Abstract
Context: Cardioprotective effects of various plants are generally attributed to their antioxidant activity. The whole fruit extract of pomegranate (WFEP), Punica granatum L. (Punicaceae), has a potent antioxidant activity.
Objective: To investigate cardioprotective effect of WFEP against doxorubicin (Dox)-induced cardiotoxicity in rats.
Materials and methods: Male Wistar rats were divided randomly into three groups of eight rats each: control (water, 5 mL/kg); Dox (10 mg/kg i.v.) and WFEP (100 mg/kg). Dox was administered in Dox and WFEP groups. After anesthetizing the animals on the last day, electrocardiogram was recorded and blood was analyzed for creatine kinase-MB isoenzyme (CK-MB), lactate dehydrogenase (LDH) and aspartate aminotransferase (AST) activities. Determinations of superoxide dismutase (SOD), reduced glutathione (GSH), lipid peroxidation (LPO) and histopathology of the heart tissues were carried out.
Results: The WFEP group showed decreased QT and increase in heart rate (p < 0.05) compared to the Dox group. Significant decrease in CK-MB (p < 0.01), LDH (p < 0.05) and no such significant decrease in AST were observed as compared to the Dox group. There was significant increase in the level of GSH (p < 0.05), whereas inhibition of LPO and increase in SOD concentration was not significant in the WFEP group compared to the Dox group. Histopathological study of the WFEP-treated group showed slight protection against myocardial toxicity induced by Dox.
Conclusion: Results indicate that WFEP has cardioprotective effect against Dox-induced cardiotoxicity in rats.
Introduction
Various parts of pomegranate, Punica granatum L. (Punicaceae), have been used for various medicinal purposes. Many studies have shown that pomegranate peel extract possesses antioxidant (CitationMurthy et al. 2002), immunomodulatory (CitationGracious Ross et al. 2001), antibacterial activities (CitationPrashanth et al. 2001), wound healing properties (CitationMurthy et al. 2004), etc. Doxorubicin (Dox), an anthracycline antibiotic, has a broad antitumor spectrum, and has been used against a wide variety of hematopoietic malignancies and solid tumors (CitationOgura 2001). Unfortunately, the cardiac toxicity of Dox, resulting in a cardiomyopathy with irreversible congestive heart failure and high mortality, is one of the main factors that limits its use. The molecular mechanisms explaining the cardiotoxicity of anthracyclines are complex, but it appears that the induction of an oxidative stress within myocardial tissue constitutes a common denominator (CitationVergely et al. 2007). Considerable efforts have been made on using antioxidants and iron chelators to protect the heart against Dox toxicity. Dexrazoxane which prevents Dox-induced cardiotoxicity is an iron chelator and possesses potent antioxidant properties. But, due to the high incidence of dexrazoxane-induced myelosuppression, its use has been limited to some advanced stages of malignant disorders (CitationSeifert et al. 1994). Cardioprotective effects of various plants (but not of pomegranate whole fruit) have been reported to be due to antioxidant activity (CitationWattanapitayakul et al. 2005). Therefore, we have hypothesized that the whole fruit extract of pomegranate (WFEP) may exert considerable cardioprotective effect. We have tested the hypothesis on the rats receiving cardiotoxic Dox.
Materials and methods
Animals
All experimental protocols of the animal studies were approved by the Institutional Animal Ethics Committee of Poona College of Pharmacy, Bharati Vidyapeeth University, Pune, India. The animals were purchased from the National Toxicology Center, Pune. Male Wistar albino rats (250–300 g) were housed under standard conditions of 25°C, relative humidity 60% and photo period of 12 h dark/12 h light. Pellet diet (Chakan Oil Mills, Pune, India) and water were provided ad libitum.
Chemicals and sample
Water extract of pomegranate aril, rind, and seeds (WFEP) was a gift from Indus Biotech, Pune, India. Dox powder was supplied by the Serum Institute of India, Pune. Creatine kinase-MB isoenzyme (CK-MB) kit was purchased from Randox Laboratories, Crumlin, UK, and lactate dehydrogenase (LDH) as well as aspartate aminotransferase (AST) from Ecoline, Merck, Mumbai. 5,5-dithiobis (2-nitrobenzoic acid) (DTNB) and thiobarbituric acid (TBA) were purchased from Sigma, St. Louis, MO. All solvents/chemicals used were of analytical grade.
Chemical analysis of WFEP
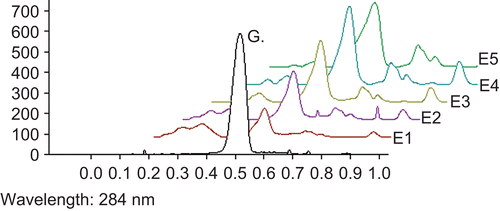
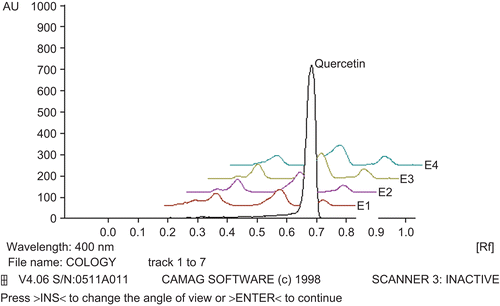
Thin-layer chromatography (TLC) fingerprint of WFEP was established using high-performance thin-layer chromatography (HPTLC). For development of the TLC fingerprint, the volumes of 3, 6, 9, 15, and 45 µL of WFEP (10 mg/mL) with 2 µL of gallic acid and quercetin standard (1 mg/mL) were spotted in the form of bands of width 6 mm on silica gel pre-coated aluminum plate 60F–254 TLC plates, (10 × 10 cm with 250 µm thickness; Merck, Darmstadt) using a Camag Linomat IV (Switzerland) automatic sample spotter. A constant application rate of 0.1 µL/s was used and the space between two bands was 5 mm. The slit dimension was kept at 5 × 0.45 mm and the scanning speed was 10 mm/s.
The monochromator bandwidth was set at 20 nm, each track was scanned three times and baseline correction was used. The plate was developed in the solvent system of ethyl acetate:toluene:formic acid (7:3.5:0.5). Densitometric scanning was performed using a Camag TLC Scanner III in the reflectance-absorbance mode at 284 nm for gallic acid and 400 nm for quercetin, and operated by CATS software (version 3.15).
Experimental procedure
The animals were divided into three groups (control, Dox, and WFEP) containing eight rats in each group. The body weights were recorded daily. WFEP powder dissolved in distilled water was administered (5 mL/kg) orally (by gavage) to the WFEP group daily for 18 days (dose: 100 mg/kg) whereas control as well as Dox groups received distilled water (5 mL/kg). The control group received sterile water for injection (1 mL/kg) through the femoral vein on day 16, whereas the Dox and WFEP groups received Dox (10 mg/kg). After 48 h from the injection, the rats were anesthetized by anesthetic ether, and electrocardiogram (ECG) was recorded using an 8-channel Power Lab System (AD Instruments, Bella Vista NSW, Australia). Changes in QT, ST interval and heart rate were computed from ECG. Blood samples were collected from the retro orbital plexus for measuring serum parameters. The animals were allowed to recover and finally euthanized on day 18. The hearts of the animals were removed and weighed. Four hearts were randomly selected for histopathology. The heart tissues from the remaining four animals were processed and homogenized with 10% chilled TRIS hydrochloride buffer (10 mM, pH 7.4) by tissue homogenizer (Remi Motors, Mumbai) and centrifuged at 7500 rpm for 15 min at 4°C using an Eppendorf 5810-R high-speed cooling centrifuge. The clear supernatant was used for the estimation of superoxide dismutase (SOD), reduced glutathione (GSH), malondialdehyde (MDA) contents and total protein.
Serum parameters
Serum levels of CK-MB, LDH, and AST enzymes were measured by automated chemistry analyzer, Micro lab 300, Merck, using reagent kits.
Tissue parameters
Lipid peroxidation (LPO) assay (MDA content)
The assay method described by CitationSlater and Sawyer (1971) was used. Freshly prepared 0.67% w/v TBA reagent was used and the absorbance was measured by UV/VIS spectrophotometer (JASCO-V-530, Japan) at 532 nm. Calibration curve was prepared using 1,1,3,3-tetra-ethoxy-propane as standard.
Estimation of GSH
The assay of GSH was carried out using DTNB reagent as per the method described by CitationMoron et al. (1979). The color developed was read at 412 nm against reagent blank. Standard GSH was in the range of (10–50 µg) and the content of reduced GSH was expressed as µg of GSH/g of protein.
Estimation of SOD activity
The SOD activity was assayed by the change in the optical density/min at 480 nm of the reaction initiated by epinephrine bitartrate (3 mM) as described by CitationMisra and Fridovich (1972). SOD activity was expressed as units/mg protein. Change in optical density/min at 50% inhibition of epinephrine to adrenochrome transition by the enzyme was taken as the enzyme unit. Calibration curve was prepared by using 10–125 units of SOD.
Determination of total proteins
Protein concentrations were determined using the method of CitationLowry et al. (1951). Diluted membrane fraction aliquots were taken and color developed by adding of 1 N Folin’s phenol reagent, which was measured at 660 nm against a reagent blank. Different concentrations (40–200 µg) of standard protein bovine serum albumin (BSA) were used for standard curve. The values were expressed as mg of protein per g of wet tissue.
Histopathological studies
Hearts were preserved in 10% formalin, processed and embedded in paraffin. Four μm thick paraffin sections were cut on glass slides and stained with hematoxylin and eosin (H&E) reagents and observed under light microscope to evaluate myocardium injury.
Heart weight to body weight ratio
In each group, heart weight to body weight ratio was calculated. Body weight was the weight of animal on the day of sacrifice. Heart weight was measured after placing the heart in cold saline and squeezing out the blood.
Statistical analysis
The data was expressed as mean ± standard error of mean (SEM). One way analysis of variance (ANOVA) was applied to test the significance of difference between average biochemical and ECG parameters of different groups, and multiple comparisons were determined by Tukey’s test. p Value less than 0.05 was considered statistically significant. The statistical analysis was performed using the program Graph Pad Prism version 4.03.
Results
Chemical analysis
The fingerprint chromatogram is shown in and .
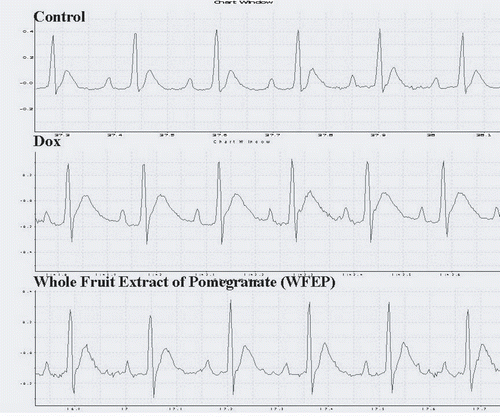
ECG changes
Dox administration significantly increased QT (p < 0.01) and ST (p < 0.05) intervals and significantly decreased heart rate (p < 0.01) compared to the control group. WFEP administration (100 mg/kg) significantly decreased QT interval and increased heart rate (p < 0.05) whereas there was no significant effect on ST interval as compared to the Dox group ( and ).
Table 1. Effect of Dox (10 mg/kg) and WFEP (100 mg/kg) + Dox on ECG (ST and QT intervals and heart rate), tissue parameters (MDA, GSH, and SOD) per mg of protein and HW to BW ratio of three experimental groups.
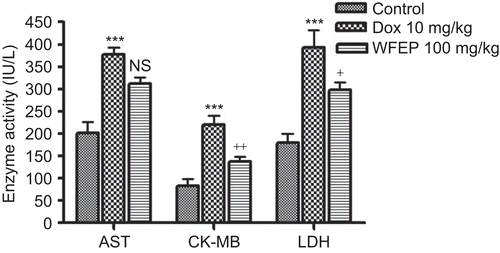
Serum parameters
Administration of Dox (10 mg/kg) increased serum CK-MB, LDH, and AST levels compared to that of control, whereas WFEP (100 mg/kg) administration decreased the levels of CK-MB and LDH significantly compared to that of the Dox group alone ().
Tissue parameters
MDA content was significantly increased whereas GSH and SOD decreased (p < 0.01 for all) in the Dox group compared to the control group. The levels of MDA content and SOD in the WFEP group were not significantly different compared to the respective levels in the Dox group. The GSH level in the WFEP group increased significantly compared to the corresponding level in the Dox group (p < 0.05) ().
Histopathological changes
The incidence of marked tissue injury with myocardial atrophy, nuclear pyknosis, cytoplasmic vacuoles and cytoplasmic eosinophilia were different in Dox and control groups. Similarly, the incidence of marked tissue injury between the Dox and WFEP groups was different. There was no change in the incidence of inflammation and vascular congestion in the three study groups ().
Table 2. Effect of WFEP (100 mg/kg/d) intake followed by Dox (10 mg/kg) administration on histopathological changes in the hearts of rats.
Discussion
The anthracycline antibiotic Dox is one of the most effective chemotherapeutic agents against a wide variety of cancers. It possesses potential for generating free radicals and causes an unusual and often irreversible cardiomyopathy. It has quinone and hydroquinone moieties that can form semiquinone radical intermediates, which in turn can react with oxygen to produce superoxide anion radicals. These can generate both hydrogen peroxide and hydroxyl radicals (·OH), which attack DNA and oxidize DNA bases (CitationSerrano et al. 1999). The production of free radicals is significantly stimulated by the interaction of Dox with iron (CitationChabner et al. 2006). Dox treatment changes ECG and causes prolongation of QT interval in rats (CitationCirillo et al. 2000). The present study has demonstrated increased myocardial injury as indicated by increase in QT interval of ECG pattern and decrease in heart rate in the Dox-treated group. Administration of WFEP along with Dox (10 mg/kg) restored QT interval close to control level and increased heart rate.
The results also showed that administration of Dox-induced cardiotoxicity manifested by a significant increase in serum CK-MB, and LDH. These results are supported by earlier studies which have reported similarly (CitationMostafa et al. 1999; CitationSayed-Ahmed et al. 2001; CitationSaad et al. 2001). In this study the significant decrease in the levels of CK-MB and LDH confirm the protective effect of WFEP against Dox-induced cardiotoxicity. This effect was found to be more pronounced for Lagenaria siceraria Molina Standley (Cucurbitaceae) fruit that was carried out earlier by our group (CitationHassanpour et al. 2008).
The relationship between cardiotoxicity and free radicals can be established by measuring the antioxidant system GSH, SOD and by product MDA. The most abundant reactive oxygen species generated in living cells are superoxide anion and its derivatives, a particularly highly reactive and damaging hydroxyl radical which induces peroxidation of cell membrane lipids (CitationHemnani and Parihar 1998). In this respect, any increase in GSH or SOD activity of the organ appears to be beneficial in the event of increased free radical generation. A single dose administration of Dox (10 mg/kg) caused a significant increase in MDA content (an index of LPO) in cardiac tissues and decrease in myocardial GSH and SOD as compared to the control group, indicating an increase in oxidative stress. These results correlate with previous studies which have demonstrated the involvement of oxidative stress and LPO in Dox-induced cardiomyopathy (CitationFadillioglu et al. 2004; CitationPatil and Balaraman 2005). Improvement in the levels of SOD, and GSH in particular, by administration of WFEP suggest its ability to combat oxidative stress. Apart from enzymes, polyphenolic compounds are commonly found in both edible and inedible plants, and they have been reported to have multiple biological effects, including antioxidant activity (CitationKähkönen et al. 1999; CitationKarou et al. 2005; CitationMaisuthisakul et al. 2007). WFEP contains large amounts of polyphenolic compounds. The total phenolics content was 157 ± 6.3 mg gallic acid equivalent/g of WFEP by Folin-Ciocalteu method. In an earlier report by CitationPanichayupakaranant et al. (2010) the method for preparation of pomegranate peel extract rich in ellagic acid has been described. The ellagic acid content of extract was increased from 7.06 to 13.63% w/w by partitioning of pomegranate peel extract between ethyl acetate and 2% aqueous acetic acid. The extract showed antioxidant activity by 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay. The authors mentioned that the extract was stable at various temperature and humidity conditions, when stored as dry powder. Our study is not similar to the above mentioned report. We have not carried out antioxidant assay by DPPH. We observed gallic acid as a major component in WFEP which is known to potentiate several pharmacological and biochemical pathways being a strong antioxidant. However, the contribution of ellagic acid in the cardioprotective effect cannot be ruled out. CitationNaz et al. (2007) have also isolated gallic acid, quercetin, cyanidin-3-glucose, and myricetin from pomegranate fruit.
Histopathological studies revealed myocardial atrophy, nuclear pyknosis, cytoplasmic vacuoles and cytoplasmic eosinophilia in the Dox-treated rat hearts. Similar observations have also been made in earlier studies on Dox-induced cardiotoxicity (CitationMorishima et al. 1998; CitationSaad et al. 2001). Histopathological observations showed less damage in the WFEP group than the Dox group. Dox also causes decrease in heart weight to body weight ratio, which indicates loss of myofibrils and cytoplasmic vacuolization in myocytes (CitationMukherjee et al. 2003). In this study the ratio of heart weight to body weight in the Dox group decreased insignificantly compared to the control group. This ratio was increased in the WFEP group as compared to the Dox-treated group.
Conclusion
It can be concluded that WFEP has the ability to reduce oxidative stress in Dox-treated animals and show cardioprotective action.
Acknowledgments
The authors acknowledge Dr. S.S. Kadam, Vice Chancellor, and Dr. K.R. Mahadik, Principal, Poona College of Pharmacy for their keen interest in this work. We also thank Dr. S. Joshi and C. Kulkarni, for their help during the study. We thank V. Mohan from Indus Biotech, Pune, for gifting the WFEP powder.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Chabner BA, Amrein PC, Druker BJ, Michaelson MD, Mitsiades CS, Goss PE, Ryan DP, Ramchandra S, Richardson PG, Supko JG, Wilson WH. (2006). Antineoplastic agents. In: Brunton LL, Lazo JS, Parker KL, 11th edition. Goodman and Gilman’s the Pharmacological Basis of Therapeutics. USA: Mc Graw- Hill Company, 1357–1359.
- Cirillo R, Sacco G, Venturella S, Brightwell J, Giachetti A, Manzini S. (2000). Comparison of doxorubicin- and MEN 10755-induced long-term progressive cardiotoxicity in the rat. J Cardiovasc Pharmacol, 35, 100–108.
- Fadillioglu E, Oztas E, Erdogan H, Yagmurca M, Sogut S, Ucar M, Irmak MK. (2004). Protective effects of caffeic acid phenethyl ester on doxorubicin-induced cardiotoxicity in rats. J Appl Toxicol, 24, 47–52.
- Gracious Ross R, Selvasubramanian S, Jayasundar S. (2001). Immunomodulatory activity of Punica granatum in rabbits–a preliminary study. J Ethnopharmacol, 78, 85–87.
- Hassanpour FM, Bodhankar SL, Dikshit M. (2008). Cardioprotective activity of fruit of Lagenaria siceraria (Molina) Standley on doxorubicin-induced cardiotoxicity in rats. Int J Pharmacol, 4, 466–471.
- Hemnani T, Parihar MS. (1998). Reactive oxygen species and oxidative DNA damage. Indian j Physiol Pharmacol, 42, 440–452.
- Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, Heinonen M. (1999). Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem, 47, 3954–3962.
- Karou D, Dicko MH, Simpore J, Traore AS. (2005). Antioxidant and antibacterial activities of polyphenols from ethnomedicinal plants of Burkina Faso. Afr J Biotechnol, 4, 823–828.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem, 193, 265–275.
- Maisuthisakul P, Suttajit M, Pongsawatmanit R. (2007). Assessment of phenolic content and free radical scavenging capacity of some Thai indigenous plants. Food Chem, 100, 1409–1418.
- Misra HP, Fridovich I. (1972). The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem, 247, 3170–3175.
- Morishima I, Matsui H, Mukawa H, Hayashi K, Toki Y, Okumura K, Ito T, Hayakawa T. (1998). Melatonin, a pineal hormone with antioxidant property, protects against adriamycin cardiomyopathy in rats. Life Sci, 63, 511–521.
- Moron MS, Depierre JW, Mannervik B. (1979). Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta, 582, 67–78.
- Mostafa AM, Nagi MN, Al Rikabi AC, Al-Shabanah OA, El-Kashef HA. (1999). Protective effect of aminoguanidine against cardiovascular toxicity of chronic doxorubicin treatment in rats. Res Commun Mol Pathol Pharmacol, 106, 193–202.
- Mukherjee S, Banerjee SK, Maulik M, Dinda AK, Talwar KK, Maulik SK. (2003). Protection against acute adriamycin-induced cardiotoxicity by garlic: Role of endogenous antioxidants and inhibition of TNF-alpha expression. BMC Pharmacol, 3, 16.
- Murthy KN, Jayaprakasha GK, Singh RP. (2002). Studies on antioxidant activity of pomegranate (Punica granatum) peel extract using in vivo models. J Agric Food Chem, 50, 4791–4795.
- Murthy KN, Reddy VK, Veigas JM, Murthy UD. (2004). Study on wound healing activity of Punica granatum peel. J Med Food, 7, 256–259.
- Naz S, Siddiqi R, Ahmad S, Rasool SA, Sayeed SA. (2007). Antibacterial activity directed isolation of compounds from Punica granatum. J Food Sci, 72, M341–M345.
- Ogura M. (2001). [Adriamycin (doxorubicin)]. Gan to Kagaku Ryoho, 28, 1331–1338.
- Panichayupakaranant P, Itsuriya A, Sirikatitham A. (2010). Preparation method and stability of ellagic acid-rich pomegranate fruit peel extract. Pharm Biol, 48, 201–205.
- Patil L, Balaraman R. (2005). Protective effect of green tea extract on doxorubicin induced cardiotoxicity in rats. OPEM, 5, 137–143.
- Prashanth D, Asha MK, Amit A. (2001). Antibacterial activity of Punica granatum. Fitoterapia, 72, 171–173.
- Saad SY, Najjar TA, Al-Rikabi AC. (2001). The preventive role of deferoxamine against acute doxorubicin-induced cardiac, renal and hepatic toxicity in rats. Pharmacol Res, 43, 211–218.
- Sayed-Ahmed MM, Khattab MM, Gad MZ, Osman AM. (2001). Increased plasma endothelin-1 and cardiac nitric oxide during doxorubicin-induced cardiomyopathy. Pharmacol Toxicol, 89, 140–144.
- Seifert CF, Nesser ME, Thompson DF. (1994). Dexrazoxane in the prevention of doxorubicin-induced cardiotoxicity. Ann Pharmacother, 28, 1063–1072.
- Serrano J, Palmeira CM, Kuehl DW, Wallace KB. (1999). Cardioselective and cumulative oxidation of mitochondrial DNA following subchronic doxorubicin administration. Biochim Biophys Acta, 1411, 201–205.
- Slater TF, Sawyer BC. (1971). The stimulatory effects of carbon tetrachloride and other halogenoalkanes on peroxidative reactions in rat liver fractions in vitro. General features of the systems used. Biochem J, 123, 805–814.
- Vergely C, Delemasure S, Cottin Y, Rochette L. (2007). Preventing the cardiotoxic effects of anthracyclines: From basic concepts to clinical data. Heart Metab, 35, 1–7.
- Wattanapitayakul SK, Chularojmontri L, Herunsalee A, Charuchongkolwongse S, Niumsakul S, Bauer JA. (2005). Screening of antioxidants from medicinal plants for cardioprotective effect against doxorubicin toxicity. Basic Clin Pharmacol Toxicol, 96, 80–87.