Abstract
Objective: Fruits of Bunium persicum (Boiss.) B. Fedtsh. (Apiaceae) are widely used in Iranian folkloric medicine for controlling colic pain and dysmenorrhea. This study was aimed to evaluate the anti-inflammatory and analgesic effects of the plant fruits and analyzing its essential oil.
Materials and methods: Hydroalcoholic extract, polyphenolic extract and essential oil were prepared using standard methods. The acetic acid and formalin tests were used in male mice (25-35 g) to assess analgesic activity. For evaluation of anti-inflammatory effect, carrageenan-induced rat paw edema and croton oil-induced ear edema were used.
Results: Hydrodistillation of the fruits of B. persicum afforded a pale yellowish oil in a yield of 2%. GC/MS analysis identified 10 compounds, and gamma-terpinene (46.1%), cuminal (23.9%) and p-cymene (15.9%) were the main components. Hydroalcoholic and polyphenolic extracts (400 and 800 mg/kg, i.p.) and essential oil (100-400 µl/kg, p.o.) significantly (p < 0.01) reduced acetic acid-induced writhings. They also significantly reduced the pain response of both early and late phases of the formalin test. In the carrageenan test and croton oil-induced ear edema both extracts and essential oil showed considerable anti-inflammatory response.
Discussion and conclusions: These results clearly show the analgesic and anti-inflammatory effects of the plant fruits, and since extracts and essential oil relieved the pain of the first phase of the formalin test, it seems that at least a part of analgesic activity is mediated centrally. Meanwhile, the effects observed in this study provide evidence for folkloric uses of the plant fruits in painful and inflammatory conditions.
Introduction
Bunium persicum (Boiss) B. Fedtsh or Carum persicum Boiss. is a grassy plant of Apiaceae family with the common name of wild Caraway. It has pink or white flowers with small brownish seeds and grows in warm climate areas of Iran (CitationGhahraman, 1993) and also in central Asia, Afghanistan, Pakistan, Pamir and Kashmir (CitationZargari, 1996).
In Iran the fruits or the aerial parts of the plant have been used traditionally as anticonvulsant, antihelmintic, anti-asthma, digestant, antiflatulent, diuretic and analgesic (CitationGhasemi, 2002).
The fruits contain about 2% (v/w) essential oil, and caryophyllene, γ-terpinene and cuminyl acetate are the major components (CitationShahsavari et al., 2008). Antifungal (CitationSekine et al., 2007), antibacterial (CitationSyed & Hanif, 1985), antioxidant (CitationShahsavari et al., 2008) and antihistaminic activity (CitationBoskabady & Moghadas, 2004) have been reported for this plant.
In Iran, Afghanistan, India and Tajikistan, dried fruits of B. persicum are widely used as a spice. Since the plant is a well-known flavoring agent and spice, and also based on Iranian folkloric claims regarding analgesic activity of the plant fruits, the purpose of the present study was to evaluate the analgesic and anti-inflammatory activities of the essential oil and extracts of the plant fruits in mice and rats using the formalin, acetic acid-induced writhing, carrageenan-induced rat paw edema and croton oil-induced ear edema tests. In addition, we describe the identification of the oil constituents by gas chromatography mass spectrometry (GC/MS) analyses.
Materials and methods
Plant material and preparation of extracts and essential oil
Fruits of B. persicum were purchased from Pakan Bazr Seed Producer (Isfahan, Iran). The identity of the fruits was confirmed at Department of Botany of Isfahan University (Isfahan, Iran). For preparation of hydroalcoholic extract, 1500 mL of EtOH: H2O (8:2) was added to powdered fruits of the plant (250 g) and left for 48 h. The extract was then filtered and the solvent was removed using a vacuum evaporator until a semi-solid extract was obtained (yield 10%) (CitationSajjadi et al., 1998). For preparation of polyphenolic extract, powdered fruits of the plant were extracted three times with EtOH:H2O (8:2). Each time enough solvent was added to make a liquid slurry and the mixture was left for 48 h. The extracts were then combined and evaporated to about one third of the initial volume. The resultant solution then was cleared by extraction in a separating funnel with chloroform and then evaporated to dryness under reduced pressure in a rotating evaporator (CitationSajjadi et al., 1998). Evaporation and solvent removal of polyphenolic fraction gave a semi-solid mass (yield 9%).
The essential oil was prepared by the hydro-distillation method recommended in the European Pharmacopoeia (CitationCoE, 2002). The volatile oil was stored at 4°C before use.
Analysis of the essential oil
The essential oil was analyzed by GC/MS using a Hewlett Packard 6890 mass selective detector coupled with a Hewlett Packard 6890 gas chromatograph, equipped with a cross-linked 5% PH ME siloxane HP-5MS capillary column (30 m × 0.25 mm, film thickness 0.25 μm). Operating conditions were as follows: carrier gas, helium with a flow rate of 2 mL/min; column temperature, 60°–275°C at 4°C/min; injector and detector temperatures, 280°C; volume injected, 0.1 μL of the oil; split ratio, 1:50. The MS operating parameters were as follows: ionization potential, 70 eV; ionization current, 1 A; ion source temperature, 200°C; resolution, 1000.
Identification of the components in the oil was based on retention indices relative to n-alkanes and computer matching with the Wiley 275.L library, as well as by comparison of the fragmentation patterns of the mass spectra with those reported in the literature (CitationAdams, 1995; CitationSandra & Bicchi, 1987).
Animals
Pain tests and croton oil-induced ear edema were carried out on male Swiss mice (25-35 g). Male Wistar rats (160-200 g) were used for the carrageenan test. Animals were housed in groups of six per standard cage, on a 12 h light/dark cycle; and air temperature was maintained at 22° ± 2°C with free access to food and water ad libitum. They were acclimatized to laboratory conditions for at least one week before testing. All experiments were performed according to guidelines for the care of laboratory animals of the Ethics Committee of Isfahan University of Medical Sciences.
Acetic acid-induced writhing test
This test was carried out according to the method of CitationKoster et al. (1959). Groups of mice (n = 8) received different doses of hydroalcoholic (200-800 mg/kg, i.p.) or polyphenolic (200-800 mg/kg, i.p.) or essential oil (100-400 µL/kg, p.o.) prior to an intraperitoneal injection of 1% acetic acid in a volume of 10 mL/kg. Control group received vehicle (10 mL/kg of 1% solution of Tween 80). Indomethacin (10 mg/kg, i.p.) was used as the reference drug.
Formalin test
The test was performed according to CitationHunskaar and Hole (1987). Briefly, 30 min after i.p. injections of vehicle, reference drug (morphine, 10 mg/kg), above-mentioned doses of the hydroalcoholic or polyphenolic extracts, or 45 min after oral administration of different doses of the essential oil, 20 μL of 2.5% formalin (v/v in 0.9% saline) was injected into the subplantar space of the right hind paw and the time spent for paw licking was determined 0-5 min (first phase) and 20-30 min (second phase) after formalin.
Carrageenan-induced rat paw edema
The anti-inflammatory activity was evaluated by the carrageenan-induced paw edema test in the rat (CitationWinter et al., 1962). After a light anesthesia with ether, animals received a subplantar injection of 0.1 mL of 1% (w/v) suspension of carrageenan in isotonic saline into their right hind paw. The left hind paws were injected with 0.1 mL saline and used as control. Paw volume was measured prior and 4 h after carrageenan administration using a mercury plethysmorgraph (Ugo Basile, Comerio, Italy).
Essential oil was emulsified by Tween 80 (1% v/v) and administered orally 1 h prior to carrageenan injection. Hydroalcoholic and polyphenolic extracts were injected i.p. 30 min prior to carrageenan. The control group received an equal volume of the vehicle. Indomethacin (10 mg/kg, i.p.) was used as positive control.
Croton oil-induced ear edema
This test was carried out as described previously (CitationTubaro et al., 1986). An acetone solution of croton oil (100 μg/ 15 μL) was carefully applied to the inner surface of the right ear of each mouse. The left ear remained untreated. Vehicle (10 mL/kg), different doses of the hydroalcoholic extract (200-800 mg/kg), polyphenolic extract (200-800 mg/ kg), essential oil (100-400 μL/kg) or indomethacin (10 mg/ kg) were administered 30 or 45 min prior to croton oil application. Essential oil was given orally and other treatments were i.p. Six hours after croton oil, the animals were sacrificed by cervical dislocation, and plugs (6 mm in diameter) were removed from both ears and weighed. The weight difference of the two plugs was taken as an index of edema formation.
Statistical analysis
Data were analyzed by SPSS (version 13) using one way analysis of variance (ANOVA) followed by Duncan test. The results are expressed as mean ± SEM and were considered significant at p <0.05.
Results
Analysis of the essential oil
The fruits of the plant yielded 2% (v/w) of a pale yellowish essential oil. Ten components were characterized by GC/MS, representing 99.8% of the total oil components detected, which are listed in with their percentage composition and retention indices. Gamma-terpinene (46.1%), cuminal (23.9%) and p-cymene (15.9%) were the main components.
Table 1. Percentage composition of the oil of Bunium persicum fruits.
Pharmacological study
In the acetic acid-induced writhing test, B. persicum hydroalcoholic extract (BPHE) and polyphenolic extract (BPPE) at doses of 400 and 800 mg/kg significantly (p < 0.001) inhibited abdominal twitches. B. persicum essential oil (BPEO) also significantly (p <0.01 for a dose of 100 μL/kg and p <0.001 for doses of 200 and 400 μL/kg) reduced abdominal writhes in a dose-dependent manner. In this test indomethacin, a reference drug at a dose of 10 mg/kg produced about 83% reduction in writhes ().
Table 2. Effect of Bunium persicum extracts and essential oil on acetic acid-induced writhing in mice.
The results of the formalin test are summarized in . Both extracts (BPHE and BPPE) and also BPEO significantly reduced paw licking time of both phases of formalin test. The antinociceptive effect of the polyphenolic extract at doses of 400 and 800 mg/kg was not significantly different and it seems that a dose of 400 mg/ kg produced the maximum response. A dose of 400 μL/ kg of B. persicum essential oil also did not produce a significantly greater response than a dose of 200 μL/kg. Morphine, a standard analgesic drug, also exhibited significant (p <0.001) reduction of paw licking time in both phases of formalin test.
Table 3. Effect of Bunium persicum extracts and essential oil in the formalin test (n = 8).
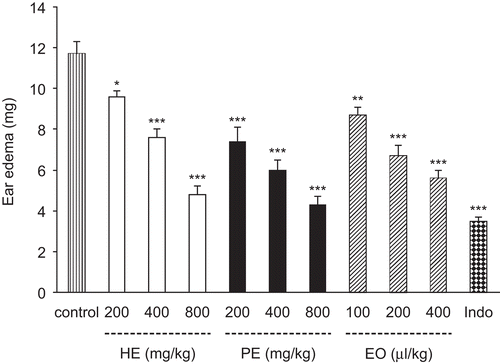
HPHE at administered doses (200-800 mg/kg) showed significant (p < 0.001) anti-inflammatory effect in carrageenan bioassay, and its potency at a dose of 200 mg/kg was not significantly different from that of indomethacin (10 mg/kg), the reference drug. Both BPPE and BPEO exhibited a dose-dependent inhibition of carrageenan-induced paw edema (). The results of croton oil-induced ear edema showed dose-dependent reduction of ear edema. BPHE at doses of 200, 400 and 800 mg/kg produced 19%, 35% and 59% inhibition of ear edema respectively and BPPE at the same doses caused 37%, 49% and 63% inhibition of ear edema. The percentage inhibitions of oral administration of B. persicum essential oil at doses of 100, 200 and 400 μL/kg were 26, 43 and 52%, respectively. Indomethacin (10 mg/kg) produced 70% inhibition of croton oil-induced inflammation and this effect was not statistically different from those observed with the maximum doses of BPHE, BPPE and BPEO ().
Table 4. Effect of Bunium persicum extracts and essential oil on carrageenan-induced rat paw edema (n = 8).
Figure 1. Effect of Bunium persicum extracts and essential oil on croton oil-induced ear edema in mice. Vehicle, extracts, essential oil or indomethacin (10 mg/kg) were administered 30 or 45 min prior to croton oil application (100 μg/ear). Essential oil was given orally and other treatments were i.p. Data are mean ± SEM of edema. *P <0.05; **P <0.01; ***P <0.001 compared with control group. HE, hydroalcoholic extract; PE, polyphenolic extract; EO, essential oil; Indo, indomethacin.
Discussion
Pharmacognosy
In the present study GC/MS analysis of B. persicum essential oil identified 10 compounds, and gamma-terpinene (46.1%), cuminal (23.9%) and p-cymene (15.9%) were the major components. In another study it was shown that caryophyllene (27.8%) was the main component of BPEO (CitationShahsavari et al., 2008). It has been reported that the composition of any plant essential oil is influenced by several factors including local, climatic, seasonal, harvesting, storage and experimental conditions (CitationDaferera et al., 2000) and these factors may explain the differences between our results and previous work.
Pharmacology
In the present study, B. persicum extracts and essential oil showed analgesic activity in acetic acid-induced writhing and formalin tests. Although the pain in the abdominal writhes induced by acetic acid is not a specific model, the involuntary muscle twitches of the abdomen may be of interest because of their similarity with some of those known in visceral disorders (CitationLe Bars et al., 2001; CitationVogel & Vogel, 1997). Also, it has been proposed that acetic acid acts indirectly by inducing the release of endogenous mediators, which stimulate the nociceptive neurons sensitive to non-steroidal anti-inflammatory drugs (NSAIDS) and opioids (CitationCollier et al., 1968).
In the formalin pain model, our results substantiate the typical biphasic behavioral response seen after s.c. formalin injection in rodents (CitationHunskaar & Hole, 1987). High nociceptive scores were recorded during the first 5 min after s.c. formalin administration and were followed by a reduction in scores for several minutes. A later licking response was also seen 20 min after formalin injection. The early phase seems to be caused predominantly by C-fiber activation due to the peripheral stimulus, while the late phase appears to be dependent on the combination of an inflammatory reaction in the peripheral tissue and functional changes in the dorsal horn of the spinal cord. These functional changes seem to be initiated by the C-fiber barrage during the early phase (CitationTjolsen et al., 1992).
Our results demonstrated that both hydroalcoholic and polyphenolic extracts and also essential oil of B. persicum reduced the pain response of both phases of formalin test. The first phase reveals an acute pain and it has been reported that centrally acting analgesic drugs such as opioids can inhibit the pain response of the first phase of the formalin test (CitationChen et al., 1995; CitationElisabetsky et al., 1995). Since extracts and essential oil of B. persicum were effective in suppression of this phase of formalin test, it seems that at least a part of analgesic activity of B. persicum fruits is mediated centrally. The late (second) phase is inflammatory in origin (CitationChen et al., 1995; CitationElisabetsky et al., 1995) and the extracts and essential oil of B. persicum were also active in suppression of the pain response of this phase and therefore it may indicate an anti-inflammatory effect of the fruits of the plant. Results obtained from carrageenan-induced paw and croton oil-induced ear edemas, which are valid animal models for assessing anti-inflammatory activity, also confirm the anti-inflammatory effect B. persicum.
In this study the yields of hydroalcoholic and polyphenolic extracts were 10% and 9%, respectively, and equal doses of these extracts produced almost equal antinociceptive and anti-inflammatory effects, and since hydroalcoholic extract also contains the polyphenolic compounds it seems that these compounds are responsible for observed pharmacological effects. The results of the present study concerning analgesic and anti-inflammatory effects of BPPE confirmed our previous studies that showed these effects for flavonoids and polyphenolic compounds of other plants (CitationGhannadi et al., 2005; CitationHajhashemi et al., 2002, Citation2003, Citation2009).
It has been shown that flavonoids and polyphenolic compounds have several pharmacological effects including inhibition of arachidonic acid metabolism (Amresh et al., Citation2007b), inhibition of histamine release from mast cells (Amresh et al., Citation2007a), and antioxidant activity (CitationBors & Saran, 1987). On the other hand it has been reported that carrageenan induces biphasic edema response. In the first phase (0-2.5 h) mediators such as histamine, serotonin and kinins are released and affect the vascular permeability, and the second phase is associated with increased production of prostaglandins and oxygen-derived free radicals (CitationAntonio & Souza Brito, 1998; CitationPanthong et al., 2004). Therefore, the anti-inflammatory effects of BPPE observed in our work may be explained by one or more of the above mechanisms and further studies are required to find the exact mechanism of action.
BPEO clearly showed analgesic and anti-inflammatory activity. Previous studies have shown analgesic activity for p-cymene (CitationIllouz & Delbarre 1964; CitationDuke et al., 2002) and anti-inflammatory and antioxidant activities for γ-terpinene (CitationMilde et al., 2004; CitationDuke et al., 2002) and since these two compounds are among the major compounds of BPEO, they may be responsible for at least a part of the observed effects. CitationShahsavari et al. (2008) reported antioxidant effect for BPEO. On the other hand it has been reported that free radicals are involved in the inflammatory process (CitationAruoma et al., 2006; CitationSoteras, 1999), and therefore the antioxidant property of BPEO may have some role in its anti-inflammatory activity.
Based on above results, it can be concluded that B. persicum has considerable anti-infiammatory and analgesic activities, and this study provides pharmacological evidence for its folkloric use in painful and inflammatory conditions. However, further studies are needed to determine the possible mechanism of action of these fractions and their potential for clinical use needs to be demonstrated in clinical trials.
Declaration of interest
This work was supported by the Research Council of the Isfahan University of Medical Sciences, Isfahan, Iran (387312). The authors alone are responsible for the content and writing of the paper.
References
- Adams RP. (1995). Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy. Carol Stream, IL: Allured Publishing.
- Amresh G, Reddy GD, Rao C, Singh PN. (2007a). Evaluation of anti-inflammatory activity of Cissampelos pareira root in rats. J Ethnopharmacol, 110, 526–531.
- Amresh G, Zeashan H, Rao ChV Singh, PN. (2007b). Prostaglandin mediated antiinflammatory and analgesic activity of Cissampelos pareira. Acta Pharm Sci, 49, 153–160.
- Antonio MA, Souza Brito AR. (1998). Oral anti-inflammatory and anti-ulcerogenic activities of a hydroalcoholic extract and partitioned fractions of Turnera ulmifolia (Turneraceae). J Ethnopharmacol, 61, 215–228.
- Aruoma OI, Grootveld M, Bahorun T. (2006). Free radicals in biology and medicine: From inflammation to biotechnology. Biofactors, 27, 1–3.
- Bors W, Saran M. (1987). Radical scavenging by flavonoid antioxidants. Free Radic Res Commun, 2, 289–294.
- Boskabady MH, Moghadas A. (2004). Inhibitory effect of Bunium persicum on histamine (H1) receptors of guinea pig tracheal chains. Phytomedicine, 11, 411–415.
- Chen YF, Tsai HY, Wu TS. (1995). Anti-inflammatory and analgesic activity from roots of Angelica pubescens. Planta Med, 61, 2–8.
- Collier HO, Dinneen LC, Johnson CA, Schneider C. (1968). The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br J Pharmacol Chemother, 32, 295–310.
- Daferera DJ, Ziogas BN, Polissiou MG. (2000). GC-MS analysis of essential oils from some Greek aromatic plants and their fungitoxicity on Penicillium digitatum. J Agric Food Chem, 48, 2576–2581.
- Duke JA, Bogenschutz-Godwin MJ, Ducellier J. (2002). CRC Handbook of Medicinal Spices. Boca Raton, FL: CRC Press.
- Elisabetsky E, Amador TA, Albuquerque RR, Nunes DS, Carvalho ACT. (1995). Analgesic activity of Psychotria colorata Muell. alkaloids. J Ethnopharmacol, 48, 77–83.
- CoE. (2002). European Pharmacopoeia. Strasbourg, France: Council of Europe.
- Ghahraman A. (1993). Plant Systematics. Cormophytes of Iran. Tehran, Iran: Nashre Daneshgahi.
- Ghannadi A, Hajhashemi V, Jafarabadi H. (2005). An investigation of the analgesic and anti-inflammatory effects of Nigella sativa seed polyphenols. J Med Food, 8, 488–493.
- Ghasemi N. (2002). Iranian Herbal Pharmacopoeia. Tehran, Iran: Ministry of Health and Medical Education.
- Hajhashemi V, Ghannadi A, Pezeshkian SK. (2002). Antinociceptive and anti-inflammatory effects of Satureja hortensis L. extracts and essential oil. J Ethnopharmacol, 82, 83–87.
- Hajhashemi V, Ghannadi A, Sharif B. (2003). Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J Ethnopharmacol, 89, 67–71.
- Hajhashemi V, Sajjadi SE, Heshmati M. (2009). Anti-inflammatory and analgesic properties of Heracleum persicum essential oil and hydroalcoholic extract in animal models. J Ethnopharmacol, 124, 475–480.
- Hunskaar S, Hole K. (1987). The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain, 30, 103–114.
- Illouz G, Delbarre F. (1964). Para-cymene, an analgesic drug with percutaneous action. Sem Hop, 40, 2902–2903.
- Koster R, Anderson M, De Beer EJ. (1959). Acetic acid for analgesic screening. Fed Proc, 18, 412–417.
- Le Bars D, Gozariu M, Cadden SW. (2001). Animal models of nociception. Pharmacol Rev, 53, 597–652.
- Milde J, Elstner EF, Grassmann J. (2004). Synergistic inhibition of low-density lipoprotein oxidation by rutin, gamma-terpinene, and ascorbic acid. Phytomedicine, 11, 105–113.
- Panthong A, Kanjanapothi D, Taesotikul T, Phankummoon A, Panthong K, Reutrakul V. (2004). Anti-inflammatory activity of methanolic extracts from Ventilago harmandiana Pierre. J Ethnopharmacol, 91, 237–242.
- Sajjadi SE, Movahedian-Atar AM, Yektaian A. (1998). Antihyperlipidemic effect of hydroalcoholic extract and polyphenolic fraction from Dracocephalum Kotschyi Boiss. Pharm Acta Helv, 73, 167–170.
- Sandra P, Bicchi C. (1987). Capillary Gas Chromatography in Essential Oil Analysis. Heidelberg, Germany: Huethig.
- Sekine T, Sugano M, Majid A, Fujii Y. (2007). Antifungal effects of volatile compounds from black zira (Bunium persicum) and other spices and herbs. J Chem Ecol, 33, 2123–2132.
- Shahsavari N, Barzegar M, Sahari MA, Naghdibadi H. (2008). Antioxidant activity and chemical characterization of essential oil of Bunium persicum. Plant Foods Hum Nutr, 63, 183–188.
- Soteras F. (1999). Free radicals and their role in inflammation. Arch Bronconeumol, 35: S3–9.
- Syed M, Hanif M. (1985). Antibacterial activity of the essential oil of the Umbellifera family. Part 1. Cuminum cyminum, Coriandrum sativum, Foeniculum vulgare and Bunium persicum oils. Pak J Sci Ind Res, 55, 116–120.
- Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K. (1992). The formalin test: An evaluation of the method. Pain, 51, 5–17.
- Tubaro A, Dri P, Delbello G, Zilli C, Della LR. (1986). The croton oil ear test revisited. Agents Actions, 17, 347–349.
- Vogel HG, Vogel WH. (1997). Drug Discovery and Evaluation. Berlin: Springer.
- Winter ChA Risley, EA, Nuss GW. (1962). Carrageenan-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med, 111, 544–547.
- Zargari A. (1996). Medicinal Plants. Tehran, Iran: Tehran University Press.