Abstract
Context: Byrsocarpus coccineus Schum. and Thonn. (Connaraceae) is a scandent shrub widely employed as a medicinal remedy for various disease conditions in West Africa.
Objective: This study evaluated fractions of B. coccineus for modulation of cytochrome P450 (CYP) enzyme activity, cytokine production, and proliferation.
Materials and methods: The BROD (benzyloxyresorufin O-debenzylase) and BFCOD (benzyloxy-4-[trifluoromethyl]-coumarin O-debenzyloxylase) assays were used to evaluate effect on CYP2B1/2 and CYP3A4 enzyme activity. Effects on cytokine production and proliferation of HT29 cells were investigated using interferon expression assay and MTT (3-3[4,5-dimethyl-2-thiazolyl]-2-5-diphenyl-2H-tetrazolium bromide) assay, respectively.
Results: Fractions derived from the organic solvent extraction of B. coccineus produced significant (p < 0.05) stimulation of human hepatic CYP2B1/2 activity in the BROD assay. The greatest effects were elicited at 1 ng/mL corresponding to ∼3-fold stimulation of enzyme activity. Enhancement of CYP3A4 enzyme activity was also observed in the BFCOD assay. Other fractions from the organic extract showed significant antiproliferative effects on HT29 cells at 100 μg/mL. Fractions obtained from the aqueous extract of B. coccineus (1 µg/µL) significantly stimulated the expression of IFNα2a and IFNβ in peripheral blood mononuclear cells (PBMC), causing a maximum 26-fold increase of IFNα2a-transcript.
Discussion and conclusion: The effect on CYP suggests that B. coccineus may reduce the therapeutic efficacy of co-administered drugs. This justifies the need for proper education of patients by healthcare practitioners on the outcomes of drug–herb interactions. This study identifies several in vitro activities that could underlie the attributed uses of this plant in traditional African medicine (TAM).
Introduction
Byrsocarpus coccineus Schum. and Thonn. (Connaraceae) is a plant commonly found across west and tropical Africa. It is a scandent shrub of savanna thickets and secondary jungle with delicate pink-tinged foliage and sweet-scented flowers (CitationBurkill, 1985). Local names in Nigeria include “tsaamiyar-kasa” (Hausa, north), “oke abolo” (Igbo, East), and “orikoteni” (Yoruba, southwest) (CitationBurkill, 1985). In addition to its popular use as an ornamental plant, various preparations of the plant using leaves, roots (scraped bark and sap) and whole plant have been used to treat diverse ailments. The decoction or infusion of the leaf of the plant has been used for skin and mouth disorders, German measles, jaundice, gonorrhea, urinary problems, impotence, anemia, primary and secondary sterility, blennorrhagia, tachycardia, and as an abortifacient (CitationNeuwinger, 1996). The plant has also been used for swellings and tumors, hemorrhage and as an emetic (CitationBurkill, 1985; CitationAdjanohoun et al., 1986; CitationNeuwinger, 1996). In previous studies, the in vivo analgesic (CitationAkindele & Adeyemi, 2006a), antidiarrhea (CitationAkindele & Adeyemi, 2006b), antipyretic (CitationAkindele & Adeyemi, 2007) and anxiolytic/sedative (CitationAkindele & Adeyemi, 2010) activities of the leaf aqueous extract of the plant have been investigated and reported.
In the present study, we investigate the effects of B. coccineus on human immune cells and a human cancer cell line in order to rationalize the reported use of this plant for tumors. Interferons are cytokines produced by some immune system cells. They possess antiviral, antiseptic, antiproliferative and immunostimulatory activities (CitationKhan et al., 1998; CitationUematsu & Akira, 2007). Both type I interferons (IFNα and β) and interferon γ have been shown to play a role in the host immune response to cancer (CitationDunn et al., 2006). Interferon therapy is used in conjunction with chemotherapy and radiation in the treatment of many cancers (CitationGilewski & Golomb, 1990; CitationClark & Weiner, 1995; CitationUematsu & Akira, 2007).
In African countries and developing countries of other continents of the world, self-medication and combination therapy using western medicines and traditional herbal medicines are frequent (CitationAgbonon et al., 2010), based on personal conviction of synergistic or additive pharmacological effect (CitationPekthong et al., 2008). Patients often do not report herbal and alternative medicine use to physicians (CitationShakeel et al., 2010), but there are a number of examples where interactions between herbal medicines and drugs can be clinically significant. Many case reports exist on irregular bleeding and unwanted pregnancies when St. John’s wort (Hypericum perforatum L. (Clusiaceae); an antidepressant herb) is taken concurrently with ethynylestradiol (CitationSchwarz et al., 2003). Clinically significant interactions have also been reported from the use of grapefruit juices with calcium antagonists, antihistamine and benzodiazepine treatment (CitationAmeer & Weintraub, 1997; CitationBailey et al., 1998; CitationKupferschmidt et al., 1998). Based on its potential for use along with conventional drugs and in view of the many examples of herbal remedies altering expression or activity of hepatic CYP450 enzymes, thus affecting beneficially or adversely the impact of co-administered drugs, an aim of this study was to investigate the effects of extracts and derived fractions of B. coccineus on cytochrome P450 enzyme activity. The hepatic microsomal cytochrome P450 group of enzymes is responsible for the metabolism of many xenobiotics and its modulation is the most common cause of drug−drug and food−drug interactions (CitationGirennavar et al., 2007).
Materials and methods
Plant material
Fresh B. coccineus plant was collected from Iju-Ogundimu a town in Ifako-Ijaiye local government area of Lagos State, Nigeria, in the month of June 2007. Botanical identification and authentication was carried out by J.D. Olowokudejo Professor of the Department of Botany, Faculty of Science, University of Lagos, and T.K. Odewo, Senior Superintendent of the Forestry Research Institute of Nigeria (FRIN), Ibadan. The voucher specimen (FHI 106623) was deposited in the herbarium of the Forestry Research Institute.
Extraction and fractionation
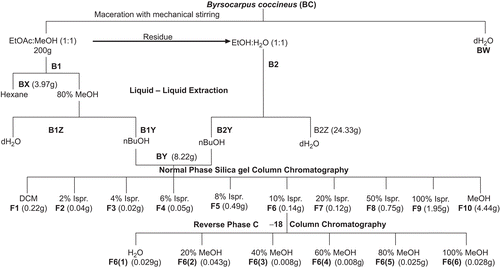
Fresh leaves of B. coccineus were air-dried until a constant weight was obtained. The dried material (200 g) was macerated with EtOAc−MeOH (1:1) for 48 h with mechanical stirring. The filtered supernatant was dried down to produce extract B1. The remaining solid residue was macerated with EtOH−H2O (1:1), also for 48 h with mechanical stirring. The filtered supernatant was dried down to produce extract B2 (see ). Another portion of the dried material (200 g) was macerated with H2O for 48 h with mechanical stirring (extract BW). Extract B1 was partitioned between hexane (fraction BX) and MeOH−H2O (80:20). The 80% MeOH fraction was then partitioned between BuOH (fraction B1Y) and H2O (fraction B1Z). Extract B2 was partitioned directly between BuOH (fraction B2Y) and H2O (fraction B2Z). B1Y and B2Y were then combined (8.22 g) and subjected to normal phase silica gel chromatography using a gradient system of increasing iPrOH in CH2Cl2 (0, 2, 4, 6, 8, 10, 20, 50, and 100% iPrOH) to obtain fractions F1–F9. The column was washed with MeOH to obtain fraction F10. Based on bioactivity obtained, fraction F6 (0.14 g) was separated to give six subfractions (F6(1)−F6(6)) through reversed phase C-18 column chromatography using a gradient system of increasing MeOH in H2O (20, 40, 60, 80, and 100% MeOH). The extracts and fractions were used in the cytochrome P450 activity study (BFCOD and BROD assays) and the MTT cell viability assay.
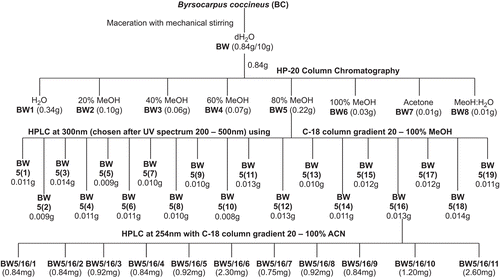
BW (0.66 g) was subjected to HP-20 column chromatography with H2O, and 20, 40, 60, 80, and 100% MeOH to obtain six fractions (BW1-BW6, see ). Fractions BW7 and BW8 were obtained with acetonitrile (ACN) and H2O−MeOH backward wash, respectively. Fraction BW5 was further fractionated by high performance liquid chromatography (HPLC) (Discovery C18 column, 5 μm particle size, 250 × 4.6 mm, Supelco; flow rate, 1.0 mL/min; detection by UV at 300 nm) using a linear MeOH−H2O gradient (20−100% MeOH over 60 min) to obtain 19 fractions (BW5(1)−BW5(19)) (see ). The extracts and fractions were used in the interferon expression study.
The choice of assays carried out on fractions was based on the bioactivity of the crude extracts.
Cytochrome P450 enzyme activity assay
The fluorogenic substrates benzyloxyresorufin (BR) and 7-benzyloxy-4-trifluoromethylcoumarin (BFC), selective for CYP2B1/2 and CYP3A4, respectively (CitationPekthong et al., 2008; CitationHuber et al., 2008; CitationStresser et al., 2000), were synthesized from the reactions of resorufin or trifluoromethylcoumarin with benzyl iodide (CitationPrough et al., 1978) and their identities were verified by 1H NMR. BR and BFC were purified to >99% pure as evaluated by HPLC. NADPH and other reagents were purchased from Sigma-Aldrich, St. Louis, MO. Human liver microsomes were obtained from de-identified transplant-quality liver obtained from the Department of Surgery, University of Florida, under a protocol approved by the Institutional Review Board.
The BROD assay measured the conversion of BR to resorufin (CitationPrough et al., 1978; CitationPohl & Fouts, 1980), while the BFCOD assay measured the conversion of BFC to 7-hydroxy-4-trifluoromethylcoumarin (HFC, CitationRenwick et al., 2000; CitationStresser et al., 2000). In a volume of 1 mL, reactions contained 100 μL test sample dissolved in methanol (evaporated under N2), 5 μM BR or 100 μM BFC, 0.1 M HEPES, pH 7.6, 2% BSA, 2 mM NADPH, and 0.25 mg (BROD) or 2 mg (BFCOD) human liver microsomes. All components except for NADPH were equilibrated at 37°C for 1 min before NADPH addition. Reactions were incubated for 10 min at 37°C before they were stopped by addition of 2.5 mL (BROD) or 3 mL (BFCOD) ice cold MeOH. The mixtures were left to stand for 20 min while protein flocculation occurred, then centrifuged for 15 min at 600 g. Reaction products were measured by fluorescence (λex/λem 550/585 nm for resorufin in the case of the BROD assay; 410/530 nm for HFC in the case of the BFCOD assay). Standard curves of known concentrations of the products were used to convert fluorescence readings to product concentrations. Specific activities were calculated as shown below. EC50 values were calculated by non-linear curve-fitting using GraphPad Prism 4 (GraphPad Software, CA).
Immunomodulation assay
Cell culture
Human peripheral blood mononuclear cells (PBMC) were isolated from the peripheral blood of healthy donors by Ficoll-Hypaque density gradient centrifugation (leukopack, peripheral blood leukocyte (PBL)) obtained from Lifesouth Community Blood Center (Gainesville, FL) using Lymphoprep (Axis-Shield, Oslo, Norway). Briefly, the contents of the leukopac were diluted three times its volume in sterile 1× phosphate buffered saline (PBS) pH 7.4 (Gibco, Carlsbad, CA). A portion of this (15 mL) was layered onto 10 mL of Lymphoprep®. The sample was then centrifuged for 25 min at 22°C and 1200 rpm. The PBMC were collected at the interface and washed twice with PBS and centrifuged each time for 10 min at 4°C and 1200 rpm. Cell viability was assessed before the last wash in a standard hematocytometer using Trypan blue exclusion. These cells were then cultured at a concentration of 1 × 106 cells/mL and maintained in DMEM medium (Sigma) supplemented with 2 mM L-glutamine (Life Technologies, Paisley, UK), 5000 U/mL penicillin (Sigma), 5000 U/mL streptomycin sulfate (Sigma), and 10% v/v fetal bovine serum (Gibco). The PBMC were cultured with nothing (control), 1 µL of 70% EtOH, or with different extracts of the plant at a final concentration of 1 µg/µL for 48 h. After culture, the cells were centrifuged, and lyzed with Trizol (Invitrogen, Carlsbad, CA) for RNA isolation.
Reverse transcription and real time polymerase chain reaction
Total cellular RNA was isolated from cells using Trizol. Final RNA concentration was measured at a 1:100 dilution in DEPC water in a Bioware DNA spectrophotometer (Biochrome, Cambridge, UK). Reverse transcription was performed using Superscript II first-strand synthesis for RT-PCR kit (Invitrogen) primed with oligo (dT) (Invitrogen), using 1 μg RNA per sample, according to the manufacturer’s instructions. Subsequently, quantitative analysis of IFNβ and IFNα2a was carried out by real-time PCR with fluorophore-labeled LUX primers (see ) and their unlabeled counterparts (Invitrogen). Type I IFNs were selected for our studies because they are the first cytokines normally produced in response to immune stimuli and possess an immunoregulatory capacity for which they are also commonly used in the therapy of many diseases (CitationHilkens et al., 2003).
Table 1. Primers used for quantitative PCR.
As previously published, the primers and PCR conditions were: 50°C, 2 min; 95°C, 2 min then (95°C, 15 s; 60°C, 30 s (IFNs) and 72°C, 1 min) for 45 cycles (CitationEksioglu et al., 2009). Reactions were conducted in a spectrofluorometric thermal cycler (MJ Research DNA Engine Opticon® 2 thermal cycler, BIORAD). Fluorescence was monitored during every PCR cycle at the annealing step. Results were analyzed with MJ Opticon Monitor 3.1 software (BIORAD). Results presented for all experiments represent triplicate determinations from separate healthy blood donors, represented as mean ± SEM.
Antiproliferative effect
HT29 cells (ATCC) were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Invitrogen) containing 10% fetal bovine serum (Hyclone, Logan, UT), in a humidified atmosphere containing 5% CO2 at 37°C. Cells were seeded into 96-well plates at a density of 10,000 cells/well (100 μL medium/well). After 24 h, fractions were added to wells at varying concentrations (as 1 μL stock solutions in EtOH−H2O, 1:1). EtOH−H2O was used as a negative control. After another 48 h, the plate was developed with MTT dye according to the manufacturer’s protocol (G4000, Promega, Madison, WI). Data are expressed as percentage viability relative to negative controls, and an extract was deemed to have a significant effect on proliferation if this value was < 50%. Results obtained were expressed as mean ± SEM. The data were analyzed using one-way ANOVA followed by Dunnett’s post hoc test. Results were considered significant when p <0.05.
Results
Cytochrome P450 enzyme activity assay
BROD assay
Fractions F6(2), F6(3), and F6(4) (see ) produced significant (p <0.05) concentration dependent stimulation of cytochrome P450 enzyme activity (see ). At the highest concentration (1 ng/mL), F6(2), F6(3), and F6(4) elicited specific activity values of 32, 26, and 27 pmol/min/mg (compared to 12 pmol/min/mg for control) corresponding to approximately 3-, 2-, and 2-fold activity enhancements, respectively. Calculated EC50 values were 6.18 × 10−3, 5.3 × 10−3, and 4.83 × 10−3 ng/mL, respectively, for F6(2), F6(3), and F6(4). The order of potency is therefore F6(4) > F6(3) > F6(2). Ketoconazole (0.01 mM) was used as a positive control for inhibition.
Table 2. Effects of B. coccineus organic solvent extract fractions on cytochrome P450 activity in the BROD and BFCOD assays.
BFCOD assay
Fractions F6(2), F6(3), and F6(4) (10−3 to 10 ng/mL) produced significant (p <0.05) stimulation of cytochrome P450 enzyme activity (see ). At the most efficacious concentrations [10 ng/mL for F6(2) and F6(3) and 10−3 ng/mL for F6(4)], F6(2), F6(3), and F6(4) elicited specific activity values of 299, 303, and 318 pmol/min/mg (compared to 160 pmol/min/mg for control), respectively, corresponding to approximately 2-fold activity enhancements. The functionality of the assay was confirmed with ketoconazole, which inhibited enzyme activity to 149 and 61 pmol/min/mg for 0.01 and 1 mM, respectively.
Immunomodulation assay
In the preliminary study, the aqueous leaf extract of B. coccineus (BW, see ) produced the greatest effect in increasing IFNβ expression at the concentration of 0.1 µg/µL relative to the organic solvent extracts (B1 and B2, data not shown). Considering the fractions obtained from BW by HP-20 column chromatography, fraction BW5 produced the greatest effects on IFNβ and IFNα2a expression, causing 7- and 4-fold increases, respectively (). Fractionating BW5 further by HPLC, fractions BW5(1) to BW5(19) were obtained. Of these fractions, BW5(4) produced the greatest significant effect on IFNα2a expression eliciting a 26-fold increase ().
Table 3. Effects of B. coccineus aqueous extract fractions on IFN expression.
Table 4. Effects of B. coccineus aqueous extract BW(5) fractions on IFNα expression.
Antiproliferative effect
The fractions generated after normal phase column chromatography, F1 to F7 (see ), produced significant inhibitory effects (>50%) on HT29 cell viability only at the highest concentration of 100 µg/mL, giving values of 34.7%, 34.67%, 36.69%, 34.71%, 20.56%, 17.74%, and 22.24%, respectively (see ). F8, F9, F10 and fractions subsequently obtained from F6 [F6(1) to F6(6)] (which produced the greatest inhibitory effect on cell viability) did not produce significant effects on HT29 cell viability (). The loss of activity of fractions derived from the active F6 can be explained by the fact that many phytomedicines exert their beneficial effects through the additive or synergistic action of several chemical compounds contrasting with synthetic pharmaceuticals based on single chemical entities (CitationBriskin, 2000). As shown in , the aqueous extract of B. coccineus (BW) and fractions obtained from it (BW1 to BW8) did not produce any significant effect on HT29 cell viability in the antiproliferative assay.
Table 5. Effects of B. coccineus extract fractions on HT29 cell viability.
Discussion
The cytochrome P450 enzyme family is involved in the metabolism and detoxification of environmental carcinogens, steroids, bile acids, fatty acids, eicosanoids, fat soluble vitamins, and drugs (CitationWrighton & Stevens, 1992; CitationGuengerich et al., 2003). These enzymes transform lipophilic drugs into more polar compounds that can be excreted in urine (CitationGirennavar et al., 2007). Depending on the nature of xenobiotics, the outcome may be activation of a prodrug, deactivation of a biologically active compound or conversion of an active drug into an active metabolite (CitationIssa et al., 2006). According to CitationGirennavar et al. (2007), induction and inhibition of drug-metabolizing enzymes are common mechanisms for drug interactions. Herbal medicines are commonly combined with modern drugs (CitationPekthong et al., 2008). Additionally, certain observations have increased interest in investigation of herb−drug interactions. St. John’s wort, which has been found to induce cytochrome P450s (particularly CYP3A4), has been reported to decrease cyclosporine plasma concentrations resulting in kidney rejection (CitationPekthong et al., 2008). Ginkgo biloba L. (Ginkgoaceae), which has been shown to display beneficial effects on the vascular system, memory, cognition and gene regulation (CitationPekthong et al., 2008), was also found in vitro to strongly inhibit CYP2C9, and to a lesser extent, CYP1A2, CYP2E1 and CYP3A4 (CitationGaudineau et al., 2004).
In light of increasing reports of drug−herb interactions, the effects of B. coccineus on cytochrome P450 activity was investigated in this study. Fractions obtained from the plant extract stimulated CYP450 activity in both the BROD and BFCOD assays. The fact that the fractions were derived from the organic extracts of the plant makes the observed result relevant in situations where the leaves are macerated in alcohol or eaten, rather than when extraction is water based. The BROD assay is reported to be selective for human CYP2B6 and CYP3A4 (CitationNiwat et al., 2003), while the BFCOD assay is linked with CYP3A4 activity (CitationStresser et al., 2000). Stimulation of activity, by heterotropic positive cooperativity, has been shown with CYP3A, but not to our knowledge with other P450 isoforms. For example, α-naphthoflavone was shown to stimulate CYP3A4 activity (CitationUeng et al., 1997). Stimulation of CYP2B1/2 and CYP3A4 activity by B. coccineus could possibly lead to reduced bioavailability and enhanced metabolism of co-administered drugs, most likely leading to a decrease in therapeutic efficacy. This finding gives further credence to the need for health care practitioners to ensure proper counseling of their patients about the possible implications of herb−drug interactions. However, it is possible that different results could be obtained in vivo. CitationZhou et al. (2007) reported that many known in vitro inhibitors of CYP proteins actually serve to induce those proteins when administered in vivo, with clotrimazole and other imidazoles, chlorpromazine, metyrapone, etc., as common examples. Therefore further in vivo studies are needed to gain a more complete understanding of the effects of this plant extract on CYP expression.
In this study, fractions from the aqueous extract of B. coccineus significantly stimulated the expression of IFNα2a and IFNβ in PBMC. The induction of interferon expression leads to diverse effects associated with antiviral, antiproliferative, and immunostimulatory activities (CitationWeinstock-Guttman et al., 1995; CitationPeters, 1996). The results obtained in this study provide a possible rationale for the anecdotal use of B. coccineus in TAM for the treatment of venereal diseases and tumors.
Significant reductions in the viability of a human colon cancer cell line (HT29) were only observed for some fractions at the highest concentration tested (100 µg/mL). It is therefore possible that the antiproliferative effect attributed to the plant results less from a direct effect on cell viability, and more from stimulation of interferon expression as evident from the results obtained from gene expression studies. Presumably in vivo this would result in a heightened immune response to cancer cells.
Conclusions
The results obtained in this study show that fractions obtained from the organic extract of B. coccineus stimulated cytochrome P450 (CYP2B1/2 and CYP3A4) metabolizing enzyme activity. This suggests that the plant may reduce bioavailability and enhance the metabolism of co-administered drugs, possibly reducing their therapeutic efficacy. Furthermore, results obtained in this study also show that fractions obtained from the aqueous extract of B. coccineus stimulated the expression of interferons and reduced the viability of HT29 cells at high concentrations. The results obtained in this study may hint at the mechanism of action in the reported uses of B. coccineus in TAM (CitationNeuwinger, 1996).
Declaration of interest
The United States Department of State Bureau of Educational and Cultural Affairs (ECA) provided sponsorship to Akindele A.J., under the Fulbright Junior Staff Development Award (JSD) program, for the conduct of research for 9 months as a Visiting Researcher in the Department of Medicinal Chemistry, College of Pharmacy, University of Florida, Gainesville, Florida 32610, USA. The authors alone are responsible for the content and writing of the paper.
References
- Adjanohoun EJ, Ahyi AMR, Ake Assi L, Akpagana K, Chibon P, El-Hadji A, Eyme J, Garba M, Gassita JN, Gbeassor M, Goudote E, Guinko S, Houdoto KK, Houngnon P, Keita A, Keoula A, KlugoOcloo WP, Lo I, Siamevi KM, Taffame KK. (1986). Contribution to ethnobotanical and floristic studies in Togo. Agency for Cultural and Technical Cooperation (ACCT). Paris: Traditional Medicine and Pharmacopoeia, 671.
- Agbonon A, Eklu-Gadegbeku K, Aklikokou K, Gbeassor M, Akpagana K, Tam TW, Arnason JT, Foster BC. (2010). In vitro inhibitory effect of West African medicinal and food plants on human cytochrome P450 3A subfamily. J Ethnopharmacol, 128, 390–394.
- Akindele AJ, Adeyemi OO. (2006a). Analgesic activity of the aqueous leaf extract of Byrsocarpus coccineus. Niger J Health Biomed Sci, 5, 43–47.
- Akindele AJ, Adeyemi OO. (2006b). Evaluation of the antidiarrhoeal activity of Byrsocarpus coccineus. J Ethnopharmacol, 108, 20–25.
- Akindele AJ, Adeyemi OO. (2007). Antipyretic activity of Byrsocarpus coccineus Schum. and Thonn. (Connaraceae). Int J Pharmacol, 3, 357–361.
- Akindele AJ, Adeyemi OO. (2010). Anxiolytic and sedative effects of Brysocarpus coccineus Schum. and Thonn. (Connaraceae). Int J Appl Res Nat Prod, 3, 28–36.
- Ameer B, Weintraub RA. (1997). Drug interactions with grapefruit juice. Clin Pharmacokinet, 33, 103–121.
- Bailey DG, Malcolm J, Arnold O, Spence JD. (1998). Grapefruit juice−drug interactions. Br J Clin Pharmacol, 46, 101–110.
- Briskin DP. (2000). Medicinal plants and phytomedicines: Linking plant biochemistry and physiology to human health. Plant Physiol, 124, 507–514.
- Burkill HM. (1985). The Useful Plants of West Tropical Africa, Vol. 1, second edition. Families A–D. Kew, UK: Royal Botanic Gardens, pp. 446–447.
- Clark JI, Weiner LM. (1995). Biologic treatment of human cancer. Curr Probl Cancer, 19, 185–262.
- Dunn GP, Koebel CM, Scheiber RD. (2006). Interferons, immunity and cancer immunoediting. Nat Rev Immunol, 6, 836–848.
- Eksioglu EA, Bess JR, Zhu H, Xu Y, Dong H-J Elyar, J, Nelson DR, Liu C. (2009). Hepatitis C virus modulates human monocyte-derived dendritic cells. J Viral Hepat. 10.1111/j.1365-2893.2009.01231.x.
- Gaudineau C, Beckerman R, Welbourn S, Auclair K. (2004). Inhibition of human P450 enzymes by multiple constituents of the Ginkgo biloba extract. Biochem Biophys Res Commun, 318, 1072–1078.
- Gilewski TA, Golomb HM. (1990). Design of combination biotherapy studies: Future goals and challenges. Semin Oncol, 17, S3–10.
- Girennavar B, Jayaprakasha GK, Patil BS. (2007). Potent inhibition of human cytochrome P450 3A4, 2D6, and 2C9 isoenzymes by grapefruit juice and its furocoumarins. J Food Sci, 72, C417–C421.
- Guengerich FP, Chun Y-J Kim, D, Gillam EMJ, Shimada T. (2003). Cytochrome P450 1B1: A target for inhibition in anticarcinogenesis strategies. Mutat Res, 523-524, 173–182.
- Hilkens CMU, Schlaak JF, Kerr IM. (2003). Differential responses to IFN-α subtypes in human T cells and dendritic cells. J. Immunol, 171, 5255–5263.
- Huber WW, Rossmanith W, Grusch M, Haslinger E, Prustomersky S, Peter-Vorosmarty B, Parzefall W, Scharf G, Schulte-Hermann R. (2008). Effects of coffee and its chemopreventive components kahweol and cafestol on cytochrome P450 and sulfotransferase in rat liver. Food Chem Toxicol 46, 1230–1238.
- Issa AY, Volate SR, Wargovich MJ. (2006). The role of phytochemicals in inhibition of cancer and inflammation: New directions and perspectives. J Food Comp Anal, 19, 405–419.
- Khan OA, Jiang H, Subramaniam PS, Johnson HM, Dhib-Jalbut SS. (1998). Immunomodulating functions of recombinant ovine interferon tau: Potential for therapy in multiple sclerosis and autoimmune disorders. Mult Scler, 4, 63–69.
- Kupferschmidt HHT, Fattinger KE, Ha HR, Follath R, Krahenbuhl S. (1998). Grapefruit juice enhances the bioavailability of the HIV protease inhibitor saquinavir in man. Br J Clin Pharmacol, 45, 355–359.
- Neuwinger HD. (1996). African Ethnobotany: Poisons and Drugs; Chemistry, Pharmacology and Toxicology. Weinheim, Germany: Chapman & Hall, pp. 377–379.
- Niwat T, Shiraga T, Yamasaki S, Ishibashi K, Ohno Y, Kagayama A. (2003). In vitro activation of 7-benzyloxyresoruin O-debenzylation and nifedipine oxidation in human liver microsomes. Xenobiotica, 33, 717–729.
- Pekthong D, Martin H, Abadie C, Bonet A, Heyd B, Mantion G, Richert L. (2008). Differential inhibition of rat and human hepatic cytochrome P450 by Andrographis paniculata extract and andrographolide. J Ethnopharmacol, 115, 432–440.
- Peters M. (1996). Actions of cytokines on the immune response and viral interactions: An overview. Hepatology, 23, 909–916.
- Pohl RJ, Fouts JR. (1980). A rapid method for assaying the metabolism of 7-ethoxyresorufin by microsomal subcellular fractions. Anal Biochem, 107, 150–155.
- Prough RA, Burke MD, Mayer RT. (1978). Direct fluorometric methods for measuring mixed-function oxidase activity. Methods Enzymol, 52, 372–377.
- Renwick AB, Surry D, Price RJ, Lake BG, Evans DC. (2000). Metabolism of 7-benzyloxy-4-trifluoromethylcoumarin by human hepatic cytochrome P450 isoforms. Xenobiotica, 30, 955–969.
- Schwarz UI, Büschel B, Kirch W. (2003). Unwanted pregnancy on self-medication with St. John’s wort despite hormonal contraception. Br J Clin Pharmacol, 55, 112–113.
- Shakeel M, Trinidade A, McCluney N, Clive B. (2010). Complementary and alternative medicine in epistaxis: A point worth considering during the patient’s history. Eur J Emerg Med, 17, 17–19.
- Stresser DM, Blanchard AP, Turner SD, Erve JCL, Dandeneau AA, Miller VP, Crespi CL. (2000). Substrate-dependent modulation of CYP3A4 catalytic activity: Analysis of 27 test compounds with four fluorometric substrates. Drug Metab Dispos, 28, 1440–1448.
- Uematsu S, Akira S. (2007). Toll-like receptors and type I interferons. J Biol Chem, 282, 15319–15323.
- Ueng Y-F Kuwabara, T, Chun Y-J Guengerich, FP. (1997). Cooperativity in oxidations catalyzed by cytochrome P450 3A4. Biochemistry, 36, 370–381.
- Weinstock-Guttman B, Ransohoff RM, Kinkel RP, Rudick RA. (1995). The interferons: Biological effects, mechanisms of action, and use in multiple sclerosis. Ann Neurol, 37, 7–15.
- Wrighton SA, Stevens JC. (1992). The human hepatic cytochromes P450 involved in drug metabolism. Crit Rev Toxicol, 22, 1–21.
- Zhou S-F Xue, CC, Yu X-Q Li, C, Wang G. (2007). Clinically important drug interactions potentially involving mechanism-based inhibition of cytochrome P450 3A4 and the role of therapeutic drug monitoring. Ther Drug Monit, 29, 687–710.