Abstract
Context: Alocasia indica Schott (Araceae) is used in several regions of India, especially in rural communities, by traditional medicine practitioners to treat diarrhea. However, no scientific data are available to justify the traditional potentials of the plant species in gastrointestinal disorders.
Objective: To evaluate the antidiarrheal and in vitro antiprotozoal activities of extracts of leaves of Alocasia indica using various pharmacological models.
Materials and methods: In vitro antidiarrheal activity of aqueous and ethanol extracts of Alocasia indica was evaluated against Escherichia coli, Salmonella typhimurium, Shigella flexneri and Staphylococcus aureus by agar well diffusion method. In vivo antidiarrheal activity of the extracts was studied against recinolic acid-induced diarrhea and magnesium sulfate-induced diarrhea. The effect of the extracts on normal intestinal transit, recinolic acid-induced intestinal transit, recinolic acid-induced intestinal fluid accumulation (enteropooling) and gastric emptying was assessed. In vitro antiprotozoal activity of aqueous and ethanol extracts of Alocasia indica was studied against Entamoeba histolytica and Giardia intestinalis.
Results: The aqueous and ethanol extracts exhibited significant in vitro antidiarrheal activity compared to the standard drug ciprofloxacine (10 µg/mL). The plant extracts showed significant (P <0.05) and dose-dependent antidiarrheal activity comparable to that of the reference drug, loperamide (10 mg/kg). The plant extracts exhibited significant in vitro antiprotozoal activity against both protozoa compared to the standard amebicidal and giardicidal drugs, metronidazole and emetine.
Discussion and conclusion: The results showed that the extracts of Alocasia indica have significant antidiarrheal and in vitro antiprotozoal activities which support its use in traditional herbal medicine practice.
Introduction
The World Health Organization (WHO) has constituted the Diarrheal Diseases Control Program (DDCP) to control the problem of diarrhea which is the leading cause of mortality in developing countries. It includes studies on traditional medicinal practices together with the evaluation of health education and prevention approaches (CitationWHO, 1964; CitationDDCP, 1979). Protozoal diseases are one of the biggest health problems and widely distributed in the world (CitationWHO, 1996). Many Indian ethnobotanical traditions propose a rich repertory of medicinal plants used by the population for the treatment, management and/or control of gastrointestinal disorders (CitationChopra et al., 1956). However, there are insufficient scientific investigations on the antidiarrheal and antiprotozoal activities conferred on these plants. One such plant from the Indian flora is Alocasia indica Schott (Araceae), an indigenous herb. The genus Alocasia consists of about 70 species occurring in Asia, Oceania, and South America. Alocasia indica has been used as a folklore remedy for gastrointestinal disorders in several regions of India. Different parts of this plant are traditionally used in diseases of the abdomen, spleen, and in inflammation (CitationKirtikar & Basu, 1975).
The juice of the leaves of the plant is used as a digestive, laxative, diuretic, astringent and is used for the treatment of rheumatic arthritis (Nadkarni & Nadkarni, 1976). It has antioxidant, hepatoprotective (CitationMulla et al., 2009a, Citation2009b), anthelmintic and antimicrobial (CitationMulla et al., 2010a, Citation2010b) properties. This plant contains flavonoids, cynogenetic glycosides, ascorbic acid, gallic acid, malic acid, oxalic acid, alocasin, amino acids, succinic acid, and β-lectins (CitationPrajapati, 2003). Since no scientific data are available to justify the traditional antidiarrheal and antiprotozoal potentials of the plant, the present study was planned to validate the therapeutic use of this plant in the management of diarrhea, dysentery and illnesses caused in some cases by enteric protozoa.
Materials and methods
Chemicals
All reagents and chemicals used were of analytical grade and purchased from Loba Chemical, Mumbai unless otherwise designated.
Experimental animals
Wistar albino rats weighing 175-225 g of either sex were obtained from Krishna Institute of Medical Sciences, Karad, District Satara (Maharashtra), India and were acclimatized for 10 days under standard housing conditions (24°± 1°C; 45-55% RH with 12:12 h light/dark cycle). The animals had free access to rat food (Hindustan Lever, Mumbai) and water. The animals were habituated to laboratory conditions for 48 h prior to experimental protocol to minimize any nonspecific stress. The experimental protocol was approved by the Institutional Animal Ethics Committee of the Government College of Pharmacy, Karad, MS, India and the animals were maintained under standard conditions in the animal house approved by Committee for the Purpose of Control and Supervision on Experiments on Animals (CPCSEA).
Plant material
Fresh leaves of Alocasia indica, collected in the month of October from different places at Karad, were authenticated by the botanist at the Botany Department, Yashwantrao Chavan College of Science, Karad. A voucher specimen (YCCK/DOB/H- 234) was deposited at the Institute’s Herbarium.
Preparation of aqueous extract
Fresh leaves of A. indica were separated from the plants and allowed to dry in sun light (30°C, 45% RH) for 15 days and then homogenized to get a coarse powder. The powder (250 g) was extracted with distilled water at room temperature by the cold maceration method (CitationWHO, 1998). The filtrate was collected and concentrated on a heating mantle at 45°C till a syrupy mass was obtained. The extract was again dried by rotary evaporator and kept under refrigeration at −4°C. The yield was found to be 6.75% with respect to the initial dried plant material. The aqueous extract of A. indica was referred to as AEAI.
Preparation of ethanol extract
The ethanol extract of leaves of A. indica (EEAI) was prepared by soxhlation. The powdered plant material (250 g) was repeatedly extracted in a 1000 mL round-bottomed flask with 500 mL ethanol (95%). The reflux time for each solvent was 40 cycles for complete extraction. The extracts were cooled at room temperature, filtered and evaporated to dryness under reduced pressure in a rotary evaporator and kept under refrigeration at −4°C. The yield was found to be 2.65% with respect to the initial dried plant material.
Phytochemical screening
Chemical tests were carried out on extracts for qualitative determination of phytochemical constituents as described by CitationHarborne (1998).
Acute toxicity study
Acute oral toxicity study was performed using the up-and-down procedure according to the Office of Prevention, Pesticide and Toxic Substances (OPPTS) (CitationEcobichon, 1997).
In vitro antidiarrheal activity
The in vitro antidiarrheal activity of aqueous and ethanol extracts of Alocasia indica was studied against Escherichia coli, Salmonella typhimurium, Shigella flexneri and Staphylococcus aureus by agar well diffusion method (CitationMulla et al., 2010b). The details of the study have been reported elsewhere. Microbial concentration of 1 × 108 CFU/mL was used for antidiarrheal activity. In each plate wells of 8 mm diameter were made using a sterile borer. The extracts were freshly reconstituted with dimethylsulfoxide to 2.5, 5 and 10 mg/mL concentrations. The test samples and standard drug ciprofloxacine (10 µg/mL) were placed in wells and plates were incubated at 37° ± 1°C for 24 h. Diameter of the zone of inhibition surrounding each well was recorded.
In vivo antidiarrheal activity
Normal intestinal transit
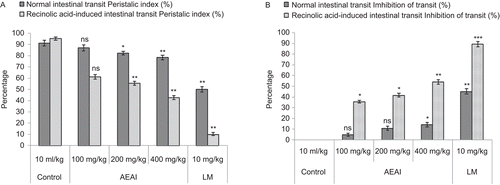
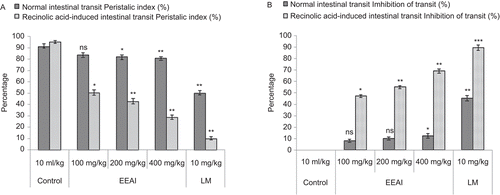
The rats were divided into eight groups of five animals each. Treatment was carried out in different groups with distilled water (10 mL/kg, p.o.), standard drug (loperamide, 10 mg/kg body weight) AEAI (100, 200, and 400 mg/kg, p.o.) and EEAI (100, 200, and 400 mg/kg, p.o.). Charcoal meal (0.2 mL/rat, p.o.) was given to each rat 30 min later. The rats were sacrificed 30 min after meal administration and the small intestine immediately isolated. The peristaltic index (PI), which is the distance traveled by the charcoal meal relative to the total length of the small intestine expressed in percent, was then determined for each rat (CitationHsu, 1982; CitationAye-Than et al., 1989).
Recinolic acid-induced diarrhea in rats
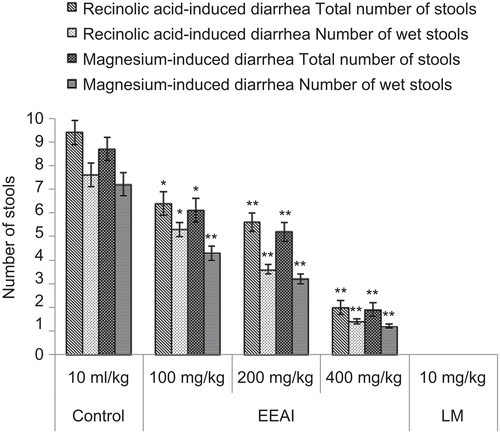
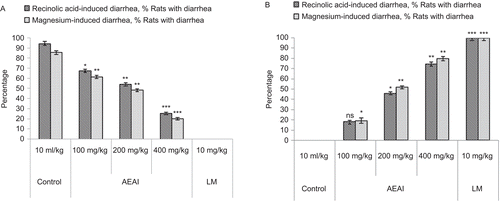
Rats were fasted for 18 h and divided into eight groups of five animals each. The first group received vehicle distilled water (10 mL/kg, p.o.) and served as control, while the second group received the standard drug, loperamide (10 mg/kg body weight). The plant extracts AEAI and EEAI at doses 100, 200 and 400 mg/kg body weight, were administered orally to groups III-VIII. All the animals received 2 mL/rat of recinolic acid in aqueous vehicle orally by gavage 1 h later. The animals were kept in separate metabolic cages with a transparent plastic container beneath the cage to collect feces (CitationAwouters et al., 1978). The severity of diarrhea was assessed each hour for 6 h. The onset of diarrhea was measured as the time interval (min) between the administration of recinolic acid and the appearance of the first diarrheic stool. The fecal matter was collected and weighed over the entire observation period of 6 h. The total score of diarrheal feces for the control group was considered as 100%. The results were expressed as a percentage of inhibition of defecation score and fecal weight calculated using the formula [control mean – treated (test) mean]/control mean × 100. The changes in body weight between the pre- and post-experimental periods were also determined.
The severity of the recinolic acid-induced diarrhea was noted and recorded as a score. A numerical score based on stool consistency was assigned as follows: normal stool (or lack of diarrhea), 1; semi-solid stool, 2; watery stool/feces, 3, respectively (Di CitationCarlo et al., 1994). The corresponding percentages and purging indices were calculated, the latter by comparison with the control group of rats. Using the formula described by CitationMbagwu and Adeyemi, (2008), the in vivo antidiarrheal index (ADI in vivo) was thereafter calculated according to formula ADI in vivo = 3√Dfreq × Gmeq × Pfreq, where Dfreq is the delay in defecation time or diarrhea onset (in % of control), Gmeq is the gut meal travel reduction (in % of control), and Pfreq is the purging frequency, as number of stool reduction (in % of control).
Magnesium sulfate-induced diarrhea in rats
A similar protocol as for recinolic acid-induced diarrhea was followed. Diarrhea was induced by oral administration of magnesium sulfate at the dose of 2 g/kg to the animals, 30 min after administration of distilled water (10 mL/kg, p.o.) to the control group, loperamide (10 mg/kg) to the positive control group, the plant extracts in doses of 100, 200 and 400 mg/kg body weight, to the test groups. All the administrations were carried out through the oral route (CitationDoherty, 1981).
Recinolic acid-induced enteropooling and gastrointestinal motility test in rats
Thirty min following oral treatments of the animals with distilled water (10 mL/kg, p.o.), graded doses of AEAI and EEAI, loperamide (10 mg/kg) and recinolic acid (2 mL/rat), each animal in the eight groups was given 3 mL/kg (p.o.) of 10% activated charcoal in distilled water. The intestinal transit and volume of intestinal fluid in the rats were measured (Di CitationCarlo et al., 1994). The rats were anaesthetized 40 min after the charcoal meal, by ether inhalation and euthanized by cervical dislocation. The abdomen of each rat was cut open and the whole length of the intestine from the pylorus to the caecum was ligated and carefully removed. The weight of the full intestine was determined. The intestinal content was expelled into a graduated measuring cylinder and its volume was determined. The weight of the empty intestine was taken and the difference between the full and empty intestine was thus calculated. The peristaltic index (PI) which is the distance traveled by the charcoal meal relative to the entire length of the small intestine was determined and expressed as a percentage of the total length of the small intestine. Percentage inhibitions of intestinal transit of the charcoal meal by AEAI, EEAI and loperamide were calculated as a function of the distilled water-treated negative control by using the formula Percentage transit inhibition = [To − Tt/To] × 100, where, To is the mean length traversed by the charcoal meal in distilled water-treated control rats; and Tt is the mean length traversed by charcoal meal in AEAI, EEAI and loperamide-treated test rats.
Gastric emptying
Rats fasted for 24 h were randomly allotted to five groups of six animals each. The different groups received distilled water (10 mL/kg, p.o.), plant extracts AEAI and EEAI at doses 200 and 400 mg/kg administered orally. A semi-solid meal of 3 mL based on methylcellulose was administered to the animals 1 h later. The rats were killed and laparatomized 1 h after with the stomachs removed. The full stomachs were weighed, opened, and rinsed. The empty stomachs were reweighed. The difference in weight between the full and empty stomachs was subtracted from the weight of 3 mL of the test meal (CitationDroppleman et al., 1980).
In vitro antiprotozoal activity
Cultures of the intestinal parasites Giardia intestinalis and Entamoeba histolytica were used in this study (CitationCedillo-Rivera et al., 2003). Their trophozoites were grown in TYI-S-33 modified medium, supplemented with 10% calf serum and TYI-S-33 medium, supplemented with 10% bovine serum, respectively. The in vitro susceptibility assays were carried out following a method previously described by CitationAndrzejewska et al. (2004). Extracts (10 mg) were dissolved in 2 mL of dimethylsulfoxide (DMSO) and added to microtubes containing 1.5 mL of medium in order to reach concentrations of 1.6, 3.3, 6.6 and 13.3 µg/mL. The solutions were inoculated with Giardia intestinalis or Entamoeba histolytica to achieve inoculums of 5 × 104 and 6 × 103 trophozoites/mL, respectively. Metronidazole and emetine were used as the standard amebicidal and giardicidal drugs, culture medium with trophozoites and DMSO was the negative control, and culture medium was the blank. Inoculated solutions were incubated for 48 h at 37°C, after which parasites were detached by chilling, and trophozoites were counted with a hemocytometer. The experiments were performed in duplicate and repeated at least three times.
Statistical analysis
The statistical significance of antidiarrheal assays was assessed using one-way of analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison tests. The values are expressed as mean ± SEM and (P <0.05) was considered significant. The data of antiprotozoal assay were analyzed using probit analysis. The percentage of trophozoite survivors was calculated by comparison with growth in the control group. The IC50 and the 95% confidence limit were computed from a plot of probit against the drug concentration.
Results
Preliminary phytochemical investigation
The preliminary phytochemical investigation of the extracts of A. indica showed that it contains flavonoids, cynogenetic glycosides, citric acid, ascorbic acid, polyphenolic compounds.
Acute toxicity studies
Aqueous and ethanol extracts of A. indica did not produce any mortality up to a dose level of 2000 mg/kg, p.o. Hence, 1/20th (100 mg/kg, p.o.), 1/10th (200 mg/kg, p.o.) and 1/5th (400 mg/kg, p.o.) of this dose were employed for further pharmacological investigations.
In vitro antidiarrheal activity
Aqueous and ethanol extracts of A. indica at 2.5, 5 and 10 mg/mL concentrations exhibited significant in vitro antidiarrheal activity against various microorganisms causing diarrhea similar to the standard drug ciprofloxacine (10 µg/mL) ().
Table 1. Effects of aqueous (AEAI, 100–400 mg/kg p.o.) and ethanolic (EEAI, 100–400 mg/kg p.o.) extracts of A. indica against various microorganisms causing diarrhea.
In vivo antidiarrheal activity
Effect of A. indica extracts on normal and recinolic acid-induced intestinal transit
AEAI and EEAI produced significant (P <0.05-0.01) and dose-dependent reductions in normal and recinolic acid-induced intestinal transit. The charcoal meal traversed 92.34% of the total length of the small intestine 30 min after intragastric administration in the control group of rats. AEAI and EEAI dose-dependently and significantly (P <0.05-0.01) decreased the propulsive movement and transit of charcoal meal through the small intestine at oral doses of 100-400 mg/kg, compared with the control group that receiving distilled water (10 mL/kg, p.o.). A similar reduction in the gastrointestinal transit of charcoal meal in rat was achieved with loperamide (10 mg/kg, p.o.). The results are shown in and .
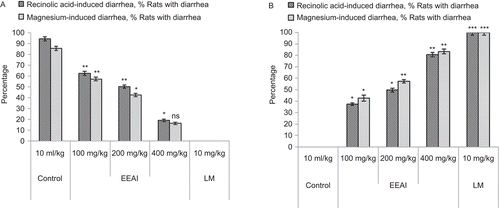
Effect of A. indica extracts on recinolic acid-induced diarrhea
In the recinolic acid-induced diarrhea experiment, AEAI and EEAI produced a marked antidiarrheal effect in the rats ). Voluminous diarrhea was observed in all the rats of the control group of animals 3 h after oral recinolic acid administration. At doses of 100, 200 and 400 mg/kg, the extracts dose-dependently and significantly (P <0.05-0.01) delayed the onset of diarrhea, reduced the frequency of defecation and the wetness of the fecal droppings (reduction in the number of wet stools and total stools) and decreased the weight of wet stools and the general diarrheal score, including the hard, mild and copious stools compared to the standard antidiarrheal drug, loperamide (10 mg/kg, p.o.).
Effect of A. indica extracts on magnesium sulfate-induced diarrhea
In the magnesium sulfate-induced diarrheal model, AEAI and EEAI at the above dose levels were found to reduce the severity of diarrhea in test animals and the results were statistically significant (P <0.05) ).
Effect of A. indica extracts on recinolic acid-induced enteropooling
Oral administration of recinolic acid (2 mL/kg, p.o.) produced a marked and significant (P <0.01) increase in the intestinal fluid volume of recinolic acid-treated groups of rats compared with the control group of animals treated with distilled water (10 mL/kg, p.o.) only. Compared with the control group of rats, pretreatment of the test groups of rats with AEAI and EEAI (100, 200 and 400 mg/kg, p.o.) dose-dependently and significantly (P <0.05-0.01) inhibited recinolic acid-induced enteropooling in rats (). The intestinal fluids of the animals pretreated with the extracts (AEAI, EEAI) and loperamide were found to be more viscous than those of the distilled water-treated control rats.
Table 2. Effects of aqueous (AEAI, 100–400 mg/kg, p.o.) and ethanolic (EEAI, 100–400 mg/kg, p.o.) extracts of A. indica on recinolic acid (2 mL)-induced enteropooling and fluid accumulation in rats.
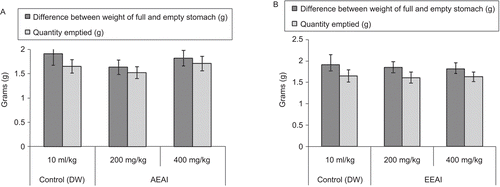
Gastric emptying
As shown in , AEAI and EEAI did not produce any significant effect on gastric emptying.
In vitro antiprotozoal activity of A. indica extracts
The aqueous extract was active against both protozoa, with IC50 value 4.78 μg/mL for Entamoeba histolytica, and 4.12 μg/mL in the case of Giardia intestinalis. The ethanol extract exhibited significant in vitro antiprotozoal activity against both protozoa with IC50 value 2.37 μg/mL for Entamoeba histolytica and 4.65 μg/mL in the case of Giardia intestinalis. The in vitro antiprotozoal activity of test extracts was compared with the standard amebicidal and giardicidal drug, metronidazole and emetine, respectively ().
Table 3. Antiprotozoal activity of aqueous (AEAI, 100–400 mg/kg p.o.) and ethanolic (EEAI, 100–400 mg/kg p.o.) extracts of A. indica.
Discussion
Diarrhea is a common and major public health problem among people with poor standards of hygiene especially in developing countries, and it remains the leading cause of morbidity and mortality in all age groups. Despite the availability of simple and cheap treatments for diarrhea (ORT), healers and patients in many communities still rely on locally available phytomedicines. These findings formed the basis of our screening of various plants, including A. indica in this study, for antidiarrheal activity.
Diarrheic conditions may result from disturbed bowel function, impaired intestinal absorption, excessive intestinal secretion of water and electrolytes, and a rapid bowel transit (CitationSuleiman et al., 2008). The antidiarrheal index (ADI) is a measure of the combined effects of the different components of diarrhea, including purging frequency, onset of diarrheal stools, and frequency of intestinal movement (CitationMbagwu & Adeyemi 2008). Recinolic acid-induced diarrhea is a secretary diarrhea since recinolic acid, the active ingredient of castor oil, induces diarrhea by a hypersecretory response (CitationStewart et al., 1975). Since the extracts of A. indica successfully inhibited the recinolic acid-induced diarrhea, it can be assumed that the antidiarrheal action was mediated by an antisecretory mechanism. This was also evident from the reduction of total number of wet feces in the test groups in the experiment. Magnesium sulfate has been reported to induce diarrhea by increasing the volume of intestinal content through prevention of reabsorption of water. It has also been demonstrated that it promotes the release of cholecystokinin from the duodenal mucosa, which increases the secretion and motility of the small intestine and thereby prevents the reabsorption of sodium chloride and water (CitationGalvez et al., 1993). Findings suggest that both extracts of A. indica increased absorption of water and electrolyte from the gastrointestinal tract.
Reduction in peristaltic index in recinolic acid-induced intestinal transit study suggests that extracts of A. indica activates the sympathetic innervations of the intestine which results in inhibition of peristaltic activity and a reduction in tone. In the enteropooling study, the plant extract significantly (P < 0.05-0.01) reduced the weight and volume of intestinal contents which may be by blocking intraluminal fluid accumulation induced by recinolic acid in a dose-related manner. All extracts were found to lessen the diarrheic condition in all models similar to the standard drug loperamide, but ethanol extract (400 mg/kg) was found to be more effective. The aqueous and ethanol extracts showed significant in vitro antidiarrheal and antiprotozoal activity against selected microbes similar to standard drugs used. The ethanol extract was found to be most active against selected microbes.
Qualitative phytochemical investigation of the extracts of A. indica showed that it contains flavonoids, gallic acid, hydrolysable tannins, citric acid, ascorbic acid, and polyphenolic compounds. Previous reports have demonstrated that antidiarrheal and antiprotozoal properties of medicinal plants were found to be due to flavonoids, tannins, alkaloids, saponins, reducing sugar, sterols and/or terpenes (CitationLonganga et al., 2000; CitationVenkatesan et al., 2005; CitationAlanis et al., 2003; CitationMittra et al., 2000). Some of these compounds have been shown to inhibit intestinal motility in a dose-related manner (CitationCarlo et al., 1994). The flavonoids have been reported to possess antidiarrheal activity through inhibition intestinal motility, antimicrobial activity and antisecretory effect (CitationDi Carlo et al., 1993; CitationRao et al., 1997). Tannins are best known to decrease the irritability of the bowel, thereby reducing peristaltic index (CitationOliver, 1960). These constituents may be responsible for the antidiarrheal and antiprotozoal activity of the aqueous and ethanol extracts of A. indica. The results obtained in the present investigation together with the reported antimicrobial activity of the leaf extracts (CitationMulla et al., 2010) and the presence of flavonoids and tannins validate the use of this plant for the treatment of gastrointestinal disorders.
Acknowledgement
We thank the Principal, Government College of Pharmacy, Karad for providing the facilities to our research work.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alanis AD, Calzada F, Cedillo-Rivera R, Meckes M. (2003). Antiprotozoal activity of the constituents of Rubus coriifolius. Phytother Res, 17, 681–682.
- Andrzejewska M, Yepez-Mulia L, Tapia A, Cedillo-Rivera R, Laudy AE, Starosciak BJ, Kazimierczuk Z. (2004). Synthesis, and antiprotozoal and antibacterial activities of S-substituted 4,6-dibromo- and 4,6-dichloro-2-mercaptobenzimidazoles. Eur J Pharm Sci, 21, 323–329.
- Aye-Than JH, Kukami W, Tha SJ. (1989). Antidiarrheal efficacy of some Burmese indigenous drug formulations in experimental diarrhea test models. J Crude Drug Res, 27, 195–200.
- Awouters F, Niemegeers CJE, Lenaerts FM, Janseen PAJ. (1978). Delay of castor oil diarrhea in rats; a new way to evaluate inhibitors of prostaglandin biosynthesis. J Pharmacol, 30, 41–45.
- Carlo DC, Mascolo N, Izzo AA, Capasso F. (1994). Effects of quercetin on the gastrointestinal tract in rats and mice. Phytother Res, 8, 42–45.
- Cedillo-Rivera R, Darby JM, Enciso-Moreno JA, Ortega-Pierres G. (2003). Genetic homogeneity of axenic isolates of Giardia intestinalis derived from acute and chronically infected individuals in Mexico. Parasitol Res, 90, 119–123.
- Chopra RN, Nayar SL, Chopra IC.(1956). Glossary of Indian Medicinal Plants. New Delhi, India: CSIR.
- DDCP. (1979). Diarrheal Disease Control Programme. Weekly Epidem Record, 16, 121.
- Di Carlo G, Autore G, Izzo AA, Maibline P, Mascolo N, Viola P, Diurno MV, Capasso F. (1993). Inhibition of intestinal motility and secretion by flavonoids in mice and rats: Structure activity relationships. J Pharm Pharmacol, 45, 1054–1059.
- Di Carlo G, Mascolo N, Izzo AA, Capasso F, Autore G. (1994). Effects of quercetin on gastrointestinal tract in rats and mice. Phytother Res, 8, 42–45.
- Doherty SS. (1981). Inhibition of arachidonic acid release, mechanism by which glucocorticoids inhibit endotoxin-induced diarrhea. Br J Pharmacol, 73, 549–554.
- Droppleman DA, Gregory R, Alphin RS. (1980). A simplified method for assessing drug effects on gastric emptying in rats. J Pharmacol Methods, 4, 227–230.
- Ecobichon DJ. (1997). Acute toxicity study, in The Basis of Toxicology Testing. New York: CRC Press, p. 43.
- Galvez J, Zarzuelo A, Crespo ME, Lorente MD, Ocete MA, Jimenez J. (1993). Antidiarrheal activity of Euphorbia hirta extract and isolation of an active flavonoid constituent. Planta Med, 59, 333–336.
- Harborne JB.(1998). Phytochemical Methods. London: Chapman & Hall Press, pp. 60–66.
- Hsu WH. (1982). Xylazine induced delay of small intestinal transit in mice. Eur J Pharmacol, 3, 55–60.
- Kirtikar KR, Basu BD.(1975). Indian Medicinal Plants. Dehradun, India: Mahendrapal Singh Press, p. 2617.
- Longanga A, Vercruysse A, Foriers A. (2000). Contribution to the ethnobotanical, phytochemical and pharmacological studies of traditionally used medicinal plant in the treatment of dysentery and diarrhea in Lomela area, Democratic Republic of Congo (DRC). J Ethnopharmacol, 71, 411–423.
- Mbagwu HOC, Adeyemi OO. (2008). Anti-diarrheal activity of the aqueous extract of Mezoneuron benthamianum Baill (Caesalpinaceae). J Ethnopharmacol, 116, 16–20.
- Mittra B, Saha A, Chowdhury AR. (2000). Luteolin, an abundant dietary component is a potent anti-leishmanial agent that acts by inducing topoisomerase II-mediated kinetoplast DNA cleavage leading to apoptosis. Mol Medicine, 6, 527–541.
- Mulla WA, Salunkhe VR, Kuchekar SB, Qureshi MN. (2009a). Free radical scavenging activity of leaves of Alocasia Indica. Indian J Pharm Sci, 71, 302–307.
- Mulla WA, Salunkhe VR, Bhise SB. (2009b). Hepatoprotective activity of hydroalcoholic extract of leaves of Alocasia indica. Indian J Exp Biol, 47, 316–321.
- Mulla WA, Thorat VS, Patil RV, Burade KB. (2010a). Anthelmintic activity of leaves of Alocasia indica. Int J Pharma Tech Res, 2, 26–30.
- Mulla WA, Sargade PB, Pawar AM, Tarkasband HA, Sayyad FJ. (2010b). Antimicrobial activity of leaves of Alocasia indica. Int J Pharma Tech Res, 2, 327–333.
- Nadkarni KM. (2000). Indian Materia Medica. India: Popular Prakashan, p. 72.
- Oliver BEP.(1960). Medicinal Plants in Nigeria. Ibadan, Nigeria: College of Arts, Science and Technology, pp. 43–53.
- Prajapati ND.(2003). A Handbook of Medicinal Plants. India: Agrobois, p. 32.
- Rao VSN, Santos FA, Sobreika TT, Souza MF, Melo LL, Silveira ER. (1997). Investigations on the gastroprotective and antidiarrheal properties of ternatin, a tetramethoxyflavone from Egletes viscose. Planta Med, 63, 146–149.
- Stewart JJ, Gaginella TS, Bass P. (1975). Actions of ricinoleic acid and structurally related fatty acids of the gastrointestinal tract. I. Effects on smooth muscle contractility in vitro. J Pharmacol Exp Ther, 195, 347–354.
- Suleiman MM, Dzenda T, Sani CA. (2008). Antidiarrheal activity of the methanol stem-bark extract of Annona senegalensis Pers. (Annonaceae). J Ethnopharmacol, 116, 125–130.
- Venkatesan N, Thiyagarajan V, Narayanan S, Arul A, Raja S, Kumar SGV, Rajarajan T, Perianayagam JB. (2005). Antidiarrheal potential of Asparagus racemous wild root extracts in laboratory animals. J Pharm Pharmaceut Sci, 8, 39–45.
- WHO Expert Committee. (1964). Technical Report Series on Enteric Infection. Geneva: World Health Organization, p. 288.
- WHO. (1996). Epidemic dysentery fact sheet. Geneva: World Health Organization.
- WHO. (1998). Methods for Preparation of Extracts in Quality Control Methods for Medicinal Plants Materials. Geneva: World Health Organization, p. 32.