Abstract
Context: Cynanchum taiwanianum T. Yamaza (Asclepiadaceae) is a medicinal herb used in folk medicine for the treatment of several inflammation-related diseases such as hepatitis and dermatitis in Taiwan.
Objective: In the present study, we investigated the anti-inflammatory effect of C. taiwanianum T. Yamaza rhizome aqueous extract (CTAE).
Materials and methods: The present study investigated the anti-inflammatory effect of CTAE using IL-1β-induced NRK-52E cells. Production of NO and PGE2 by ELISA, the mRNA and protein expression of iNOS and COX-2, phosphorylation of IκBα, and activation of NF-κB by RT-PCR and western blotting were determined.
Results: The CTAE significantly (P < 0.05) inhibited NO and PGE2 production (decreased by 46.1% and 51%, respectively), and also significantly (P < 0.05) attenuated protein and mRNA expression of iNOS and COX-2 (decreased by 90% and 55% for iNOS and by 72% and 74%% for COX-2, respectively) in IL-1β-induced NRK-52E cells, in a dose-dependent manner, without obvious cytotoxic effects. Furthermore, the CTAE suppressed the NF-κB nuclear translocation, in terms of inhibition of IκBα phosphorylation.
Discussion and conclusion: Our results provided evidence for its folkloric uses and suggest that the anti-inflammatory activities of CTAE may result from the inhibition of inflammatory mediators, such as NO and PGE2, and an upstream suppression of a NF-κB-dependent mechanism, might be involved.
Introduction
Inflammatory reaction is not only the response of living tissues to infection and injury but also relevant to diseases such as atherosclerosis and cancer (Citationde Boer et al., 2000; CitationCoussens & Werb, 2002). Several reports indicate that overproduction of nitric oxide (NO) is cytotoxic (CitationRadi et al., 1991; CitationLipton et al., 1993; CitationKim et al., 1999) and is related to cell injury or tissue damage in a number of diseases such as inflammation and carcinogenesis (CitationMordan et al., 1993; CitationVodovotz et al., 1993). Therefore, a potent nitric oxide synthase (NOS) inhibitor might be an effective therapeutic strategy for inflammation-related diseases (CitationKoo et al., 2001). In inflammation, prostaglandin E2 (PGE2) is also one of the major mediators and is a well-known oncogenic signal (CitationAlmekinders & Temple, 1998; CitationNarumiya et al., 1999).
In the inflammation process, two inducible enzymes, inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), are responsible for the synthesis of NO and PGE2 (CitationSripanidkulchai et al., 2009), respectively. Recently, these two enzymes have been reported to be inhibited by several plant extracts such as green tea (CitationNakagawa & Yokozawa, 2002), kaempferol, and quercetin (CitationBanerjee et al., 2002), Phyllanthus amarus Schumach (Euphorbiaceae) (CitationKiemer et al., 2003), pine (CitationYen et al., 2008), Chrysanthemum indicum Linn. (Asteraceae) (CitationCheon et al., 2009), and wild bitter gourd (CitationLii et al., 2009). At inflamed sites, NO and PGE2 have pleiotropic effects and produced, respectively, by COX-2 and iNOS, which are induced in response to different stimuli including cytokines (CitationSmith et al., 2000). Therefore, the inhibitors of these two enzymes may also be considered as anti-inflammatory agents.
It is well known that the phosphorylation of I kappa B-alpha (IκBα) results in the degradation of IκBα and consequent dissociation of nuclear factor-kappa B (NF-κB) from IκBα (CitationPonnappan, 1998; CitationHayden & Ghosh, 2004). Subsequently, the free NF-κB translocates into the nucleus and then activates its target genes (CitationBaeuerle & Henkel, 1994; CitationVinayagamoorthi et al., 2005), including iNOS and COX-2 (CitationAppleby et al., 1994; CitationStylianou & Saklatvala, 1998), increasing the expression of inflammatory mediators (CitationLa Grutta et al., 2003). Hence, phosphorylation of IκBα to phospho-IκBα (p-IκBα) was a decisive step in the NF-κB activation pathway (CitationYin et al., 1998). In addition, the induction of COX-2 and iNO synthase by cytokines, such as interleukin-1β (IL-1β), is well known to be partly mediated by NF-κB (CitationMitchell et al., 1997; CitationNewton et al., 1997b; CitationGouze et al., 2002).
Cynanchum taiwanianum T. Yamaza (Asclepiadaceae) is a known and popular herb, the rhizome of which has been used as a folk medicine in Taiwan. Many therapeutic effects of C. taiwanianum T. Yamaza have been studied, such as antifebrile, diuretic, antitussive, expectorant, anodyne, and tonic activities in China (CitationChen et al., 1991) and hepatoprotection in Taiwan (CitationLin et al., 1995). Recently, many researchers focused on the chemical constituents (CitationHuang et al., 1995, Citation1999; CitationLin et al., 1997a,Citationb, Citation1998) or found the biologically active compounds of C. taiwanianum T. Yamaza (CitationChen et al., 1991); however, little is known about the actual mechanisms of anti-inflammatory activities of C. taiwanianum T. Yamaza. The objective of the present study was to investigate the potential anti-inflammatory mechanism of C. taiwanianum T. Yamaza rhizome aqueous extract (CTAE) by rat kidney cell line NRK-52E induced by interleukin 1-β (IL-1β).
Methods
Plant material
The C. taiwanianum T. Yamaza rhizome was collected from Neimen Township, Kaohsiung County, in Southern Taiwan, in April 2008. The plant was identified by Dr. Yan of the National Museum of Natural Science, Taichung, Taiwan. The voucher specimen of C. taiwanianum T. Yamaza was deposited in the Department of Management and Utilization, Fengshan Tropical Horticultural Experiment Branch. The herbarium number is FTHES-08-01.
The rhizome was sliced, hot-dried (50°C), and pulverized into powder. The CTAE was prepared as follows: the C. taiwanianum T. Yamaza powder (100 g) was soaked in 500 mL double-distilled water at 60°C and shaken for 1 h. The CTAE was concentrated using a vacuum concentrator and subjected to freeze-drying. The yield of the aqueous extract was 6.7% (w/w). The CTAE was filtered through a 0.45-μm filter and kept at 4°C.
Chemicals and regents
Anti-serum iNOS, I-κB, p-IκB, and NF-κB were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Avidin-horseradish peroxidase (Av-HRP) was obtained from BD Pharmingen (San Diego, CA). Bovine serum albumin, sodium bicarbonate, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) formazan, sulfanilamide, N-(1-naphthyl)ethylenediamine dihydrochloride, ethidium bromide, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), potassium chloride, magnesium chloride, ethylene diamine tetraacetic acid (EDTA), dithiothreitol (DTT), phenyl methane sulfonyl fluoride (PMSF), leupeptin, aprotinin, NP-40 lysis buffer (NP-40), 3,3-diaminobenzidine (DAB), β-actin, and glycerol were purchased from Sigma (St. Louis, MO). COX-2, PGE2, and PGE2-acetylcholinesterase conjugates were obtained from Cayman (Ann Arbor, MI). Isopropanol was obtained from Merck (Germany). Easy-BLUE™ RNA extraction kit was obtained from INtRON Biotechnology (Korea). Fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), TRIzol® reagent, penicillin–streptomycin, and trypsin–EDTA were purchased from Gibco (Grand Island, NY). Interleukin-1β (IL-1β) was obtained from R&D Systems (Minneapolis, MN). Taq DNA polymerase, oligo-dT primer, and dNTPs were obtained from Invitrogen (Grand Island, NY).
Cell culture
The normal rat kidney cell line NRK-52E (BCRC 60086) was purchased from the Bioresource Collection and Research Center of Food Industry Research and Development Institute in Taiwan and maintained in DMEM containing 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 1% l-glutamine, at 37°C with 5% CO2 in humidified air. Cells were preincubated with and without various concentration of CTAE (0.1, 0.5, 1.0 mg/mL) followed by IL-1β (5 ng/mL) 30 min later. Supernatants were collected after IL-1β treatment for 18 h for NO/PGE2 assays, respectively.
Cell viability assay
Cell viability was evaluated by the mitochondrial-dependent MTT reduction method (CitationMosmann, 1983). The CTAE-treated cells were washed with phosphate-buffered saline (PBS) twice and the medium was replaced with DMED without containing phenol red. MTT solution (0.5 mg/mL) was added to the cell cultures. After incubation for 3 h at 37°C, the medium was removed again and the formazan crystals in viable cells were dissolved with 1 mL of isopropanol. After shaking for 10 min, the solution was centrifuged at 5000 g for 5 min. The absorbance of the supernatant of each sample was determined at 570 nm. The optical density of formazan formed in control (untreated) cells was taken as 100% viability.
Stimulation experiment
Cells were harvested by gentle scraping, plated into 24-well plates at a density of 8 × 104 cells per well, and allowed to adhere for 24 h at 37°C under 5% CO2 atmosphere. For stimulation, the culture medium was replaced with fresh DMEM containing 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, and 1% l-glutamine in the presence or absence of 5 ng/mL IL-1β (CitationLee et al., 2007). To evaluate the effects of the extracts, cells were first incubated with the CTAE at the concentrations of 0.1, 0.5, or 1.0 mg/mL for 5 h, and with or without 5 ng/mL IL-1β as above for 18 h. As reference controls, assays were also performed with medium containing PBS or IL-1β only (CitationGuo et al., 2006).
Nitrite assay
As an indicator of NO production, we determined the nitrite (NO2−) concentration in the culture medium by the Griess reaction (CitationGreen et al., 1982). Each culture supernatant (100 μL), assayed in triplicate, was reacted with an equal volume of Griess reagent (1% sulfanilamide and 0.1% N-(1-naphthyl)ethylenediamine dihydrochloride in 2.5% phosphoric acid solution) at 20°C in darkness. After incubation for 10 min, the absorbance was read at 540 nm and calculated nitrite content based on the standard calibration curve.
PGE2 measurement
PGE2 production was measured in culture medium in order to determine COX-2 activity (CitationNantel et al., 1999). The culture medium of control and treated cells was collected, centrifuged, and stored at -80°C until tested. The level of PGE2 released into culture medium was quantified by using a commercial competitive enzyme immunoassay kit (EIA; Cayman, Ann Arbor, MI) according to the manufacturer’s instruction.
Western blotting
Whole-cell extracts containing equal quantities of proteins (30−50 μg) were electrophoresed in 10% polyacrylamide gel. Subsequently, the separated proteins were transferred to PVDF membrane using semi-dry transfer cell (Trans-Blot, Bio-Rad, Hercules, CA). In brief, the membrane was blocked for 30 min with blocking buffer (5% skim milk in 50 mM Tris–HCl, 200 mM NaCl, and 0.05% Tween 20, pH 7.5), and was incubated with appropriate dilutions of primary antibodies (against iNOS, COX-2, IκB, p-IκB, and NF-κB) overnight at 4°C. After washing twice with the above buffer, the membrane was further incubated for 2 h with 1:1000 dilution of biotin-conjugated goat anti-mouse antibody (Biotain, Dublin, Ireland), and developed with Av-HRP and DAB solution. In the case of COX-2, an enhanced chemiluminescence (ECL) method was used as western blot detection system (Amersham Biosciences, Piscataway, NJ) according to the manufacturer’s instruction.
Nuclear protein preparation
Cells were preincubated with each of the CTAE for 3 h before the addition of 5 ng/mL IL-1β for 90 min. Nuclear protein extracts were prepared from NRK-52E cells according to a modification of Dignam’s method (CitationWen & Han, 2000). Cells were washed twice and scraped with cold PBS and centrifuged. The pellets were resuspended in the hypotonic extraction buffer containing 10 mM HEPES, 10 mM KCl, 1 mM MgCl2, 1 mM EDTA, 0.5 mM DTT, 0.2 mM PMSF, 4 μg/mL leupeptin, 20 μg/mL aprotinin, and 0.5% NP-40 for 15 min on ice and centrifuged at 6000 g for 15 min. Nuclear proteins were extracted by gently mixing with 50 μL hypertonic extraction buffer containing 10 mM HEPES, 0.4 mM KCl, 1 mM MgCl2, 1 mM EDTA, 0.5 mM DTT, 0.2 mM PMSF, 4 μg/mL leupeptin, 20 μg/mL aprotinin, and 10% glycerol at 4°C for 30 min. The samples were centrifuged at 10,000 g for 15 min. The supernatant fluid containing nuclear proteins was collected and estimated by the CitationBradford (1976) method.
RNA extraction and reverse transcription-polymerase chain reaction
Total cellular RNA was isolated using an easy-BLUE™ RNA extraction kit according to the manufacturer’s instructions. In brief, total cellular RNA was extracted with TRIZOL® reagent according to the manufacturer’s instructions. RNA concentrations were calculated from absorbance at 260 and 280 nm. Total RNA (2 mg) was converted to cDNA by treatment with 200 units of reverse transcriptase and 500 ng of oligo-dT primer in 50 mM Tris–HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM DTT, and 1 mM dNTPs at 42°C for 1 h. The reaction was stopped by incubating the solution at 70°C for 15 min, followed by addition of 3 mL of the cDNA mixture for enzymatic amplification. Polymerase chain reaction (PCR) was performed using a reaction mixture comprised of 50 mM KCl, 10 mM Tris–HCl (pH 8.3), 1.5 mM MgCl2, 0.2 mM dNTPs, 2.5 units of Taq DNA polymerase, and 0.1 mM each of primers specific for iNOS, COX-2, and β-actin. The amplification conditions were as follows: denaturation at 94°C for 3 min for the first cycle and then 35 cycles of 94°C for 45 sec, annealing of iNOS at 56°C for 45 sec or annealing of COX-2 at 53°C for 45 sec with a final extension at 72°C for 7 min. The PCR products were electrophoresed on a 1.5% agarose gel and stained with ethidium bromide. β-Actin served as an internal control. The primers used in this study were as follows: iNOS, 5′-AGCCCAACAATACAAATGACCCTA-3′ (sense) and 5′-TTCCTGTTGTTTCTATTTCCTTTGT-3′ (antisense); Cox-2, 5′-CACTCAGTTTGTTGAGTCATTC-3′ (sense) and 5′-GATTAGTACTGTAGGGTTAATG-3′ (antisense); β-actin, 5′-ATGAAGATCCTGACCGAGCGT-3′ (sense) and 5′-AACGCAGCTCAGTAACAGTCCG-3′ (antisense).
Statistical analysis
All data presented in this study were obtained from at least three independent experiments and were expressed as mean values ± SD. The Duncan’s t-test was used for evaluating statistical significance. The P < 0.05 was considered to be statistically significant.
Results
Effect of CTAE on cell viability
To exclude possible cytotoxic effects of CTAE on NRK-52E cells in either the presence or the absence of IL-1β, the MTT assay was employed as above in “Methods” section. Cell viability of NRK-52E cells was not significantly (P < 0.05) altered and the cell viabilities were above 95% up by an 18 h incubation with various concentrations of CTAE (0.1, 0.5, and 1.0 mg/mL) with or without IL-1β (data not presented).
Effect of CTAE on IL-1β-induced NO and PEG2 production
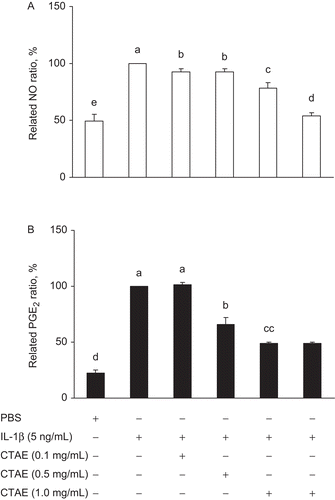
To estimate the effects of CTAE on IL-1β-induced NO and PGE2 production in NRK-52E cells, cells were treated with 0.5 ng/mL IL-1β and various concentrations of CTAE for 18 h. IL-1β stimulation generated a noticeable accumulation of nitrite in the culture medium; however, CTAE significantly (P < 0.05) reduced the IL-1β-induced nitrite production in a dose-dependent manner (). Agreeing with the effect of CTAE on NO production, the CTAE also generated a remarkable decrease in PGE2 in a dose-dependent manner (). When CTAE concentration was 1 mg/mL, the reduction percentage of IL-1β-induced NO and PGE2 were 46.1% and 51%, respectively. Inhibition of both NO and PGE2 correlated with the inhibition of iNOS and COX-2 activities.
Figure 1. Effects of C. taiwanianum T. Yamaza rhizome aqueous extract (CTAE) on (A) NO and (B) PGE2 productions in IL-1β-induced NRK-52E cells. NRK-52E cells were treated with IL-1β (5 ng/mL) alone or with various concentrations of CTAE (0.1, 0.5, or 1 mg/mL) for 18 h, respectively. The production of NO (78%) and PGE2 (49%) were significantly decreased after 1 mg CTAE combined IL-1β treatment, as compared with IL-1β control group (100%). Data are the means ± SD from three or five independent experiments. Values not sharing the same letter are significantly different (P < 0.05).
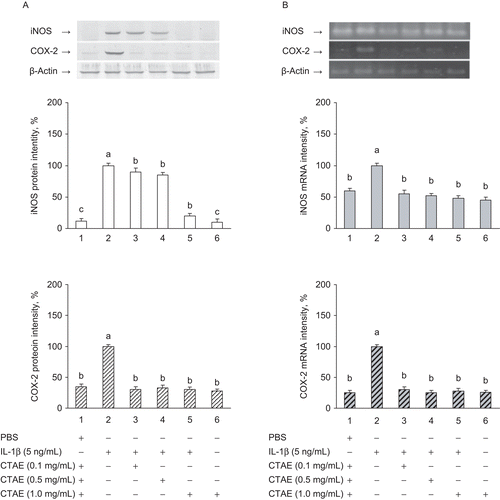
Effect of CTAE on IL-1β-induced mRNA and protein expression of iNOS and COX-2
To verify whether the inhibitory effects of CTAE on NO and PGE2 production originate from decreased mRNA and protein expression of IL-1β-induced overexpression of iNOS and COX-2 in NRK-52 cells, the reverse transcription PCR (RT-PCR) and western blot analysis were executed. The expression of the iNOS and COX-2 mRNA and protein were significantly (P < 0.05) lower in unstimulated cells than in stimulated cells; however, these were significantly (P < 0.05) increased after IL-1β-induced treatment. CTAE had a significant (P < 0.05) and dose-dependent inhibitory effect on mRNA and protein expression of iNOS and COX-2 in IL-1β-induced NRK-52E cells ( and 2B). When CTAE concentration was 1 mg/mL, CTAE significantly (P < 0.05) decreased protein and mRNA expression of iNOS and COX-2 (by 90% and 55% for iNOS and by 72% and 74% for COX-2, respectively).
Figure 2. Effects of C. taiwanianum T. Yamaza rhizome aqueous extract (CTAE) on iNOS and COX-2 protein (A) and mRNA (B) expression in NRK-52E cells. NRK-52E cells were treated with 5 ng/mL IL-1β alone or with various concentrations of CTAE for 18 h, respectively. Protein extracts from cell pellets were subjected to SDS-PAGE followed by western blot analysis using anti-COX-2 and anti-iNOS antibodies. Total mRNAs were prepared from the cell pellets using TRIzol. The relative levels of mRNAs were assessed by RT-PCR. Results were normalized to β-actin. The level of iNOS and COX-2 protein and mRNA expression induced by IL-1β were expressed as 100%. Data are the means ± SD from three or five independent experiments. Values not sharing the same letter are significantly different (P < 0.05). When CTAE concentration was 1 mg/mL, CTAE significantly (P < 0.05) decreased protein and mRNA expression of iNOS and COX-2 (by 90% and 55% for iNOS and by 72% and 74% for COX-2, respectively).
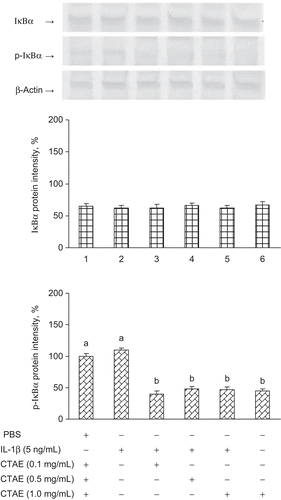
Effect of CTAE on IL-1β-induced phosphorylation of IκBα
Phosphorylation and degradation of IκBα to p-IκBα results in IκBα and the release of NF-κB that translocates to the nucleus (CitationHenkel et al., 1993; CitationAggarwal et al., 2004). We investigate whether CTAE could preclude the phosphorylation of IκBα induced by IL-1β. The p-IκBα is a marker of NF-κB pathway activation; we have determined the expression of p-IκBα in this study. shows that IκBα phosphorylation was remarkably increased upon exposure to IL-1β alone, and that this phosphorylation was significantly (P < 0.05) inhibited in the presence of CTAE.
Figure 3. Effects of C. taiwanianum T. Yamaza rhizome aqueous extract (CTAE) on IκBα and p-IκBα protein level on NRK-52E induced by IL-1β. NRK-52E cells were treated with 5 ng/mL IL-1β alone or with various concentrations of CTAE for 18 h, respectively. Results were normalized to β-actin. Data are the means ± SD from three or five independent experiments and are expressed as the percentage of the phosphate-buffered saline (PBS) vehicle control. Values not sharing the same letter are significantly different (P < 0.05).
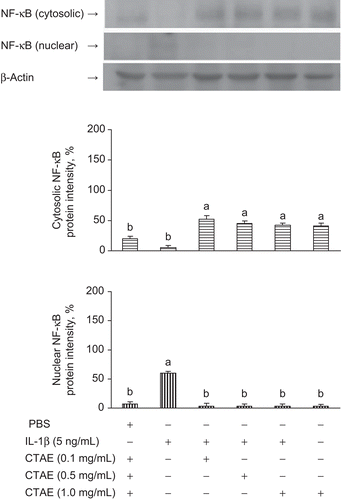
Effect of CTAE on IL-1β-induced nuclear translocation of the NF-κB p65
To elucidate the mechanisms underlying the inhibition of iNOS and COX-2 expression in IL-1β-induced NRK-52E cells, we studied the effects of CTAE on the NF-κB nuclear translocation. Since NF-κB p65 is the major component of the activated NF-κB, we have evaluated the levels of NF-κB p65 in nuclear extracts by western blotting analysis. CTAE inhibited IL-1β-induced nuclear translocation of NF-κB p65, in a dose-dependent manner (). Taken together, these data suggest that the inhibitory effect of CTAE on the IL-1β-induced translocation of NF-κB p65 might be involved in the suppression of IκBα phosphorylation.
Figure 4. Effects of C. taiwanianum T. Yamaza rhizome aqueous extract (CTAE) on cytosolic and nuclear NF-κB protein level on NRK-52E induced by IL-1β. NRK-52E cells were treated with 5 ng/mL IL-1β alone or with various concentrations of CTAE for 18 h, respectively. Results were normalized to β-actin. Data are the means ± SD from three or five independent experiments and are expressed as the percentage of the phosphate-buffered saline (PBS) vehicle control. Values not sharing the same letter are significantly different (P < 0.05).
Discussion
Several reports demonstrated that IL-1β stimulation significantly enhanced the production of NO and PGE2 (Citationvan der Kraan & van den Berg, 2000; CitationAhmed et al., 2002; CitationTamura et al., 2002; CitationWu et al., 2005) by enhanced the expression of COX-2 and iNOS (CitationBlanco et al., 1995; CitationWu & Guo, 2007); however, the stimulation was prevented by either transcriptional or translational blockers (CitationNewton et al., 1997a). The IL-1β was employed as a stimulator to induce inflammatory response in NRK-52E cells in this study. The results indicated that IL-1β successfully induced inflammatory response and increased the production of NO and PGE2 in NRK-52E cells. As expected, the CTAE significantly (P < 0.05) reduced IL-1β-induced NO and PGE2 production in a dose-dependent manner in NRK-52E cells.
The NO production was regulated by constitutive NOS (cNOS) under normal physiological conditions (CitationNakagawa & Yokozawa, 2002); however, NO was also overproduced at inflamed sites by iNOS in inflammatory events (CitationKim et al., 2005; CitationLee et al., 2007). Therefore, regulation of iNOS in tissues is very important for the treatment of inflammation and tumorigenesis (CitationChan et al., 1998). On the other hand, COX-2, one of the inducible enzymes also, is crucial in the inflammatory response, converts arachidonic acid to PGE2, and is required in introducing and sustaining reactions during the inflammatory course (CitationFitzGerald & Patrono, 2001; CitationSurh et al., 2001). CitationPark et al. (2004) noted that overproduction of PGE2 mediated by COX-2 was connected to the development of inflammation and carcinogenesis. In the study, we found the expression of iNOS and COX-2 protein and mRNA were inhibited by CTAE, confirming the suppressive effect of CTAE on the NO and PGE2 production. The present work also showed that the inhibitory effect of CTAE on IL-1β-induced iNOS and COX-2 expression may have resulted from the transcriptional inhibition of iNOS and COX-2 gene, respectively.
Our data showed that IκBα was phosphorylated and was degraded to p-IκBα as well as the NF-κB complex being present both in cytosol and nucleus. Because the phosphorylation and degradation of IκBα is necessary for NF-κB activation (CitationHenkel et al., 1993) and NF-κB signaling pathway involved in the regulation of inflammatory responses (CitationSilverman & Maniatis, 2001; CitationAggarwal et al., 2004). Herein, based on our results, we not only considered that IL-1β successfully induced the inflammation but also showed CTAE blocked the NF-κB signaling pathway in IL-1β-induced NRK-52E cells. In terms of the CTAE anti-inflammatory mechanism, we showed that CTAE exerted an inhibitory effect on the expression of iNOS and COX-2, as well as the production of NO and PGE2; moreover, the inhibitory effect was accompanied by a reduced phosphorylation of IκBα, confirming the inhibition resulted from the blockage of NF-κB pathway. C. taiwanianum T. Yamaza might be a potential candidate as a new drug agent.
Conclusion
The suppression of NO and PGE2 production by CTAE in IL-1β-induced NRK-52E cells could have resulted from the inhibition of protein expression and mRNA transcription of iNOS and COX-2, respectively. Moreover, the capacity of inhibition of NF-κB nuclear protein DNA-binding activity and of IκBα phosphorylation may account, at least in part, for the anti-inflammatory mechanism of C. taiwanianum T. Yamaza.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aggarwal BB, Takada Y, Shishodia S, Gutierrez AM, Oommen OV, Ichikawa H, Baba Y, Kumar A. (2004). Nuclear transcription factor NF-kappa B: Role in biology and medicine. Indian J Exp Biol 42:341–353.
- Ahmed S, Rahman A, Hasnain A, Lalonde M, Goldberg VM, Haqqi TM. (2002). Green tea polyphenol epigallocatechin-3-gallate inhibits the IL-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radic Biol Med 33:1097–1105.
- Almekinders LC, Temple JD. (1998). Etiology, diagnosis, and treatment of tendonitis: An analysis of the literature. Med Sci Sports Exerc 30:1183–1190.
- Appleby SB, Ristimäki A, Neilson K, Narko K, Hla T. (1994). Structure of the human cyclo-oxygenase-2 gene. Biochem J 302(Pt 3):723–727.
- Baeuerle PA, Henkel T. (1994). Function and activation of NF-kappa B in the immune system. Annu Rev Immunol 12:141–179.
- Banerjee T, Van der Vliet A, Ziboh VA. (2002). Downregulation of COX-2 and iNOS by amentoflavone and quercetin in A549 human lung adenocarcinoma cell line. Prostaglandins Leukot Essent Fatty Acids 66:485–492.
- Blanco FJ, Ochs RL, Schwarz H, Lotz M. (1995). Chondrocyte apoptosis induced by nitric oxide. Am J Pathol 146:75–85.
- Bradford MM. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254.
- Chan MM, Huang HI, Fenton MR, Fong D. (1998). In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties. Biochem Pharmacol 55:1955–1962.
- Chen ZS, Lai JS, Kuo YH. (1991). The constituents of Cynanchum taiwanianum. J Chin Chem Soc (Taiwan) 38:393–396.
- Cheon MS, Yoon T, Lee do Y, Choi G, Moon BC, Lee AY, Choo BK, Kim HK. (2009). Chrysanthemum indicum Linné extract inhibits the inflammatory response by suppressing NF-kappaB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol 122:473–477.
- Coussens LM, Werb Z. (2002). Inflammation and cancer. Nature 420:860–867.
- de Boer OJ, van der Wal AC, Becker AE. (2000). Atherosclerosis, inflammation, and infection. J Pathol 190:237–243.
- FitzGerald GA, Patrono C. (2001). The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med 345:433–442.
- Gouze JN, Bianchi A, Bécuwe P, Dauça M, Netter P, Magdalou J, Terlain B, Bordji K. (2002). Glucosamine modulates IL-1-induced activation of rat chondrocytes at a receptor level, and by inhibiting the NF-kappa B pathway. FEBS Lett 510:166–170.
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. (1982). Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem 126:131–138.
- Guo JY, Huo HR, Zhao BS, Liu HB, Li LF, Ma YY, Guo SY, Jiang TL. (2006). Cinnamaldehyde reduces IL-1beta-induced cyclooxygenase-2 activity in rat cerebral microvascular endothelial cells. Eur J Pharmacol 537:174–180.
- Hayden MS, Ghosh S. (2004). Signaling to NF-kappaB. Genes Dev 18:2195–2224.
- Henkel T, Machleidt T, Alkalay I, Krönke M, Ben-Neriah Y, Baeuerle PA. (1993). Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature 365:182–185.
- Huang PL, Lu CM, Yen MH, Wu RR, Lin CN. (1995). Acetophenones from Cynanchum taiwanianum. Phytochemistry 40:537–541.
- Huang PL, Won SJ, Day SH, Lin CN. (1999). A cytotoxic acetophenone with a novel skeleton, isolated from Cynanchum taiwanianum. Helv Chim Acta 82:1716–1720.
- Kiemer AK, Hartung T, Huber C, Vollmar AM. (2003). Phyllanthus amarus has anti-inflammatory potential by inhibition of iNOS, COX-2, and cytokines via the NF-kappaB pathway. J Hepatol 38:289–297.
- Kim AR, Cho JY, Zou Y, Choi JS, Chung HY. (2005). Flavonoids differentially modulate nitric oxide production pathways in lipopolysaccharide-activated RAW264.7 cells. Arch Pharm Res 28:297–304.
- Kim HK, Cheon BS, Kim YH, Kim SY, Kim HP. (1999). Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure–activity relationships. Biochem Pharmacol 58:759–765.
- Koo TH, Lee JH, Park YJ, Hong YS, Kim HS, Kim KW, Lee JJ. (2001). A sesquiterpene lactone, costunolide, from Magnolia grandiflora inhibits NF-kappa B by targeting I kappa B phosphorylation. Planta Med 67:103–107.
- La Grutta S, Gagliardo R, Mirabella F, Pajno GB, Bonsignore G, Bousquet J, Bellia V, Vignola AM. (2003). Clinical and biological heterogeneity in children with moderate asthma. Am J Respir Crit Care Med 167:1490–1495.
- Lee MH, Lee JM, Jun SH, Lee SH, Kim NW, Lee JH, Ko NY, Mun SH, Kim BK, Lim BO, Choi DK, Choi WS. (2007). The anti-inflammatory effects of Pyrolae herba extract through the inhibition of the expression of inducible nitric oxide synthase (iNOS) and NO production. J Ethnopharmacol 112:49–54.
- Lii CK, Chen HW, Yun WT, Liu KL. (2009). Suppressive effects of wild bitter gourd (Momordica charantia Linn. var. abbreviata ser.) fruit extracts on inflammatory responses in RAW264.7 macrophages. J Ethnopharmacol 122:227–233.
- Lin CC, Yen MH, Wu YW, Xu GJ. (1995). The histological and biological studies on Cynanchum taiwanianum. Proceedings of a Symposium on Development and Utilization of Resources of Medicinal Plants in Taiwan. (Du CC., Lu HS, Liu SY, Eds.). TARI Special Publication 48:313–320.
- Lin CN, Huang PL, Wang JJ, Day SH, Lin HC, Wang JP, Ko YL, Teng CM. (1998). Stereochemistry and biological activities of constituents from Cynanchum taiwanianum. Biochim Biophys Acta 1380:115–122.
- Lin CN, Huang PL, Lu CM, Yen MH, Wu RR. (1997a). Revised structure for five acetophenones from Cynanchum taiwanianum. Phytochemistry 44:1359–1363.
- LinYL, Wu YM, Kuo YH. (1997b). Revised structures for four acetophenones from Cynanchum taiwanianum. Phytochemistry 45:1057–1061.
- Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS. (1993). A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 364:626–632.
- Mitchell JA, Saunders M, Barnes PJ, Newton R, Belvisi MG. (1997). Sodium salicylate inhibits cyclo-oxygenase-2 activity independently of transcription factor (nuclear factor kappaB) activation: role of arachidonic acid. Mol Pharmacol 51:907–912.
- Mordan LJ, Burnett TS, Zhang LX, Tom J, Cooney RV. (1993). Inhibitors of endogenous nitrogen oxide formation block the promotion of neoplastic transformation in C3H 10T1/2 fibroblasts. Carcinogenesis 14:1555–1559.
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63.
- Nakagawa T, Yokozawa T. (2002). Direct scavenging of nitric oxide and superoxide by green tea. Food Chem Toxicol 40:1745–1750.
- Nantel F, Denis D, Gordon R, Northey A, Cirino M, Metters KM, Chan CC. (1999). Distribution and regulation of cyclooxygenase-2 in carrageenan-induced inflammation. Br J Pharmacol 128:853–859.
- Narumiya S, Sugimoto Y, Ushikubi F. (1999). Prostanoid receptors: structures, properties, and functions. Physiol Rev 79:1193–1226.
- Newton R, Kuitert LM, Slater DM, Adcock IM, Barnes PJ. (1997a). Cytokine induction of cytosolic phospholipase A2 and cyclooxygenase-2 mRNA is suppressed by glucocorticoids in human epithelial cells. Life Sci 60:67–78.
- Newton R, Stevens DA, Hart LA, Lindsay M, Adcock IM, Barnes PJ. (1997b). Superinduction of COX-2 mRNA by cycloheximide and interleukin-1beta involves increased transcription and correlates with increased NF-kappaB and JNK activation. FEBS Lett 418:135–138.
- Park EJ, Min HY, Ahn YH, Bae CM, Pyee JH, Lee SK. (2004). Synthesis and inhibitory effects of pinosylvin derivatives on prostaglandin E2 production in lipopolysaccharide-induced mouse macrophage cells. Bioorg Med Chem Lett 14:5895–5898.
- Ponnappan U. (1998). Regulation of transcription factor NF kappa B in immune senescence. Front Biosci 3:d152–d168.
- Radi R, Beckman JS, Bush KM, Freeman BA. (1991). Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem 266:4244–4250.
- Silverman N, Maniatis T. (2001). NF-kappaB signaling pathways in mammalian and insect innate immunity. Genes Dev 15:2321–2342.
- Smith WL, DeWitt DL, Garavito RM. (2000). Cyclooxygenases: Structural, cellular, and molecular biology. Annu Rev Biochem 69:145–182.
- Sripanidkulchai B, Junlatat J, Wara-aswapati N, Hormdee D. (2009). Anti-inflammatory effect of Streblus asper leaf extract in rats and its modulation on inflammation-associated genes expression in RAW 264.7 macrophage cells. J Ethnopharmacol 124:566–570.
- Stylianou E, Saklatvala J. (1998). Interleukin-1. Int J Biochem Cell Biol 30:1075–1079.
- Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. (2001). Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res 480–481:243–268.
- Tamura M, Sebastian S, Yang S, Gurates B, Fang Z, Bulun SE. (2002). Interleukin-1beta elevates cyclooxygenase-2 protein level and enzyme activity via increasing its mRNA stability in human endometrial stromal cells: an effect mediated by extracellularly regulated kinases 1 and 2. J Clin Endocrinol Metab 87:3263–3273.
- van der Kraan PM, van den Berg WB. (2000). Anabolic and destructive mediators in osteoarthritis. Curr Opin Clin Nutr Metab Care 3:205–211.
- Vinayagamoorthi R, Koner BC, Kavitha S, Nandakumar DN, Padma Priya P, Goswami K. (2005). Potentiation of humoral immune response and activation of NF-kappaB pathway in lymphocytes in experimentally induced hyperthyroid rats. Cell Immunol 238:56–60.
- Vodovotz Y, Bogdan C, Paik J, Xie QW, Nathan C. (1993). Mechanisms of suppression of macrophage nitric oxide release by transforming growth factor beta. J Exp Med 178:605–613.
- Wen JK, Han M. (2000). Comparative study of induction of iNOS mRNA expression in vascular cells of different species. Biochemistry (Mosc) 65:1376–1379.
- Wu MH, Wang CA, Lin CC, Chen LC, Chang WC, Tsai SJ. (2005). Distinct regulation of cyclooxygenase-2 by interleukin-1beta in normal and endometriotic stromal cells. J Clin Endocrinol Metab 90:286–295.
- Wu Y, Guo SW. (2007). Suppression of IL-1beta-induced COX-2 expression by trichostatin A (TSA) in human endometrial stromal cells. Eur J Obstet Gynecol Reprod Biol 135:88–93.
- Yen GC, Duh PD, Huang DW, Hsu CL, Fu TY. (2008). Protective effect of pine (Pinus morrisonicola Hay.) needle on LDL oxidation and its anti-inflammatory action by modulation of iNOS and COX-2 expression in LPS-stimulated RAW 264.7 macrophages. Food Chem Toxicol 46:175–185.
- Yin MJ, Yamamoto Y, Gaynor RB. (1998). The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature 396:77–80.