Abstract
Context: The role of natural products as a source for remedies has been recognized since the beginning of mankind. Nevertheless, a minority of folkloricly used medicinal plants have been evaluated for their pharmacological activities.
Objectives: The purpose of this study is to evaluate 33 selected Yemeni plants for their in vitro anticancer, antimicrobial, and antioxidant activities.
Materials and methods: The plants were extracted with methanol and hot water. The obtained 66 extracts were tested for their in vitro cytotoxic activity using the neutral red uptake assay against two cancer cell lines (5637 and MCF-7). The antimicrobial activity was determined using the agar diffusion method and MIC-determination. The DPPH radical method was used for the determination of antioxidant activity.
Results: Interesting cytotoxic activity was observed for Hypoestes forskalei (Vahl) R. Br. (Acanthaceae), Lycium shawii Roem. & Schult. (Solanaceae), Pergularia tomentosa L. (Asclepiadaceae), Psiadia punctulata (DC.) Vatke (Compositae), Pulicaria petiolaris Jaub. & Spach (Compositae) and Rosmarinus officinalis L. (Labiatae) (IC50 values < 50 μg/mL). Antimicrobial activity with MIC values ≤ 125 μg/mL was exhibited against Gram-positive bacteria by Chrozophora oblongifolia (Del.) A.Juss. ex Spreng. (Euphorbiaceae), Myrtus communis L. (Myrtaceae), Phragmanthera regularis (Steud. ex Sprague) M.G. Gilbert (Loranthaceae) and R. officinalis. Antioxidant activity was observed for C. oblongifolia, M. communis, and P. regularis.
Conclusion: The results justified the use of some investigated plants in the Yemeni ethnomedicine. These findings demonstrated that some of the investigated plants could be a source of new cytotoxic and antibiotic compounds; however, further work is needed.
Introduction
Plants have been used as a folkloric source of medicinal agents since the beginning of mankind. Despite major scientific progress in chemistry, drugs derived from plants still make an enormous contribution to drug discovery today and continue to be an important source to fight serious diseases, especially in developing countries (CitationZhang, 2004). Infections caused by microorganisms and parasites as well as malignant diseases are still a serious threat to public health, despite the great development in modern medicine. The relative unavailability of medicines in developing countries and the current problems associated with the use of antibiotics makes the impact of infectious diseases particularly large and significant (CitationOkeke et al., 2005). Thus, the interest in herbal drugs with antimicrobial properties has been revived. With the increasing prevalence of multi-resistant bacteria, the search for plant extracts against these organisms offers an important potential for the development of new agents effective against infections which are difficult to treat (CitationCowan, 1999; CitationRios & Recio, 2005).
Currently, cancer is one of the most conspicuous diseases leading to death in humans and recently there has been considerable scientific interest in the continuing discovery of new anticancer agents from natural product sources. Plants have played an important role as a source of effective anticancer agents, and at present over 60% of currently used anticancer agents are derived in one way or another from natural sources, including plants (CitationKinghorn et al., 2003; CitationClardy & Walsh, 2004; CitationCragg & Newman, 2000; CitationRethy et al., 2007).
Generally, the role of free-radical reactions in biology and in disease pathology has become an area of great interest, proposing that such reactions are essential for several metabolic processes and could be in many cases harmful to health as well. It is also known that free radicals such as reactive oxygen species (ROS) e.g. superoxide anions, hydroxyl radicals, and hydrogen peroxide play an important role in the development of tissue damage in living organisms. Moreover, growing evidence relates the occurrence of cancer to the oxidative damage to DNA, proteins and lipid in the body caused by radicals and other carcinogens (CitationHalliwell, 1996). Although medicinal plants are rarely used as ‘antioxidants’ in traditional medicine, their claimed therapeutic properties could be due, in part, to their capacity for scavenging oxygen free radicals which may be involved in many diseases, as for example, in the case of plants used to treat inflammatory diseases, gastric ulcers, diabetes, atherosclerosis, and ischemic heart disease which could act by reducing the oxidative stress that takes place in the cells undergoing such processes (CitationHalliwell & Gutteridge, 2007; CitationHarman, 1988; CitationMaxwell, 1995; CitationSreejayan & Rao, 1996). Thus, the interest in natural antioxidants is on the increase, particularly from medicinal and dietary plants, which possess preventative oxidative damage capacity.
In Yemen, there is a rich tradition of the use of herbal medicine for the treatment of various diseases, including inflammations, infections and other diseases (CitationAl-Dubai & Al-khulaidi, 1996; CitationFleurentin & Pelt, 1982; CitationSchopen, 1983). Despite some investigations during the last decade (CitationAl-Fatimi et al., 2007; CitationAwadh et al., 2001; CitationMothana & Lindequist, 2005; CitationMothana et al., 2006, Citation2007, Citation2009), a minority of traditionally used medicinal plants have been evaluated for their pharmacological and chemical properties. Considering the importance of these untapped research areas, the aim of the present study was to evaluate the in vitro antimicrobial, antitumor, and antioxidant activities of 33 important medicinal plants, which are used in the Yemeni traditional medicine for various disorders including infectious diseases as well as different types of inflammations.
Materials and methods
Plant materials
The plants were collected from different localities of Yemen in July 2005 and identified by Ramzi Mothana at the Pharmacognosy Department, Faculty of Pharmacy, Sana’a University and by Peter Koenig, at the Botanical Garden, Ernst-Moritz-Arndt-University, Greifswald, Germany. Voucher specimens were deposited at the Pharmacognosy Department, Faculty of Pharmacy, Sana’a University.
Extraction of plant material
The air-dried and powdered plant materials (10 g of each) were extracted with 400 mL methanol by using a Soxhlet apparatus (Witeg, Germany) for 8 h. The residue was dried overnight and then extracted with 250 mL water by using a shaking water-bath (GFL, Burgwedel, Germany) at 70°C for 2 h. The extraction with water was repeated three times. The methanol and water extracts obtained were filtered and evaporated by using a rotary evaporator (Buechi, Flawil, Switzerland) and freeze dryer (Labconco, Kansas City) to give the crude dried extract. The dried extracts were stored at −20°C until use.
Determination of antimicrobial activity
Microorganisms
The following microorganisms were used as test organisms: Staphylococcus aureus (ATCC 6538), Bacillus subtilis (ATCC 6059), Micrococcus flavus (SBUG 16), Escherichia coli (ATCC 11229), Pseudomonas aeruginosa (ATCC 27853) and Candida maltosa (SBUG). Furthermore, three multi-resistant Staphylococcus strains, namely Staphylococcus epidermidis 847, Staphylococcus haemolyticus 535, and Staphylococcus aureus north German epidemic strain (Institute of Hygiene of Mecklenburg-Vorpommern, Greifswald, Germany) were also subjected to the screening.
Agar diffusion method
The disc-diffusion assay as described by CitationBauer et al. (1966) was used to determine the antimicrobial activity of the investigated extracts. Nutrient agar (Oxoid, Basingstoke, UK) was prepared by dissolving of 27 g/L in water. The sterile nutrient agar was inoculated with microbial cells (200 μL microbial cell suspension in 20 mL agar medium) and poured into sterile Petri dishes. Sterile filter paper discs of 6 mm diameter (Schleicher & Schuell, Dassel, Germany) were impregnated with 20 μL of the extract solution (equivalent to 4 mg of the dried extract). The paper discs were allowed to evaporate and after that placed on the surface of the inoculated agar plates. Plates were refrigerated for 2 h to enable prediffusion of the extracts into the agar. Then the plates were incubated overnight (18 h) at 37°C. In contrast, M. flavus was incubated at room temperature for 48 h and C. maltosa was incubated at 28°C for 48 h. Ampicillin, gentamicin and amphotericin B (Sigma, Deisenhofen, Germany) were used as positive controls. Negative controls were performed using paper discs loaded with 20 μL of methanol. At the end of the incubation period the inhibition zones were measured. The test extracts showing inhibition zones of ≥15 mm were considered significantly active, while those giving ≤10 mm diameter zones were considered inactive.
Broth micro-dilution assay for minimum inhibitory concentrations
The broth micro-dilution method described by CitationMann and Markham (1998) with modifications was used to determine the minimum inhibitory concentrations (MIC) of extracts against the three standard Gram-positive strains. Using sterile round-bottom 96-well plates, duplicate two-fold serial dilutions of extract (100 µL/well) were prepared in the appropriate broth containing 5% (v/v) DMSO to produce a concentration range of 2000 to 15.6 µg of extract/mL. Two-fold dilutions of ampicillin were used as a positive control. 100 µL of a bacterial cell suspension (prepared in the appropriate broth) corresponding to 1 × 106 CFU/mL was added to all wells except those in column 10, 11 and 12 which served as saline, extract and media sterility controls, respectively. Controls for bacterial growth without plant extract were also included on each plate. The final concentration of bacteria in the assay was 5 × 105 CFU/mL. The final concentration of extracts was 1000 to 7.8 µg/mL. Plates were then incubated at 37°C for 18 h overnight. After incubation, the MIC of each extract was determined as the lowest concentration at which no growth was observed in the duplicate wells. An aliquot of 20 µL of p-iodonitrotetrazolium violet solution (0.04%, w/v) (Sigma) was then added to the wells. The plates were incubated for a further 30 min, and estimated visually for any change in color from yellow to pink indicating reduction of the dye due to bacterial growth. The highest dilution (lowest concentration) that remained yellow corresponded to the MIC. Experiments were performed in duplicate.
Determination of cytotoxic activity on human cancer cell lines
For the estimation of the in vitro cytotoxic potency of the extracts, an established microtiter plate assay according to CitationLindl and Bauer (1989) was used with two human cancer cell lines. Human urinary bladder carcinoma cells 5637 [ATCC HTB-9] were grown in Roswell Park Memorial Institute medium (RPMI) (Lonza, Verviers, Belgium) supplemented with 10% fetal bovine serum (Sigma) and 1% penicillin-streptomycin solution (penicillin 10,000 IU/mL; streptomycin 10,000 µg/mL, Biochrom, Berlin). Human breast cancer cells MCF-7 [ATCC HTB-22] were cultivated in Iscove’s modified Dulbecco’s medium (IMDM) (Lonza) supplemented as before but with the further addition of 1% non-essential amino acids (Biochrom), 10 µg/mL bovine insulin (Sigma) and 1 mM sodium pyruvate (Biochrom). Both cell lines were cultured at 37°C in 95% air humidity, and 5% CO2, and sub-cultured twice weekly using trypsin/EDTA (trypsin/ethylenediaminetetra-acetic) (0.05%/0.02%, Lonza). The cells were seeded into 96-well microtiter plates (TPP, Trasadingen, Switzerland) at a density of 1000 cell/well for 24 h. After washing with Hanks’ Buffered Salt Solution (HBSS) (PAA, Cölbe, Germany), fresh medium was added. Extracts were diluted in assay medium using a stock solution (40 mg/mL in DMSO or water). Final vehicle concentration did not exceed 1.25%. Possible effects on cell performance were considered in calculation. Plates were left undisturbed for 48 h at 37°C. Finally, plates were washed twice with HBSS and incubated with neutral red in RPMI or IMDM (3.3 µg/mL) in the incubator for 3 h. After removing the supernatant and extensive washing, remaining neutral red was dissolved in acidic ethanol and the optical density (OD) at 550 nm was measured. Etoposide (4 µM or 10 µM) was used as toxic control, cell culture medium as non-toxic control. Cell viability was calculated as percentage of vehicle control after background reduction. All experiments were carried out three times with six replicates for each concentration tested. Where applicable, IC50 values were calculated by linear regression.
Determination of antioxidant activity
In order to measure antioxidant activity, 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging assay was used. The method was carried out as previously described by CitationBrand et al. (1995). This assay measures the free radical scavenging capacity of the investigated extracts. DPPH (Sigma) is a molecule containing a stable free radical. In the presence of an antioxidant which can donate an electron to DPPH, the purple color, which is typical for free DPPH radical decays and the change in absorbency at 517 nm is followed spectrophotometrically. This test could provide information on the ability of a compound to donate a hydrogen atom, on the number of electrons a given molecule can donate, and on the mechanism of antioxidant action. The methanol and aqueous extracts were redissolved in methanol and 5% ethanol respectively and various concentrations (10, 50, 100, 500 and 1000 µg/ mL) of each extract were used. The assay mixture contained in total volume of 1 mL, 500 µL of the extract, 125 µL prepared DPPH (1 mM in methanol) and 375 µL solvent (methanol or 5% ethanol). After 30 min incubation at 25°C, the decrease in absorbance was measured at Λ = 517 nm. Ascorbic acid was used as a positive control. The radical scavenging activity was calculated from the equation:
Phytochemical screening
The preliminary screening of the chemical constituents was performed on the methanol extracts by using chemical methods and thin-layer chromatography (TLC) according to CitationWagner and Bladt (1996).
Results
The present work extends our evaluation on the in vitro antimicrobial, anticancer, and antioxidant activities of a number of plants collected from different locations of Yemen. A number of methanol and hot aqueous extracts (66) representing 33 plant species of 22 families were studied. The botanical names of the plants, parts used and the traditional uses are shown in .
Table 1. List of plant species studied.
Antimicrobial activity
The inhibition zones of the 66 extracts are summarized in . The MIC values are presented in . The antimicrobial activity of the studied plant extracts was shown mainly against the Gram-positive bacteria. Only three plants demonstrated an activity against Gram-negative bacteria. Furthermore, only two plants exhibited a considerable antifungal effect against C. maltosa. It was interesting to note that the multi-resistant Staphylococcus strains showed more sensitivity to the investigated extracts than the antibiotic susceptible Gram-positive bacteria. For the most part, among the investigated extracts the methanol extracts exhibited the highest antibacterial effect. The most remarkable antibacterial activity with inhibition zones higher than 15 mm and MIC values between 62.5 and 250 µg/mL was noticed for the methanol extracts of Aristolochia bracteata Retz (Aristolochiaceae), Chrozophora oblongifolia, Myrtus communis, Phragmanthera regularis, Pulicaria jaubertii Gamal Ed Din (Compositae) and Rosmarinus officinalis. Other plants like Achillea biebersteinii Afan. (Compositae), Cissus rotundifolia (Forssk.) Vahl (Vitaceae), Echinops spinosissimus Turra (Compositae), Fagonia indica Burm. f. (Zygophyllaceae), Kleinia pendula (Forssk.) DC. (Compositae), Lycium shawii, Oncoba spinosa Forssk. (Salicaceae), Otostegia fruticosa (Forssk.) Briq. (Labiatae), Oxalis corniculata L. (Oxalidaceae), Psiadia punctulata, Tecoma stans (L.) H.B.K. (Bignoniaceae), and Zizyphus spina-christi (L.) Willd. (Rhamnaceae) were less active with inhibition zones between 10 and 15 mm and MIC values between 500 and 1000 µg/mL ( and ). Only the methanol extracts of Hypoestes forskalei and Myrtus communis exhibited antifungal activity, with inhibition zones of 18 and 16 mm ().
Table 2. Antimicrobial activity of the crude extracts in agar diffusion assay.
Table 3. MIC values (µg/mL) for antimicrobial activity and IC50 values ± SD (µg/mL) for cell growth inhibition on two human cancer cell lines of the investigated plants.
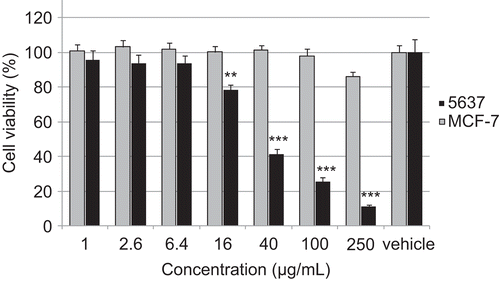
In vitro cytotoxicity
reports the IC50 values for the in vitro cytotoxic activity of the investigated extracts. As expected the epithelial 5637 cell line showed a higher sensitivity than the MCF-7. Compared with methanol extracts, the aqueous extracts had little cytotoxicity, with IC50 values more than 500 µg/mL. The majority of the hot aqueous extracts of the active plants did not express any activity or exhibited only low activity. The results suggested that the main anticancer constituents existed in the methanol extracts. It was demonstrated that 8 methanol extracts out of 33 exhibited a noticeable cytotoxic effect against both tested cancer cell lines (). The most remarkable results were found for Hypoestes forskalei, Lycium shawii, Pergularia tomentosa, Psiadia punctulata, Pulicaria petiolaris, and Rosmarinus officinalis with IC50 values between 14.3 and 48.3 µg/mL ().
Antioxidant activity
The results of the antioxidant activity are shown in . Six plants demonstrated a considerable radical scavenging activity at low concentrations. Whereas the most aqueous extracts showed no noteworthy antioxidant activity even at high concentrations; high free radical scavenging activity (between 41% and 94%) was observed for Achillea biebersteinii, Chrozophora oblongifolia, Myrtus communis, Oxalis corniculata, Phragmanthera regularis, and Tecoma stans at 50 µg/mL ().
Table 4. Free radical scavenging activity and phytochemical screening.
Discussion
The present study is the first report about antimicrobial, cytotoxic, and antioxidant activities of Aristolochia bracteata, Chrozophora oblongifolia, Echinops spinosissimus, Hypoestes forskalei, Kleinia pendula, Oncoba spinosa, Otostegia fruticosa, Phragmanthera regularis, and Pulicaria petiolaris, while other plants are partially investigated. The existing knowledge about them is in many cases very limited. The results of the screening confirmed the use of some investigated plants such as Chrozophora oblongifolia, Hypoestes forskalei, Myrtus communis, Phragmanthera regularis, and Rosmarinus officinalis in Yemeni traditional medicine.
CitationSokmen et al. (2004) reported previously the antimicrobial and antioxidant effect of Achillea biebersteinii. Our results are in agreement with earlier work with the exception that our methanol extract exhibited a moderate antibacterial effect whereas CitationSokmen et al. (2004) found antibacterial activity in the essential oil. The observed activities could be attributed to the volatile oil and flavonoids present in the methanol extract. The antibacterial effect of the roots of Aristolochia bracteata was previously reported (CitationNegi et al., 2003). In the present investigation a potent antibacterial effect was noticed for the leaves. This property could be attributed to the presence of phenanthrene derivatives known as aristolochic acids (CitationZhang et al., 2005).
One of the most interesting plants was Chrozophora oblongifolia. Both extracts demonstrated a high activity against all tested microorganisms including Gram-negative bacteria and C. maltosa (MIC values between 62.5 and 125 µg/mL) (). A noticeable antioxidant effect was also detected at low concentration (41% and 78% at 50 and 100 µg/mL). Whereas no literature data were available for Chrozophora oblongifolia, other plants of the genus Chrozophora were phytochemically and biologically evaluated. So a strong antimicrobial activity of C. verbascifolia, and C. senegalensis has been reported (CitationBonjar et al., 2004; CitationUsman et al., 2007). Moreover, the study on Chrozophora brocchiana revealed an antioxidant effect and the isolated brocchlin carboxylic acid and its methyl ester were responsible for the observed effect (CitationHawas, 2007). Our phytochemical screening revealed the presence of flavonoids and tannins, which could be mainly responsible for the antioxidant effect as well as terpenoids also enhance the antimicrobial effect. These findings justified the primary use as an antiseptic agent for wounds.
Although earlier studies (CitationMuhammad et al., 1997, Citation1998) isolated fusicoccane diterpene ketones from Hypoestes forskalei, no reports on pharmacological activities were found. The phytochemical investigation revealed the presence of terpenoids. The methanol extract of Hypoestes forskalei tested showed a cytotoxic effect against both tested cancer cell lines (IC50 values: 14.3 and 32.1 µg/mL) (). Apparently the observed activity is correlated with the presence of the diterpenoid ketones isolated before. The terpenoid derivatives may also be the cause of the strong antifungal effect obtained in the present screen.
It was previously reported that Myrtus communis and Rosmarinus officinalis have strong antimicrobial and antioxidant activities (CitationAngioni et al., 2004; CitationAppendino et al., 2006; CitationBonjar, 2004; CitationBozin et al., 2007; CitationMoreno et al., 2006; CitationOkamura et al., 1994; CitationRomani et al., 2004; CitationRosa et al., 2003; CitationSantoyo et al., 2005). The results obtained in the present screen are in agreement with literature data found, and justified the folkloric use. Moreover, the remarkable in vitro anticancer activity observed for Rosmarinus officinalis (48.3 µg/mL) is in agreement with literature data found (CitationCheung & Tai 2007). These findings are mostly attributed to the presence of essential oil, flavonoids, tannins and other phenolic compounds found in both plants. The presence of cardiac glycosides in Pergularia tomentosa (CitationAl-Said et al., 1988) could be responsible for the remarkable cytotoxic effect obtained in current screen.
One of the most noteworthy plants was Phragmanthera regularis, which demonstrated a strong antimicrobial effect against Gram-positive bacteria (MIC values between 125 and 250 µg/mL) as well as the strongest antioxidant effect with 94% at a concentration of 50 µg/mL. To the best of our knowledge, this is the first report on pharmacological and chemical investigation of plants of the genus Phragmanthera. The flavonoids revealed in our phytochemical screening could be responsible for some of the indicated activities. The high radical scavenging effect found may confirm the traditional use as antidiabetic agent.
In earlier studies, a strong antimicrobial effect was observed for extracts of Capparis spinosa, Melia azedarach and Peganum harmala (CitationKhan et al., 2001; CitationMahasneh, 2002; CitationShahverdi et al., 2005). Additionally, CitationBonina et al. (2002) reported a high radical scavenging effect for C. spinosa. On the contrary, the extracts of these plants in our screen demonstrated no antimicrobial effect and only a weak antioxidant effect.
An interesting effect had been observed for different plants of the genus Pulicaria. P. jaubertii extracts did not exert any cytotoxic activity. On the other hand the methanol extract of P. petiolaris is quite toxic but exerts a selective effect. Whereas 5637 cell line showed a high sensitivity against the extract (IC50: 42 µg/mL), we could not establish an IC50 value for MCF-7 cells below 250 µg/mL, indicating a missing toxicity (). Here, more detailed investigation will be necessary to clarify the mode of action.
Conclusions
In conclusion, plants still remain a prime source of drugs for the treatment of infections and cancers. The present screen justifies the use of some investigated plants in Yemeni ethnomedicine. Based on the obtained data, the results suggest that some of these plants e.g. C. oblongifolia, M. communis, P. petiolaris, P. regularis and R. officinalis could be promising sources of new potential anticancer and antimicrobial agents; however, bio-guided fractionation and isolation must be carried out in order to identify the compounds responsible for the observed activity.
Acknowledgments
Thanks go to Peter Koenig for supporting in the identification of the investigated plants, and to Jutta Fenske for tests with multi-resistant bacteria strains.
Declaration of interest
The first author (Ramzi A. Mothana) would like to extend deep thanks to the Alexander von Humboldt Foundation for a George Foster scholarship enabling the stay at Ernst-Moritz-Arndt University Greifswald. The authors alone are responsible for the content and writing of the paper.
References
- Al-Dubai AS, Al-khulaidi AA. (1996). Medicinal and Aromatic Plants of Yemen (In Arabic). Sana’a, Yemen: Obadi Center for Studies and Publishing.
- Al-Fatimi M, Wurster M, Schroeder G, Lindequist U. (2007). Antioxidant, antimicrobial and cytotoxic activities of selected medicinal plants from Yemen. J Ethnopharmacol, 111, 657–666.
- Al-Said MS, Hifnawy MS, McPhail AT, McPhail DR. (1988). Ghalakinoside, A cytotoxic cardiac glycoside from Pergularia tomentosa. Phytochemistry, 27, 3245–3250.
- Angioni A, Barra A, Cereti E, Barile D, Coïsson JD, Arlorio M, Dessi S, Coroneo V, Cabras P. (2004). Chemical composition, plant genetic differences, antimicrobial and antifungal activity investigation of the essential oil of Rosmarinus officinalis L. J Agric Food Chem, 52, 3530–3535.
- Appendino G, Maxia L, Bettoni P, Locatelli M, Valdivia C, Ballero M, Stavri M, Gibbons S, Sterner O. (2006). Antibacterial galloylated alkylphloroglucinol glucosides from myrtle (Myrtus communis). J Nat Prod, 69, 251–254.
- Awadh NA, Juelich W-D Kusnick, C, Lindequist U. (2001), Screening of Yemeni medicinal plants for antibacterial and cytotoxic activities. J Ethnopharmacol, 74, 173–179.
- Bauer AW, Kirby WMM, Sheriss, JC, Turck, M. (1966). Antibiotic susceptibility testing by standardized single method. Am J Clin Pathol, 45, 493–496.
- Bonina F, Puglia C, Ventura D, Aquino R, Tortora S, Sacchi A, Saija A, Tomaino A, Pellegrino ML, de Caprariis P. (2002). In vitro antioxidant and in vivo photoprotective effects of a lyophilized extract of Capparis spinosa L buds. J Cosmet Sci, 53, 321–335.
- Bonjar GH. (2004). Antibacterial screening of plants used in Iranian folkloric medicine. Fitoterapia, 75, 231–235.
- Bonjar GH, Aghighi S, Nik AK. (2004). Antibacterial and antifungal survey in plants used in indigenous herbal-medicine of south east regions of Iran. J Bio Science, 4, 405–412.
- Bozin B, Mimica-Dukic N, Samojlik I, Jovin E. (2007). Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J Agric Food Chem, 55, 7879–7885.
- Brand WW, Cuvelier HE, Berset C. (1995). Use of a free radical method to evaluate antioxidant activity. Food Sci Technol, 82, 25–30.
- Cheung S, Tai J. (2007). Anti-proliferative and antioxidant properties of rosemary Rosmarinus officinalis. Oncol Rep, 17, 1525–1531.
- Clardy J, Walsh C. (2004). Lessons from natural molecules. Nature, 432, 829–837.
- Cowan MM. (1999). Plant products as antimicrobial agents. Clin Microbiol Rev, 12, 564–582.
- Cragg GM, Newman DJ. (2000). Antineoplastic agents from natural sources: Achievements and future directions. Expert Opin Investig Drugs, 9, 1–15.
- Fleurentin J, Pelt J-M. (1982). Repertory of drugs and medicinal plants of Yemen. J Ethnopharmacol, 6, 85–108.
- Halliwell B, Gutteridge JMC. (2007). Free Radicals in Biology and Medicine, fourth edition, Oxford: Clarendon Press.
- Halliwell B. (1996). Antioxidants in human health and disease. Annu Rev Nutr, 6, 33–50.
- Harman D. (1988). Free radical theory of aging: Current status. In: Nagy IZ, ed. Lipofuscin-1987: State of the Art. Budapest: Akademiai kiado, and Amsterdam: Elsevier, 3–21.
- Hawas UW. (2007). Antioxidant activity of brocchlin carboxylic acid and its methyl ester from Chrozophora brocchiana. Nat Prod Res, 21, 632–640.
- Khan MR, Kihara M, Omoloso AD. (2001). Antimicrobial activity of Horsfieldia helwigii and Melia azedarach. Fitoterapia, 72, 423–427.
- Kinghorn AD, Farnsworth NR, Soejarto DD, Cordell GA, Swanson SM, Pezzuto JM, Wani MC, Wall, ME, Kroll NH, Kramer RA, Rose WC, Vite GD, Fairchild CR, Peterson RW, Wild R. (2003). Novel strategies for the discovery of plant-derived anticancer agents. Pharm Biol, 41, 53–67.
- Lindl T, Bauer J. (1989). Zell und Gewebekultur. Berlin: Gustav-Fischer-Verlag Jena, 181.
- Mahasneh AM. (2002). Screening of some indigenous Qatari medicinal plants for antimicrobial activity. Phytother Res, 16, 751–753.
- Mann CM, Markham JL. (1998). A new method for determining the minimum inhibitory concentration of essential oils. J Appl Microbiol, 84, 538–544.
- Maxwell SRJ. (1995). Prospects for the use of antioxidant therapies. Drugs, 45, 345–361.
- Moreno S, Scheyer T, Romano CS, Vojnov AA. (2006). Antioxidant and antimicrobial activities of rosemary extracts linked to their polyphenol composition. Free Radic Res, 40, 223–231.
- Mothana RAA, Grünert R, Bednarski PJ, Lindequist U. (2009). Evaluation of the in vitro anticancer, antimicrobial and antioxidant activities of some Yemeni plants used in folk medicine. Pharmazie, 64, 260–268.
- Mothana RAA, Grünert R, Lindequist U, Bednarski PJ. (2007). Study of the anticancer potential of Yemeni plants used in folk medicine. Pharmazie, 62, 305–307.
- Mothana RAA, Lindequist U. (2005). Antimicrobial activity of some medicinal plants of the island Soqotra. J Ethnopharmacol, 96, 177–181.
- Mothana RAA, Mentel R, Reiss C, Lindequist U. (2006). Phytochemical screening and antiviral activity of some medicinal plants of the island Soqotra. Phytother Res, 20, 298–302.
- Muhammad I, Mossa JS, Al-Yahya MA, El-Feraly FS, McPhae AT. (1997). Hypoestenone: A fusicoccane diterpene ketone from Hypoestes forskalei. Phytochemistry, 44, 125–129.
- Muhammad I, Mossa JS, Ramadan AF, El-Feraly FS, Hufford CD. (1998). Additional diterpene ketones from Hypoestes forskalei. Phytochemistry, 47, 1331–1336.
- Negi PS, Anandharamakrishnan C, Jayaprakasha GK. (2003). Antibacterial activity of Aristolochia bracteata root extracts. J Med Food, 6, 401–403.
- Okamura N, Haraguchi H, Hashimoto K, Yagi A. (1994). Flavonoids in Rosmarinus officinalis leaves. Phytochemistry, 37, 1463–1466.
- Okeke IN, Laxmaninarayan R, Bhutta ZA, Duse AG, Jenkins P, O’Brien TF, Pablos-Mendez A, Klugman KP. (2005). Antimicrobial resistance in developing countries. Part1: Recent trends and current status. Lancet Infect Dis, 5, 481–493.
- Rethy B, Csupor-Loeffler B, Zupko I, Hajdu Z, Mathe I, Hohmann J, Redei T, Falkay G. (2007). Antiproliferative activity of Hungarian Asteraceae species against human cancer cell lines. Part I. Phytother Res, 21, 1200–1208.
- Rios J, Recio M. (2005). Medicinal plants and antimicrobial activity. J Ethnopharmacol, 100, 80–84.
- Romani A, Coinu R, Carta S, Pinelli P, Galardi C, Vincieri FF, Franconi F. (2004). Evaluation of antioxidant effect of different extracts of Myrtus communis L. Free Radic Res, 38, 97–103.
- Rosa A, Deiana M, Casu V, Corona G, Appendino G, Bianchi F, Ballero M, Dessi MA. (2003). Antioxidant activity of oligomeric acylphloroglucinols from Myrtus communis L. Free Radic Res, 37, 1013–1019.
- Santoyo S, Cavero S, Jaime L, Ibanez E, Senorans FJ, Reglero G. (2005). Chemical composition and antimicrobial activity of Rosmarinus officinalis L. essential oil obtained via supercritical fluid extraction. J Food Prot, 68, 790–795.
- Schopen A. (1983). Traditionelle Heilmittel in Jemen [Traditional remidies in Yemen]. Berlin: Franz Steiner.
- Shahverdi AR, Monsef-Esfahani HR, Nickavar B, Bitarafan L, Khodaee S, Khoshakhlagh N. (2005). Antimicrobial activity and main chemical composition of two smoke condensates from Peganum harmala seeds. Z Naturforsch, 60, 707–710.
- Sokmen A, Sokmen M, Daferera D, Polissiou M, Candan F, Unlu M, Akpulat HA. (2004). The in vitro antioxidant and antimicrobial activities of the essential oil and methanol extracts of Achillea biebersteini Afan. (Asteraceae). Phytotherapy Res, 18, 451–456.
- Sreejayan N, Rao M. (1996). Free radical scavenging activity of curcuminoids. Drug Res, 46, 169–171.
- Usman H, Musa YM, Ahmadu AA, Tijjani, MA. (2007). Phytochemical and antimicrobial effects of Chrozophora senegalensis. Afr J Trad Complement Altern Med, 4, 488–494.
- Wagner, H., Bladt, S. (1996). Plants Drug Analysis: A Thin Layer Chromatography Atlas, second edition. Berlin: Springer, 306–364.
- Zhang C-Y Wang, X, Su T, Ma C-M Wen, Y-J, Shang M-Y Li, X-M, Liu G-X Cai, S-Q. (2005). New aristolochic acid, aristololactam and renal cytotoxic constituents from the stem and leaves of Aristolochia contorta. Pharmazie, 60, 785–788.
- Zhang X. (2004). Traditional medicine: Its importance and protection. In: Twarog S, Kapoor P, eds. Protecting and Promoting Traditional Knowledge: Systems, National Experiences and International Dimensions. Part 1. The Role of Traditional Knowledge in Healthcare and Agriculture. New York: Aristolochia contorta United Nations, 3–6.