Abstract
Context: Glycosmis pentaphylla (Retz.) DC (Rutaceae) is a shrub, traditionally used to treat anemia, rheumatism, as an anthelmintic, febrifuge and vermifuge, for jaundice, and liver complaints.
Objective: This study evaluated the hepatoprotective activity of G. pentaphylla against paracetamol-induced hepatotoxicity in Swiss albino mice.
Materials and methods: Effect of methanol extract (200 and 400 mg/kg) and petroleum ether extract (200 and 400 mg/kg) were studied on paracetamol-induced (250 mg/kg intraperitoneally) hepatic damage in mice for estimating the serum marker enzymes as alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin, and total protein. Then the sections of liver were observed for histopathological changes in liver architecture including necrosis, steatosis, and fatty change of hepatic cells.
Results: Mice were protected from the hepatotoxic action of paracetamol as evidenced by significant reduction in the elevated serum level of ALT (P < 0.001), AST (P < 0.001), ALP (P < 0.001), total bilirubin (P < 0.01) and an increased level of total protein (P < 0.01) with a significant reduction in liver weight (P < 0.01) when compared with paracetamol treated group and silymarin (50 mg/kg) was used as a positive control.
Discussion and conclusion: These biochemical observations were supplemented by histopathological examination of liver sections from different experimental groups and corroborated the hepatoprotective efficacy of methanol and petroleum ether plant extract. The methanol extract (400 mg/kg) of G. pentaphylla is able to alter the toxic condition of the hepatocytes so as to protect the membrane integrity against paracetamol-induced leakage of marker enzymes.
Introduction
Liver disease is still a worldwide health problem. Unfortunately, conventional or synthetic drugs used in the treatment of liver diseases are inadequate and sometimes can have serious side effects (CitationGuntupalli et al., 2006). Drug-induced hepatotoxicity is a major cause of new drug withdrawal from the market. It also limits further development of promising therapeutic agents even prior to clinical trials. Over-the-counter drugs are not exempt from hepatotoxic liability; for example, acetaminophen (paracetamol), a widely used (and misused) analgesic/antipyretic agent, when taken acutely in large doses or chronically in greater than recommended dosages, can lead to liver and kidney damage (CitationLorelle et al., 2003). In view of severe undesirable side effects of synthetic agents, there is growing focus to follow systematic research methodology and to evaluate scientific basis for the traditional herbal medicines that are claimed to possess hepatoprotective activity (CitationManokaran et al., 2008). The plant Glycosmis pentaphylla (Retz.) DC (Rutaceae), commonly known as “tooth brush plant” in English and “ashvashakota” in Sanskrit, is an aromatic shrub that grows from 0.5 to 2 m in height and distributed throughout India (CitationKirtikar & Basu, 1998). It is common in waste places, villages, road-sides, and throughout the tropical and subtropical areas of India. The whole plant is beneficial in anemia, jaundice, rheumatism, and cough. The leaf juice is used as an anthelmintic, febrifuge, vermifuge, in liver complaints, and applied in the form of paste to eczema and other skin diseases (CitationChatterjee & Pakrashi, 1997). In homeopathy, leaves are used in biliary colic, worm-colic, diarrhea, and dysentery; while the ethanol extract (50%) of aerial parts is used as spasmolytic, diuretic, and the homeopathic mother tincture of leaves is claimed to cure throat cancer (CitationAsolkar et al., 2000). Wood brushed with water is administered internally as an antidote for snakebite (CitationChopra et al., 1999). The plant has been reported to contain glycosminine, glycosmicine, γ-fagarine, isoarborinol, and arborinine (CitationRastogi, 1999); the leaves contain furoquinoline, methyl acridone, arborinine, arborine, mupamine, and phlobaphene (CitationChatterjee & Pakrashi, 1997). The literature reveals that G. pentaphylla is used orally in the treatment of jaundice in traditional Indian medicine and, moreover, the hepatoprotective activity of the stem and leaf water extract of G. pentaphylla against carbon tetrachloride-induced liver toxicity was reported by CitationMitra and Sur (1997). To date, no systematic study has been reported in an acute model regarding the paracetamol-induced hepatotoxicity in mice. In the present study, an effort has been made to establish the scientific validity to the hepatoprotective property of methanol and petroleum ether leaf extracts of G. pentaphylla against paracetamol-induced hepatotoxicity in mice.
Materials and methods
Plant material
The leaves of Glycosmis pentaphylla (Retz.) DC (Rutaceae) were collected during the month of September 2008 from the rural belt of Mohuda in Ganjam District of Orissa, India; identified and authenticated by taxonomist S.K. Dash, Department of Bioscience, College of Pharmaceutical Sciences, Mohuda, and samples were kept in the laboratory for future reference. After authentication, the collected plant leaves were washed and air-dried under shade, cut into small pieces, powdered by a mechanical grinder and passed through a 40-mesh sieve and stored in a closed container for future use.
Preparation of G. pentaphylla leaves extracts
Coarsely powdered leaves (1 kg) were extracted with 1200 mL of methanol by cold maceration (CitationMinistry of Health and Welfare, 1985). The methanol extract was dried and concentrated by evaporating the solvent completely under vacuum using rotatory evaporator (Jain Scientific Glass Works, DTC 201, Ambala cantonment, India) to produce a brownish semisolid mass after drying (16%, w/w) and the methanol extract (GPME) was further extracted with 600 mL of petroleum ether (60–80°C) to produce a greenish brown semisolid mass petroleum ether extract (GPPE) after drying (5.04%, w/w) and both the extracts were stored in a desiccator. The chemical constituents of the extract were identified by qualitative analysis and confirmed by thin layer chromatography (i.e., hRf values). The dried extracts were made into emulsion by triturating the accurately weighed quantity (8 g) of the extract with 1% gum acacia and suspended in distilled water at definite concentrations separately used for the pharmacological study (CitationSabir & Rocha, 2008).
Chemicals
Paracetamol (acetaminophen) was obtained from ALPA Laboratories, Indore, India and silymarin was obtained from Micro labs, Hosur, India as free samples. Assay kits were used for alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin and total protein estimations and purchased from Autospan Diagnostic Reagent, Surat, India. All extractive solvents are of analytical grade reagents (AR) and purchased from S.D. Fine Chemicals, Mumbai, India.
Preliminary phytochemical analysis
The GPME and GPPE extracts were subjected to preliminary phytochemical screening for detection of major chemical groups. In each case test 10% w/v solution of the extracts were used unless otherwise mentioned in individual test (CitationPatil et al., 2001). The qualitative analysis was performed by using Mayer’s, Dragendorff’s, or Wagner’s reagent for tests of alkaloids and terpenoids; Liebermann-Burchard reagent for test of steroids; using 1% lead acetate solution for test of saponins; using 1 mL dilute ammonia or 1 mL dilute sodium carbonate or 1 mL dilute sodium hydroxide or using 25% basic lead acetate solution for the test of flavonoids and polyphenols; using Fehling’s A & B solution or Benedict’s solution for test of reducing sugar; also by using 1 mL of 5% ferric chloride or 10% lead acetate solution for the test of tannins.
Experimental animals
All animal procedures were in strict accordance with the CPCSEA Guidelines (Committee for the Purpose of Control and Supervision of Experiment on Animals). Healthy Swiss albino mice of 7–8 weeks old weighing 20–35 g of either sex were used. They were obtained from Jawaharlal Nehru Cancer Hospital and Research Centre, Bhopal (Madhya Pradesh, India). The mice were housed at a temperature of 20–25°C under a 12 h light/dark cycle with 50% of relative humidity. They were fed standard animal feed (Lipton’s, India) and water ad libitum. This study complied with current ethical regulations on animal research in Jawaharlal Nehru Cancer Hospital and Research Centre, Bhopal. Approval for experimental work was as per the ethical committee of Jawaharlal Nehru Cancer Hospital and Research Centre (Reference No. 670/225-IAEC/2008/Project No. 50).
Acute toxicity study
The LD50 values of GPME and GPPE were derived as described by CitationLitchfield and Wilcoxon (1949). The maximum non-lethal doses of both the extracts were found to be 4000 mg/kg body weight (bw), orally. The 1% gum acacia was used as a vehicle and showed no mortality. By adopting fixed dose the guideline of CPCSEA and 1/10th of LD50 cut off values (CitationPatil et al., 2001; CitationShivakumar et al., 2006) the determination of acute toxicity of the extracts were taken as screening dose, i.e., 200, 400 mg/kg for subsequent studies (GPME-200, 400 mg/kg and, GPPE-200, 400 mg/kg).
Paracetamol-induced hepatotoxicity in mice (acute model)
Animals were randomized and divided into seven groups (1–7) with six mice in each group. Animals of group 1 (control) received only vehicle (1% gum acacia in distilled water). Animals of group 2 (paracetamol control) received acetaminophen 250 mg/kg only. Animals of group 3 fed with acetaminophen 250 mg/kg and the standard drug silymarin 50 mg/kg. Animals of groups 4 to 7 were fed with a single dose of acetaminophen 250 mg/kg; whereas groups 4 and 5 were fed with GPME, 200 and 400 mg/kg. Similarly, animals of groups 6 and 7 were fed with GPPE, 200 and 400 mg/kg. Plant extracts were administered 3 h after the administration of acetaminophen. All the treatments were done by means of a gavage or gastric tube. The treatments were continued for seven consecutive days and on the eighth day of the experiment, the animals were anesthetized with ether and blood samples were collected by puncturing the retro-orbital plexus, and serum was separated by centrifugation at 1500 rpm for 10 min. The serum was then analyzed for alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin and total protein level using assay kits according to the supplier (Autospan Diagnostic Reagent, Surat, India) specifications (CitationSabir & Rocha, 2008). The estimation was done by using a photoanalyser instrument (FT-2; AMS); each determination was carried out with a suitable kit (Autospan Diagnostic Reagent, Surat, India) containing the necessary reagents at a predetermined wavelength for each parameter.
Biochemical estimation of marker enzymes
Effect of treatment with paracetamol on the serum ALT activities
The estimation was done by using a photoanalyser (FT-2; AMS); serum ALT determination was carried out with a suitable kit (Autospan Diagnostic Reagent) containing the necessary reagents at a predetermined wavelength 340 nm where the rate of oxidation of NADH is measured kinetically by monitoring the decrease in absorbance at 340 nm (CitationMurray, 1994).
Effect of treatment with paracetamol on the serum AST activities
The estimation was done by using a photoanalyser (FT-2; AMS); serum AST activity was carried out by suitable kit (Autospan Diagnostic Reagent) containing the necessary reagents. The rate of oxidation of NADH is measured kinetically by monitoring the decrease in absorbance at 340 nm and is directly proportional to GOT activity in the sample (CitationMurray, 1994).
Effect of treatment with paracetamol on the levels of ALP activities
The level of ALP activities were carried out using the kit Autospan Diagnostic Reagent analyzed in a photoanalyser (FT-2; AMS). By using a specific reagent at pH 10.3, alkaline phosphatase (ALP) catalyses the hydrolysis of colorless p-nitrophenyl phosphate (p-NPP) to yellow colored p-nitrophenol and phosphate. Change in absorbance due to yellow color formation is measured kinetically at 405 nm and is proportional to ALP activity in the sample (CitationMoss & Henderson, 1994).
Effect of treatment with paracetamol on the serum bilirubin levels
Serum bilirubin levels in paracetamol-induced liver damage were estimated using an analytical kit from Autospan Diagnostic Reagent. The assay principle is based on the fact that total bilirubin reacts in the presence of caffeine with diazotized sulfanilic acid to form azobilirubin. The determination of direct bilirubin at 546 nm is proportional to the concentration of bilirubin (CitationWillard & Meites, 1982).
Effect of treatment with paracetamol on hepatic total protein levels
The total protein level was estimated using an analytical kit from Autospan Diagnostic Biuret Reagent analyzed in a photoanalyser (FT-2; AMS). Peptide bonds of proteins react with cupric ions in alkaline solutions to form a colored chelate, the absorbance of which is measured at 578 nm. The biuret reagent contains sodium potassium tartrate to complex cupric ions and maintains their solubility at alkaline pH. Absorbance data are proportional to protein concentrations (CitationTietz, 1983).
Histopathological studies
One animal from the treated groups showing maximal activity as indicated by improved biochemical parameters from each test, positive control, hepatotoxin and control groups were utilized for this purpose. The animals were sacrificed and the abdomen was cut open to remove the liver; 5 mm thick pieces of the liver were fixed in Bouin’s solution (mixture of 75 mL of saturated picric acid, 25 mL of 40% formaldehyde and 5 mL of glacial acetic acid) for 12 h, then embedded in paraffin wax and cut into 5 μ thick sections were made using microtome and stained using hematoxylin-eosin dye and finally mounted in diphenyl xylene. Then the sections were observed for histopathological changes in liver architecture and their photomicroscopic observation including necrosis, steatosis and fatty change of hepatic cells (CitationGray, 1964; CitationShai et al., 2001; CitationSaeed & Sabir, 2002).
Statistical analysis
The results were presented as mean ± SEM and statistical significance between treated and a control group was evaluated by one-way analysis of variance (ANOVA) followed by Dunnett’s t-test.
Results
Preliminary phytochemical analysis
Results of different chemical tests on the GPME showed the presence of phytoconstituents, i.e., reducing sugars, flavonoids, polyphenols, alkaloids, tannins, and saponins, whereas GPPE showed a positive tests for the presence of alkaloids, steroids, and terpenoids.
Effect of treatment with paracetamol on serum ALT activities
Rats treated with paracetamol in group 2 alone developed significant liver cell damage as was evident from a significant (P < 0.001) increase in serum alanine aminotransferase enzyme activities. Oral administration of GPME and GPPE at two different doses, 200 and 400 mg/kg (groups 4 to 7), were seen to lower significantly (P < 0.01) the activities of marker enzymes; comparable to the group 3 of standard drug silymarin (50 mg/kg; P < 0.01) as shown in .
Table 1. Effect of extracts of Glycosmis pentaphylla on paracetamol (acetaminophen)-induced hepatotoxicity in mice.
Effect of treatment with paracetamol on the serum AST activities
In the paracetamol treated group 2; there was a significant (P < 0.001) increase in aspartate aminotransferase activity than in that of the normal group 1. AST activity of GPME and GPPE groups of two different doses 200 and 400 mg/kg (groups 4 to 7) significantly (P < 0.01) recovered the level of enzyme activities which is comparable with standard drug silymarin group-3 (P < 0.01) as shown in .
Effect of treatment with paracetamol on the levels of ALP activities
Rats treated with paracetamol (group 2) alone developed significant liver cell damage as was evident from a significant (P < 0.001) increase in serum enzyme levels of ALP compared to that of normal group 1. GPME and GPPE groups of two different doses, 200 and 400 mg/kg significantly (P < 0.01 group 4, 5, 7; P < 0.05 group 6), recovered the ALP level as compared to the standard drug silymarin group 3 (50 mg/kg; P < 0.01) as shown in .
Effect of treatment with paracetamol on the serum bilirubin levels
A significant (P < 0.001) increase in serum bilirubin level was seen in paracetamol-treated animals in group 2 compared to that of the normal group. Bilirubin levels for GPME and GPPE groups of two different doses 200 and 400 mg/kg significantly (P < 0.01 group 4, 5, 7; P < 0.05 group 6) recovered against the paracetamol group 2 and both doses were comparable with the standard drug silymarin at 50 mg/kg (P < 0.01) as shown in .
Effect of treatment with paracetamol on hepatic total protein levels and liver weight
Rats treated with paracetamol group 2 alone developed significant liver cell damage as was evident from a significant (P < 0.001) decrease in the total protein levels when compared to normal group 1. But the administration of GPME and GPPE groups of two different doses 200 and 400 mg/kg have showed the significant (P < 0.01 groups 4, 5, 7; P < 0.05 group 6) recovery of total protein level as compared to the standard drug silymarin (50 mg/kg; P < 0.01) as shown in , whereas there was a significant reduction (P < 0.01) of liver weight of groups 4 to 7, when the GPME and GPPE groups of two different doses 200 and 400 mg/kg was given along with paracetamol.
Histopathological studies
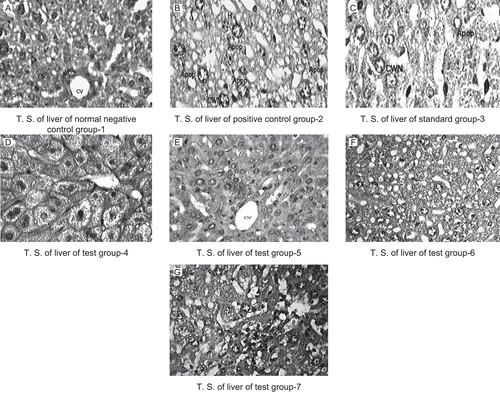
Histopathological examination of liver sections of control group 1 and standard group 3 showed normal cellular architecture with distinct hepatic cells, sinusoidal spaces and central vein (). Disarrangement of normal hepatic cells with centrilobular necrosis, vacuolization of cytoplasm and fatty degeneration were observed in the paracetamol treated mice of group 2 in . The liver sections of the mice treated with GPME (200 and 400 mg/kg) of groups 4 and 5 showed signs of protection as was evident by the absence of necrosis and vacuoles () while the liver sections of the mice treated with GPPE (200 and 400 mg/kg) of groups 6 and 7 showed reduction in fatty degeneration and absence of necrosis ().
Figure 1. Histopathological study of rats liver treated with the methanol and petroleum ether extracts of Glycosmis pentaphylla. Photomicrographs of mice liver obtained from different treatment groups. T.S Figure (A) revealed normochromatic hepatocytes (H), occasionally apoptosis, cart wheel nucleus (CWN) and lipid vacuoles (CV). Figure (B) T.S. revealed hyper chromatic highly damaged hepatocytes (H), large numbers lipid vacuoles (LV), frequent apoptosis (Apop), necrosis (CWN). Figure (C) Standard group T.S. revealed normochromatic hepatocytes (H), occasionally apoptosis (Apop), cart wheel nucleus (CWN) and lipid vacuoles (LV). Figure (D) & (E) T.S. revealed normochromatic hepatocytes (H) with good mitotic index, occasionally lipid vacuoles (LV) without apoptosis, necrosis and cart wheel nucleus (S). Figure (F) & (G) T.S. of liver revealed hyper chromatic hepatocytes (H) with average mitotic index, lipid vacuoles (LV) and cart wheel nucleus (CWN), few apoptotic hepatocyts (AH). H and E staining (X400). Group 1(Control), Group 2 (paracetamol 250 mg/kg), Group 3 (paracetamol 250 mg/kg + silymarin 50 mg/kg), Group 4 (paracetamol 250 mg/kg + GPME-200 mg/kg), Group 5 (paracetamol 250 mg/kg + GPME-400 mg/kg), Group 6 (paracetamol 250 mg/kg + GPPE-200 mg/kg) and Group 7 (paracetamol 250 mg/kg + GPPE-400 mg/kg), H & E staining (400X).
Discussion
In the acute toxicity studies, oral administration of both GPME and GPPE of G. pentaphylla did not produce any mortality in mice up to a dose level of 4000 mg/kgbw. This may be due to the broad non-toxic range of the plant, where the plant extract showed a high LD50 and relative safety. Paracetamol (acetaminophen)-induced hepatic injuries are commonly used models for hepatoprotective drug screening (CitationPlaa & Hewitt, 1982). It is a well-known antipyretic and analgesic agent, which produces hepatic necrosis at higher doses because of the reaction metabolite N-acetyl-p-benzoquinoneimine (NAPQI), which causes oxidative stress and glutathione depletion (CitationBoyd & Bereczky, 1966). Introduction of cytochrome or depletion of hepatic glutathione is a prerequisite for paracetamol-induced hepatotoxicity (CitationMoron et al., 1979; CitationGupta et al., 2006). Assessment of liver function can be made by estimating the activities of serum ALT, AST, ALP, bilirubin and total protein which are enzymes originally present in higher concentration in cytoplasm (CitationManokaran et al., 2008). When there is hepatopathy, these enzymes leak into the blood stream which serves as an indicator for the liver damage (CitationNkosi et al., 2005).
The abnormally high level of serum ALT, AST, ALP, total bilirubin and decrease in total protein content observed in group 2 in our study are the consequence of paracetamol-induced liver dysfunction and denotes the damage to the hepatic cells (). Treatment with GPME and GPPE at dose levels of 200 and 400 mg/kg reduced the enhanced level of serum ALT, AST, ALP, total bilirubin and increased the total protein level which seemed to offer protection and maintain the functional integrity of hepatic cells. The elevated levels of serum marker enzyme activities such as ALT, AST enzyme levels are a direct measure of hepatic injury and they show the status of liver. The elevated levels of serum enzymes are indicative of cellular leakage and loss of functional integrity of cell membrane in liver. Thus the lowering of the enzyme content in serum is a definite indication of the hepatoprotective action of a drug.
GPME and GPPE at a dose of 200 mg/kg (P< 0.01) and 400 mg/kg decreased significantly (P < 0.01) the activity of both AST and ALT as compared to the standard drug silymarin. Serum ALP and bilirubin levels are also related to the status and function of hepatic cells. Increase in serum ALP level is due to increased synthesis, in the presence of increasing biliary pressure (CitationMoss & Butterworth, 1974). In the present study, GPME and GPPE at a dose of 200 mg/kg (P < 0.01 & P < 0.05), and GPME and GPPE at a dose 400 mg/kg, were found to reduce significantly (P < 0.01) both serum ALP and bilirubin levels in the treated groups which are comparable to the standard drug silymarin (). A reduction in total serum protein () observed in the paracetamol-treated rats may be associated with a decrease in the number of hepatocytes, which in turn may result in decreased hepatic capacity to synthesize protein and consequently increase liver weight (). When the GPME and GPPE groups of two different doses 200 and 400 mg/kg was given along with paracetamol, a significant increase (P < 0.05 & P < 0.01) in total serum protein was observed indicating the hepatoprotection activity of the extract and also accounting for the decrease in the liver weight most probably through the hepatic cell regeneration.
Hepatoprotective activity of G. pentaphylla was shown by its ability to inhibit paracetamol-induced liver damage in mice. The fall in serum marker level by GPME (400 mg/kg) was significantly (P < 0.01) able to alter the toxic condition of the hepatocytes as compared to GPPE at a dose of 200 and 400 mg/kg, so as to protect the membrane integrity against acetaminophen-induced leakage of marker enzymes. This means there was a potential functional change in the hepatocytes of the hepatic cells. Hence, it has been concluded that the methanol extract of G. pentaphylla (GPME) at dose levels of 400 mg/kg revealed its potential significance as a hepatoprotection against acetaminophen-induced toxicity which was further supported by the presence of phytoconstituents such as flavonoids, polyphenols, tannins, and saponins. All the results can be comparable with the standard drug silymarin. A comparative histopathological study of liver from different experimental groups corroborated the hepatoprotective efficacy of methanol extract which was more effective than the petroleum ether extract of Glycosmis pentaphylla. Further detailed studies may, however, confirm the utility profile of this drug.
Acknowledgements
The authors thankfully acknowledge Jawaharlal Nehru Cancer Hospital and Research Centre, Bhopal, India.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Asolkar LV, Kakkar KK, Chakre OJ. (2000). Glossary of Indian Medicinal Plants with Active Principles (A-K), New Delhi: Council for Scientific and Industrial Research, 332.
- Boyd EH, Bereczky GM. (1966). Liver necrosis from paracetamol. Br J Pharmacol, 26, 606–614.
- Chatterjee A, Pakrashi SC. (1997). The Treatise on Indian Medicinal Plants. New Delhi, National Institute of Science Communication, 99.
- Chopra RN, Nayar SL, Chopra IC. (1999). Glossary of Medicinal Plants. New Delhi: Council for Scientific and Industrial Research, 126.
- Gray P (1964): Hand Book of Basic Micro Technique. Vol 3, New York: McGraw Hill, 85–145.
- Guntupalli M, Mohana R, Chandana VR, Palpu P, Annie S. (2006). Hepatoprotective effects of rubiadin, a major constituent of Rubia cordifolia Linn. J Ethnopharmacol 103, 484–490.
- Gupta AK, Chitme H, Dass SK, Misra N. (2006). Hepatoprotective activity of Rauwolfia serpentina rhizome in paracetamol intoxicated rats. J Pharmacol Toxicol,1, 82–88.
- Ministry of Health and Welfare. (1985). Indian Pharmacopoeia, Vol 2, second edition. Delhi, Government of India, Ministry of Health and Welfare.
- Kirtikar KR, Basu BD. (1998). Indian Medicinal Plants. India: International Book Distributors, 470.
- Litchfield JT, Wilcoxon F. (1949). A simplified method of evaluating dose effect experiments. J Pharmacol Exp Ther, 96, 99–133.
- Lorelle IB, Jonathan FC, Daune LC, Frances NS, Herbert T. (2003). Hepatoprotection by l-cysteine-glutathione mixed disulfide, a sulfhydryl-modified prodrug of glutathione. J Biochem Mol Toxic, 17, 95–97.
- Manokaran S, Jaswanth A, Sengottuvelu S, Nandhakumar J, Duraisamy R, Karthikeyan D, Mallegaswari R. (2008). Hepatoprotective activity of Aerva lanata Linn. against paracetamol induced hepatotoxicity in rats. Res J Pharm Technol, 1, 398–400.
- Mitra S, Sur RK. (1997). Hepatoprotection with Glycosmis pentaphylla (Retz). Indian J Exp Biol, 35, 1306–1309.
- Moron MS, Depierre JW, Mannervik B. (1979). Levels of glutathione, glutathione reductase and glutathione-S-transferase activities in rat lung and liver. Biochim Biophys Acta, 582, 67–78.
- Moss DW, Butterworth PJ. (1974). Enzymology and Medicine. London: Pitman Medical, 139.
- Moss DW, Henderson AK. (1994). Clinical enzymology. In: Burtis CA and Ashwood ER, eds. Tietz Textbook of Clinical Chemistry, third edition. Philadephia: WB Saunders, 617–721.
- Murray RL. (1994). Enzymes. In: Kaplan LA, & Pesce AJ, eds. Clinical Chemistry; Theory, Analysis and Correlation, Toronto: Mosby, 1079–1134.
- Nkosi CZ, Opoku AR, Terblanche SE. (2005). Effect of pumpkin seed (Cucurbita pepo) protein isolate on the activity levels of certain plasma enzymes in CCl4-induced liver injury in low protein fed rats. Phytother Res, 19, 341–345.
- Patil MB, Jalalpure SS, Ashraf A. (2001). Preliminary phytochemical investigation and wound healing activity of the leaves of Argemone mexicana Linn. Indian Drugs, 38, 288–293.
- Plaa GL, Hewitt WR. (1982). Quantitative evaluation of indices of hepatotoxicity. In: Zakin D, Bayer TD, eds. Toxicology of the Liver. New York: Raven Press, pp.103–120.
- Rastogi RP (1999). Compendium of Indian Medicinal Plants, Vol 1, pp. 199–200.
- Sabir SM, Rocha JBT. (2008). Water-extractable phytochemicals from Phyllanthus niruri exhibit distinct in vitro antioxidant and in vivo hepatoprotective activity against paracetamol-induced liver damage in mice. Food Chem, 111, 845–851.
- Saeed MA, Sabir AW. (2002). Irritant potential of triterpenoids from Ficus carica leaves. Fitoterapia,73, 417–420.
- Shai R, Yoel K, Ruth R, Michael S, Raphael M. (2001). Suppressors of cancer cell proliferation from fig (Ficus carica) resin: Isolation and structure elucidation. J Nat Prod, 64, 993–996.
- Shivakumar H, Sankara SLVJ, Vaidya VP. (2006). Anti-inflammatory activity of the unripe fruits of Ficus glomerata. Indian Drugs, 44, 48–50.
- Tietz NW. (1983). Clinical Guide to Laboratory Tests, London, WB Saunders, 416.
- Willard RF, Meites S. (1982). Selected Methods for the Small Clinical Chemistry Laboratory, Vol 9, 113–118.