a qualitative and quantitative chemo profiling of a potent plant species used for antidysenteric activity in the Indian subcontinent which can be used in quality control of raw material, formulations and finished products. The study also provides a stage and preliminary data on the toxicity profile of ethanol and aqueous extracts of this medicinally important plant for future preclinical and clinical studies, and results, along with the previous studies, provide a rationale for its apparent clinical usefulness in treating diarrheal disease.
Berberine is known to inhibit the intestinal secretary response induced by enterotoxins of Shigella species (CitationSabir et al., 1978; Sack & Froehlich, 1982).The remarkable dose-dependent reduction in castor oil-induced diarrhea in mice is a demonstration of the efficacy of Berberis aristata plant as an antidiarrheal medicine. It is well known that castor oil causes motility and secretory diarrhea by affecting both intestinal motility as well as water and electrolyte transport across the intestinal mucosa (CitationRouf et al., 2003). Our results seem to suggest an antisecretory mechanism to be the basis of the antidiarrheal action of extracts of Berberis aristata.
Acknowledgements
The authors are grateful to Anchrom India, Mumbai, for providing berberine standard as a gift sample.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Amin AH, Subhash TV, Abbasi KM. (1969). Berberine sulphate: Antimicrobial activity, bioassay and mode of action. Can J Microbiol, 15, 1067–1076.
- Ayurvedic Pharmacopeia (2001). Ayurvedic Pharmacopeia of India. New Delhi, India: Government of India Publications.
- Birdsall TC, Kelly GS. (1997). Berberine: Therapeutic potential of an alkaloid found in several medicinal plants. Alternat Med Rev, 2, 94–96.
- CSIR (1985). Wealth of India. New Delhi, India: CSIR Publications.
- Edeoga HO, Okwo DE, Mbaebie BO. (2005). Phytochemical constituents of Nigerian medicinal plants. Afr J Biotechnol, 4, 685–688.
- Fukuda K, Hibiya Y, Mutoh M, Koshiji K, Akaos S, Fujiwara, H. (1999). Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J Ethnopharmacol, 66, 227–233.
- Gacche RN, Dhole NA. (2006). Antioxidant and possible anti-inflammatory potential of selected medicinal plants prescribed in the Indian traditional system of medicine. Pharm Biol, 44, 389–395.
- Gerald NT, Jules RK, Omer BN, Donatein G. (2007). Antidiarrheal and antimicrobial activities of Emilli coccinea (Sims) G. Don extracts. J Ethnopharmacol, 112, 278–283.
- Harborne J. (1973). Phytochemical Methods. London: Chapman & Hall.
- Havagiray R, Ramesh C, Mehrd AD, Sadhana K. (2004). Studies on anti-diarrhoeal activity of Calotropis gigantea R.Br. in experimental animals. J Pharm Pharmaceut Sci, 7, 70–73.
- Heinrich M, Heneka B, Ankli A, Rimpler H, Sticher O, Kostiza T.(2005). Spasmolytic and antidiarrhoeal properties of the Yucatec Mayan medicinal plant Casimiroa tetrameria. J Pharm Pharmacol, 57, 1081–1085.
- IHP (1999). Indian Herbal Pharmacopoeia. Jammu, India: Indian Drugs Manufacturer’s Association and Regional Research Laboratory.
- Jia Q, SuW, PengW, Li P, Wang Y. (2008). Anti-diarrhoea and analgesic activities of the methanol extract and its fractions of Jasminum amplexicaule Buch.-Ham (Oleaceae). J Ethnopharmacol, 10, 7–14.
- Joshi S, Sing AK, Dhar DN. (1989). Isolation and structural elucidation of potential active principles of Curcuma zedoria rhizomes. Herba Hung, 28, 95–98.
- Marek R, Seckarova P, Hulova D, Marek J, Dostal J, Sklenar V. (2003). Palmitate and berberine isolation artifacts. J Nat Prod, 66, 481–482.
- Mukherjee PK, Wahile A, Kumar V, Rai S, Mukherjee K, Saba BP. (2006). Marker profile of botanicals used for hepatoprotection in Indian system of medicine. Drug Information J USA, 40, 131–139.
- Maity TK, Mandal S, Mukherjee PK, Saha K, Saha BP, Das J, Pal M. (1997). Evaluation of the hepatoprotective potential of Cassia tora leaf extract. Nat Prod Sci, 3, 122–125.
- Mukherji PK. (2002). Quality Control of Herbal Drugs: An Approach to Evaluation of Botanicals. New Delhi: Business Horizons.
- Mukherji PK, Balsubramaniyan R, Saha K, Saha BP, Pal M. (1995). Antibacterial efficiency of Nelumbo nucifera (Nymphaeaceae) rhizomes extract. Indian Drugs, 32, 274–276.
- NCCLS (2008). Performance Standards for Antimicrobial Susceptibility Testing; Ninth Informational Supplement. NCCLS document M100-S9. Wayne, PA, National Committee for Clinical Laboratory Standards.
- OECD (2001). The OECD Guidelines for Testing of Chemicals: 425 Acute Oral Toxicity. Paris, Organization of Economic Co-operation Development, 12–18.
- Perez V, Garcia JF, Badiola JJ. (1996). Description and classification of different types of lesions associated with natural paratuberculosis infection in sheep. J Comparative Pathol, 114, 107–122.
- Rouf AS, Islam MS, Rahman MT. (2003). Evaluation of antidiarrhoeal activity of Rumex maritimus roots. J Ethnopharmacol, 84, 307–310.
- Sabir M, Akhtar MH, Bhinde NK. (1978). Further studies on pharmacology of berberine. Ind J Physiol Pharmacol, 22, 9–23.
- Sack BR, Froehlich JL. (1981). Berberine inhibits intestinal secretory response of Vibrio cholerae and Escherichia coli enterotoxins. Infect Immun, 35, 471–475.
- Singh M, Srivastava S, Rawat AK. (2007). Antimicrobial activities of Indian Berberis species. Fitoterapia, 78, 574–576.
- Uddin SJ, Shilpi JA, Alam SMS, Alamgir M, Rahman M, Sarker SD. (2005). Antidiarrheal activity of the methanol extract of the barks of Xylocarpus moluccensis castor oil- and magnesium sulphate-induced diarrhoea models in mice. J Ethnopharmacol, 101, 139–143.
- WHO (1979). Diarrhoea disease control programme. Wkly Epidemiol Rec, 16, 121
- Williamson EM, Okpako DT, Evans FJ. (1996). Pharmacological Methods in Phytotherapy Research: Selection, Preparation and Pharmacological Evaluation of Plant Material. New York: Wiley.