Abstract
Context: Penstemon gentianoides (Kunth) Poir. and Penstemon campanulatus (Cav.) Willd. (Plantaginaceae) are important medicinal plants in Mexico used by indigenous people for their anti-inflammatory effects and to also reduce rheumatic pains.
Objective: In addition to radical scavenging activity, the anti-inflammatory activity of the extracts, fractions and compounds of these plants were investigated and reported here for the first time.
Materials and methods: The anti-inflammatory activities of MeOH, CH2Cl2, and ethyl acetate extracts and iridoid, flavonoids, and phenylpropanoids from Penstemon gentianoides and P. campanulatus were studied in the TPA-induced mouse ear edema model. In addition, antioxidant activity against DPPH, crocin and β-carotene were investigated.
Results: All extracts were tested and a selection of known compounds significantly (p <0.05) inhibited mouse ear edema. The results showed that CH2Cl2 extracts of roots and stems from P. gentianoides and ethyl acetate extracts of leaves from P. gentianoides and P. campanulatus, as well as luteolin, diosmetin, penstemide and verbascoside produced the most positive results. Of all substances tested, the CH2Cl2 extract of P. gentianoides roots was the most powerful inhibitor (ED50 = 0.07 mg/ear), with activity comparable to that of indomethacin. These extracts, compounds purified, as well as known compounds, inhibited oxidation of β-carotene and crocin.
Discussion and conclusion: These findings showed that the iridoid monoterpenes, flavonoids and phenylpropanoids present in these plants species may all contribute to the observed anti-inflammatory activity. Additionally, the observed antioxidant activity is correlated with the anti-inflammatory activity of these plants and the phytochemicals derived from them.
Introduction
As a continuation of our systematic search for biological activities of compounds from the Mexican flora, we wish to report the isolation and anti-inflammatory activities of a series of iridoid monoterpenes, flavonoids, and phenylpropanoids from Penstemon gentianoides (Kunth) Poir. and Penstemon campanulatus (Cav.) Willd. (Plantaginaceae). Both species of Penstemon are shrubs 1 to 3 m in height that grow in mountainous regions of Guatemala, Mexico, and the US. These plants are used by indigenous people of Mexico under the common names of “jarritos”, “parrito”, and “campanitas” for their anti-inflammatory, emollient, balsamic, and laxative effects, as well as to treat rheumatic pains (CitationDomínguez et al., 2005). Previous studies of these plants reported the presence of iridoid monoterpenes (CitationJiménez-Estrada et al., 2006) such as catalpol, aucubin, and penstemide. Extracts of these plants had pronounced antioxidant activity (CitationDomínguez et al., 2005). One of these compounds, penstemide, exhibited antitumor activity and laxative effects (CitationJiménez-Estrada et al., 2006). In previous studies, iridoid monoterpenes have been found to have other biological activities such as antimicrobial activity (CitationLi et al., 2009), as feeding stimulants for butterfly larvae, and as compounds that offer protection against avian predators (CitationBowers, 1983; CitationSeigler, 1998). Large foliar concentrations of iridoid monoterpenes may provide specialist herbivores with defensive compounds that they sequester from the plants for their own defense and make the plants unavailable to generalist herbivores (CitationPeñuelas et al., 2006).
In this paper, we report the anti-inflammatory activity of MeOH, CH2Cl2, EtOAc extracts from roots and aerial parts, as well as the purified compounds catalpol 1, penstemide 2, pensteminoside 3, plantarenaloside 4, globularisicin 5, martinoside 6, verbascoside 7, luteolin 8 and diosmetin 9 isolated from P. campanulatus and P. gentianoides. The anti-inflammatory effect was determined by means of the 12-O-tetradecanoylphorbol acetate (TPA)-induced mouse ear edema test (CitationTubaro et al., 1985; CitationDe Young et al., 1989; CitationPaya et al., 1993; CitationRecio et al., 2000).
Materials and methods
Plant material
Plant material of P. campanulatus (4.9 kg) was collected in Desierto de Los Leones National Park, and that of P. gentianoides (10.1 kg) in Los Dinamos National Park, both in the mountains to west of Mexico City at 2890 m in March 2006. Samples were identified by Francisco Ramos, Instituto de Biología at UNAM, Ciudad Universitaria, Coyoacán, Mexico City. Voucher samples have been deposited in the Ethnobotanical Collection of the Herbarium of the Universidad Nacional Autónoma de Mexico, Instituto de Biología. Voucher: Francisco Ramos: MEXU, Gen: 7508, G-No. 7. All plant material was dried in shade.
Animals
Male Wistar rats (200–250 g body weight; 10 weeks old) were obtained from the Instituto de Fisiología Celular of the Universidad Nacional Autónoma de Mexico. Prior to the experiments the rats were fed with standard food and water ad libitum. All procedures and experiments reported here were carried out following the guidelines stated in Guide for the Care and Use of Laboratory Animals (CitationNIH, 1985; CitationNOM, 1999).
Chemicals and solvents
All reagents used were either analytical reagent (including TPA) or chromatographic grade, purchased from Sigma, Toluca, Mexico. Methanol, CH2Cl2, CHCl3, KCl, CuSO4, silica gel GF254 analytical chromatoplates, silica gel grade 60, (70-230, 60A°) for column chromatography, n-hexane, and ethyl acetate were purchased from Merck, Mexico City. Indomethacin (Sigma-Aldrich, Toluca) and ovatifolin obtained during a previous work (CitationCéspedes et al., 2001) were used as standard bioactive compounds.
Apparatus
1H-NMR spectra were recorded at 300 MHz, 13C-NMR at 75 at MHz, on a Varian VXR-300S spectrometer, chemical shifts (ppm) are relative to (CH3)4Si as internal reference, CDCl3 and acetone-d6 from Aldrich, Toluca, were used as solvents, coupling constants are quoted in Hz. IR spectra were obtained in KBr on a Perkin Elmer 283-B and an FT-IR Nicolet Magna 750 spectrophotometers. UV spectra of pure compounds were determined on a Shimadzu UV-160 spectrophotometer. A Spectronic model Genesys 5 spectrophotometer was used for measurement of biological activities. Melting points were obtained on a Fisher-Johns melting point apparatus and are uncorrected.
General experimental procedures
HPLC was performed on a Waters Model 600E chromatograph, equipped with Bondapack RP 18 column, 250 mm × 8 mm, at a flow rate of 1.5 mL/min, paper speed 5 mm/min, UV detector 280 nm, mobile phase MeOH/H20 7:3 v/v. Analytical TLC was performed on silica gel 60 F254 Merck plates (Mexico City). The plates were visualized by spraying with a 10% solution of H2SO4, followed by heating at 110°C.
Extraction and isolation of iridoid monoterpenes, flavonoids and phenylpropanoids
Plant material of P. gentianoides and P. campanulatus was separated into various morphological parts (roots, stems, leaves and flowers), which were dried (in shade), milled and extracted with methanol. The samples of plant material studied were based on previous reports concerning the popular use of the plants as ethnomedicines (CitationDomínguez et al., 2005, Citation2007; CitationJiménez-Estrada et al., 2006). As an example, leaves (of P. gentianoides) (1.2 kg) were extracted with methanol. The methanol extract (180 g) (A) was dried, redissolved in MeOH-H2O (6:4), and subsequently partitioned with n-hexane (C), CH2Cl2 (D) and ethyl acetate (E). The remaining liquid yielded a residue (B) (CitationDomínguez et al., 2007).
The ethyl acetate partition (46.22 g) exhibited strong antioxidant activity. The amorphous residue was further separated into eight fractions (I-VIII), by open column chromatography on silica gel (type G, 10-40 μm, Sigma-Aldrich, Toluca) (CitationDomínguez et al., 2005). The fractions were eluted with different ratios of n-hexane-ethyl acetate (from n-hexane 100% increasing in 10% with ethyl acetate until 100% of ethyl acetate and finally addition of MeOH (up to 100%) to increase the polarity of the gradient. Each fraction was analyzed by TLC using ceric sulfate as the visualization system.
A series of iridoid monoterpenes, flavonoids, and phenylpropanoids were isolated from various fractions by conventional phytochemical procedures and collected for bioassay. In each case, the compounds were separated by vacuum chromatography column with silica gel (type G, 10-40 µm, Sigma-Aldrich) using a CH2Cl2-Me2CO elution system (6:4). Another series of compounds were isolated from fractions by chromatography on Sephadex LH-20 with n-hexane-CH2Cl2-MeOH 2:1:1. Compounds 3 (110 mg), 6 (60 mg) and 7 (40 mg) were obtained from this type of column with CH2Cl2-MeOH (8:2). Compound 2 was isolated from crude methanol extract by column chromatography on Sephadex LH-20 using mixtures of EtOAc-MeOH (8:2) as the elution system.
The structural elucidation of each of these compounds has been previously reported (CitationDomínguez et al., 2007; CitationJiménez-Estrada et al., 2006). All data were compared to literature values, those of our data base, and with authentic samples.
Anti-inflammatory activity
The TPA-induced mouse ear edema assay was based on the described method (CitationTubaro et al., 1985; CitationMerlos et al., 1991; CitationDella Loggia et al., 1996). Groups of 5 male CD-1 mice (25–30 g, were used, 5 units due to their existence in the laboratory) were anesthetized with Imalgen®. A solution of 12-O-tetradecanoylphorbol-13-acetate (TPA, 2.5 μg) in acetone (10 μL) was applied topically to both faces (5 μL each face) of the right ear of the mice. The left ear faces received only acetone. Solutions of the extracts, compounds 1-7, diosmetin, luteolin, ovatifolin and indomethacin (as standard compounds) were prepared at concentrations of 0.05, 0.1 and 0.5 mg in 20 μL of acetone. These solutions were applied to both faces of the right ear (10 μL each face) 10 min after TPA treatment. The ears of control animals received only acetone. Four hours later, the animals were killed by cervical dislocation. A 9 mm diameter plug was removed from each ear. Swelling was assessed as the difference in weight between the right and left ear plugs. The percentage inhibition of edema was calculated by the equation: % = (edema A – edema B/edema A) × 100, where edema A = edema induced by TPA alone and edema B = edema induced by TPA plus sample.
Reduction of the 2,2-diphenyl-1-picrylhydrazyl radical
Extracts and partitions were chromatographed on TLC and examined for antioxidant effects by spraying the TLC plates with 2,2-diphenyl-1-picrylhydrazyl radical (DPPH) reagent. Specifically, the plates were sprayed with 0.2% DPPH in methanol. Quercetin and α-tocopherol were used as standards (CitationDomínguez et al., 2005).
Reduction of crocin radical
Each of the solutions above was placed under UV254 light. Following the decrease of absorbance, bleaching of crocin and fluorescence emission at 440 and 470 nm were monitored each 0.5 min (CitationCéspedes et al., 2008; CitationDomínguez et al., 2005).
Reduction of hydroxyl radical
Briefly, measurement of hydroxyl radical was based on the reduction of the absorbance of β-carotene treated with OH radicals generated from the reaction between hydrogen peroxide and ferrous ions (CitationSoares et al., 2003).
Statistical analysis
Data shown in consists of the mean results obtained from means of five animals and are presented as the percentage (%) of mean ± standard errors of the mean (SEM). Data were subjected to analysis of variance (ANOVA) with significant differences between means identified by GLM procedures and Student’s t-test analysis. The results are given in the text as probability values, with p <0.05 adopted as the criterion of significance, differences between treatment means were established with a Dunnett’s test. The EC50 values for each activity were calculated by Probit analysis on the basis of the percentage of inhibition obtained at each concentration of the samples. EC50 is the concentration producing 50% inhibition. All statistical analyses were performed by means of the MicroCal Origin 8.0 statistical and graphs PC program.
Table 1. Inhibitory effect of extracts and compounds 1-9 and indomethacin on the TPA-induced inflammation in a mouse modela.
Results and discussion
Structural determination of compounds 1-9
The identification of compounds was achieved by comparison of their spectroscopic and physical data with those previously published (CitationDomínguez et al., 2007; CitationJiménez-Estrada et al., 2006).
Anti-inflammatory activity
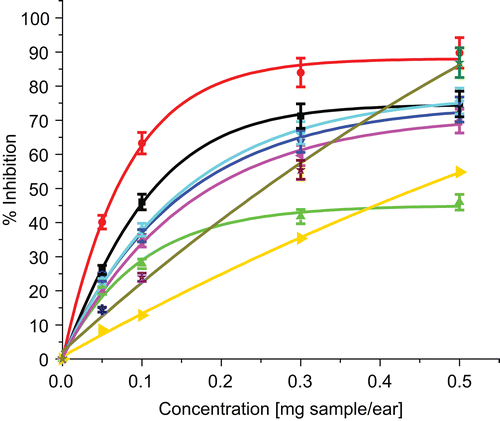
TPA-induced inflammation determined with the 12-O-tetradecanoylphorbol-13-acetate mouse ear method was inhibited most strongly by CH2Cl2 extracts of roots and stems from P. gentianoides and EtOAc extracts of leaves from P. campanulatus and P. gentianoides, with IC50 values of 0.07, 0.16, 0.18 and 0.15 mg/ear, respectively (, ). Luteolin, diosmetin, penstemide, catalpol, verbascoside and plantarenaloside exhibited an inhibition of 86.9, 54.77, 44.3, 29, 40.3 and 35.6% at 0.5 mg/ear, respectively. This bioassay was carried out at 0.1 and 0.5 mg/ear with all compounds. The CH2Cl2 extract from roots of P. gentianoides, luteolin, and diosmetin proved to be the most active samples, therefore a dose-response curve was made with these substances, which had IC50 values of 0.07, 0.25 and 0.45 mg/ear, respectively. Luteolin, diosmetin, and CH2Cl2 extract possessed dose-dependent anti-inflammatory activity that was compared to that produced by the commercially available anti-inflammatory drug indomethacin and a natural compound, ovatifolin, previously isolated from Podanthus ovatifolius Lag. (Asteraceae) (CitationCéspedes et al., 2001) (). Each of the compounds assayed inhibited TPA-induced inflammation at 0.5 mg/ear. The CH2Cl2 extract from roots of P. gentianoides was even more active than indomethacin, a selective cyclo-oxygenase (COX) inhibitor, at 0.1 mg/ear (). Decrease in the anti-inflammatory activity was observed when iridoid monoterpenes with sugar moieties at C-1, catalpol 1, penstemide 2, and plantarenaloside 4, were assayed. Surprisingly, penstemide, which also has a sugar moiety at C-1, had intermediate activity; at 0.5 mg/ear this iridoid glucoside showed 48.9% inhibition. A similar, but less potent activity was observed when diosmetin (with a 3-O-methoxy group) was used instead luteolin (which possesses a 3-O-hydroxyl group). The compounds pensteminoside 3 and globularisicin 5 were not examined in this assay due to minute amounts available (although they were examined in the accompanying antioxidant activity).
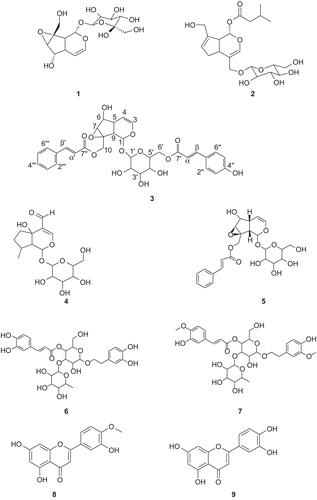
Figure 1. Chemical structures of catalpol 1, penstemide 2, pensteminoside 3, plantarenaloside 4, globularisicin 5, verbascoside 6, martinoside 7, diosmetin 8 and luteolin 9.
Interestingly, all extracts produced a curve of inhibition of logarithmic form, sometimes called the Monod function (CitationDockery & Klapper, 2001) (). The activity of the compounds follows a linear form (from 0 to 0.9 mg of sample), but at higher concentrations (>1 mg sample) the plot changes to a horizontal line. We are presently studying the kinetics of inhibition of these plant extracts and compounds ().
Figure 2. Percentage of inhibition of edema by extracts and compounds from Penstemon gentianoides and P. campanulatus. (▪) indomethacin, (•) CH2Cl2 extract from roots of P. gentianoides, (▴) MeOH extract from leaves of P. gentianoides, (▾) CH2Cl2 extract from stems of P. gentianoides, (♦) Ethyl acetate extract from leaves of P. gentianoides, (◂) ethyl acetate extract from leaves of P. campanulatus, (▸) diosmetin, (★) luteolin.
Antioxidant capacity radical scavenging properties
Radical scavenging properties of extracts and compounds 1-9 were evaluated against the DPPH radical, using DPPH as a TLC spray reagent. Compounds 6-9 (10 μM) appeared as yellow spots against a purple background, whereas the same amounts of compounds 1-5 did not react with the radical. Compounds 6-9 were also tested against DPPH in a spectrophotometric assay. This method confirmed that compounds 6-9 exhibited the strongest radical-scavenging activity (). Compounds 1-5 were less sensitive to oxidation than compounds 6-9.
Table 2. Amounts of phenolic content (mg/L ± standard error) from extracts and compounds from P. gentianoides and P. campanulatus needed to inhibit oxidative damage by 50%a.
The antioxidant activity of the methylene chloride extract from roots of P. gentianoides and compounds 1-9 was also evaluated spectrophotometrically by an assay involving bleaching of the H2O-soluble carotenoid crocin. Alkoxyl radicals were generated from t-BuOOH by UV photolysis of aqueous solutions containing 10 μM crocin and 1 mM t-BuOOH. t-BuOH (0.5 M) was added to scavenge the HO• radicals produced. Gallic acid was added as a reference compound. Compounds 6-9 were all active and exhibited activities comparable to that of gallic acid ().
Finally, compounds 6-9 showed the greatest capacity to scavenge hydroxyl radicals generated by the interaction between hydrogen peroxide, ferrous ions and β-carotene as percentage inhibition of β-carotene oxidation ().
It should be noted that the criteria used for measurement of antioxidant activity were valid with concentrations from 0 to 500 ppm. For extracts greater than 500 ppm, anti-inflammatory activity was not correlated with antioxidant activity.
Conclusions
The CH2Cl2 extract of root of P. gentianoides was the best inhibitor of inflammation (ED50 = 0.07 mg/ear) and had greater activity than the well-known COX inhibitor indomethacin (ED50 = 0.11 mg/ear) (). Interestingly, this extract had the lowest antioxidant activity (I50 >1000 ppm) (). We are presently examining the mechanism of action responsible for the anti-inflammatory activity. In this instance, we believe that the activity may be attributed to presence of steroidal compounds.
The flavonoids and phenylpropanoids that occur in these two Penstemon species have been considered as the active principles of other anti-inflammatory plants. Many iridoid monoterpenes and flavonoids have known inhibitory activity on the biosynthesis of nitric oxide, a compound implicated in physiological and pathological process such as chronic inflammation (CitationMatsuda et al., 2000; CitationOdontuya et al., 2005; CitationLópez-Lázaro, 2009).
Acknowledgements
We thank M. Teresa Ramírez-Apan, Luis Velasco and Rocio Patiño for technical assistance; Chemistry Institute, UNAM [Universidad Nacional Autonoma de Mexico]. The correspondence author is indebted to David S. Seigler, Plant Biology Department, University of Illinois at Urbana-Champaign, for his useful comments and assistance in the revision of this paper. This work was supported in part by grant MEXUS-CONACYT, PAPIIT-DGAPA-UNAM Grants: IN243802-2 and IN211105-3. We also acknowledge and are grateful for an internal grant of the Departamento de Ciencias Básicas, Universidad del Bío-Bío, Chile.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bowers MD. (1983). The role of inflammatory glycosides in host-plant specificity of checkerspot butterflies. J Chem Ecol, 9, 475–493.
- Céspedes CL, Hoeneisen M, Bittner M, Becerra J, Silva M. (2001). Comparative study of ovatifolin antioxidant and growth inhibition activities. J Agric Food Chem, 49, 4243–4251.
- Céspedes CL, El-Hafidi M, Pavón N, Alarcón J. (2008). Antioxidant and cardioprotective activities of phenolic extracts from fruits of Chilean blackberry Aristotelia chilensis (Elaeocarpaceae), maqui. Food Chem, 107, 820–829.
- Dockery J, Klapper I. (2001). Finger formation in biofilm layers. SIAM J Appl Math, 62, 853–869.
- Della Loggia R, Sosa S, Tubaro A, Morazzoni P, Bombardelli E, Griffini A. (1996). Anti-inflammatory activity of some Ginkgo biloba constituents and their phospholipid-complexes. Fitoterapia, 67, 257–264.
- De Young LM, Kheifets JB, Ballaron SJ, Young JM. (1989). Edema and cell infiltration in the phorbol ester-treated mouse ear are temporally separate and can be differentially modulated by pharmacologic agents. Inflamm Res, 26, 335–341.
- Domínguez M, Nieto A, Marín JC, Keck AS, Jeffery E, Céspedes CL. (2005). Antioxidant activities of extracts from Barkleyanthus salicifolius (Asteraceae) and Penstemon gentianoides (Scrophulariaceae). J Agric Food Chem, 53, 5889–5895.
- Domínguez M, Marín JC, Esquivel B, Céspedes CL. (2007). Pensteminoside, an unusual catalpol-type iridoid monoterpenes from Penstemon gentianoides HBK (Plantaginaceae). Phytochemistry, 68, 1762–1766.
- Jiménez-Estrada M, Saad I, Sánchez-Contreras A, Reyes-Chilpa R. (2006). Estudios sobre monoterpenos e iridoides. In: Romo de Vivar A, ed. Química de la Flora Mexicana. Investigaciones en el Instituto de Química, UNAM. Mexico City: Sociedad Química de Mexico, 39–62.
- Li J, Huang X, Du X, Sun W, Zhang Y. (2009). Study of chemical composition and antimicrobial activity of leaves and roots of Scrophularia ningpoensis. Nat Prod Res, 23, 775–780.
- López-Lázaro M. (2009). Distribution and biological activities of the flavonoids luteolin. Mini-Rev Med Chem, 9, 31–59.
- Matsuda H, Kagerura T, Toguchida I, Ueda H, Morikawa T, Yoshikawa M. (2000). Inhibitory effects of sesquiterpenes from bay leaf on nitric oxide production in lipopolysaccharide-activated macrophages: Structure requirement and role of heat shock protein induction. Life Sci, 66, 2151–2157.
- Merlos M, Gómez LA, Giral M, Vericat ML, Garcia-Rafanell J, Forn J. (1991). Effects of PAF-antagonists in mouse ear oedema induced by several inflammatory agents. Br J Pharmacol, 104, 990–994.
- NIH. (1985). Guide for the Care and Use of Laboratory Animals. NIH Publication No. 85-23, US Department of Health, Education and Welfare, Rockville, Maryland, USA.
- NOM. (1999). Mexican Official Guidelines “Norma Oficial Mexicana NOM-062-ZOO-1999”. Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación, [SAGARPA], Mexico DF, Mexico.
- Odontuya G, Hoult JRS Houghton, PJ. (2005). Structure-activity relationship for anti-inflammatory effect of luteolin and its derived glycosides. Phytother Res, 19, 782–786.
- Peñuelas J, Sardans J, Stefanescu C, Parella T, Filella I. (2006). Lonicera implexa leaves bearing naturally laid eggs of the specialist herbivore Euphydryas aurinia have dramatically greater concentrations of iridoid glycosides than other leaves. J Chem Ecol, 32, 1925–1933.
- Payá M, Ferrándiz ML, Sanz MJ, Bustos G, Blasco R, Rios JL, Alcaraz MJ. (1993). Study of the antioedema activity of some seaweed and sponge extracts from the Mediterranean coast in mice. Phytother Res, 7, 159–162.
- Recio MC, Giner RM, Uriburu L, Mañez S, Cerda M, De la Fuente JR, Rios JL. (2000). In vivo activity of pseudoguaianolide sesquiterpene lactones in acute and chronic inflammation. Life Sci, 66, 2509–2518.
- Seigler DS. (1998). Plant Secondary Metabolism. Norwell, MA: Kluwer Academic Publishers.
- Soares DG, Andreazza AC, Salvador M. (2003). Sequestering ability of butylated hydroxytoluene, propyl gallate, resveratrol, and vitamins C and E against ABTS, DPPH, and hydroxyl free radicals in chemical and biological systems. J Agric Food Chem, 51, 1077–1080.
- Tubaro A, Dri P, Delbello G, Zilli C, Della Loggia R. (1985). The croton oil ear test revisited. Agents Actions, 17, 347–349.