Abstract
Context: Enicostemma hyssopifolium Verdoon (Gentianaceae) has been documented for various therapeutic effects in traditional systems of medicine; the hypoglycemic and hypolipidemic activities are also well reported.
Objective: Bioactivity guided fractionation of methanol extract of E. hyssopifolium to test the hypothesis that E. hyssopifolium and its constituents influence cells and systemic glucose homeostasis.
Materials and methods: Derived fraction and isolated compounds were studied for (1) aldose reductase (AR) inhibition, (2) α-glucosidase inhibition, (3) effect on gluconeogenesis in rat hepatoma, (4) cytoprotection against streptozotocin (STZ)-induced toxicity on RINm5F cells, (5) normalization of glycemic control in acute hyperglycemic rat model, and (6) insulin-releasing effect both in vitro and in vivo.
Results: The results indicated that E. hyssopifolium can modify the glucose homeostasis at the cellular level. Two bioactive constituents were identified. Swertisin was found to inhibit AR (IC50 1.23 μg/mL) and α-glucosidase (IC50 1.89 μg/mL). It also possessed a significant cytoprotective action of RINm5F cell line against toxicant STZ. Swertiamarin was found to have hepatic gluconeogenesis inhibiting and insulin-releasing effect on rat hepatoma and RINm5F cells, respectively. The results of the in vivo study showed that swertiamarin, unlike the in vitro effect, produced no significant raise of insulin secretion. Swertisin normalized the serum glucose 60 min after high dose of glucose (2 g/kg, i.p.) in rats.
Discussion and conclusion: These findings demonstrate that the fraction derived from the aerial part of E. hyssopifolium achieve normoglycemic status in hyperglycemic conditions via various mechanisms. The constituents swertiamarin and swertisin are responsible for bioactivity.
Introduction
Enicostemma hyssopifolium Verdoon (Gentianaceae) is a perennial herb attaining a height of 5–20 inches. It produces white colored flowers, which are arranged in clusters. The extract of E. hyssopifolium has been reported to affect numerous activities resulting in hypoglycemic, antioxidant, and hypolipidemic effects in non-insulin-dependent diabetes mellitus patients (CitationVasu et al. 2003): the antidiabetic effect is also reported by other workers (CitationVijayvargia et al. 2000; CitationMaroo et al. 2002; CitationMurali et al. 2002; CitationUpadhyay and Goyal 2004; CitationSrinivasan et al. 2005). It is cited in ancient literature as an antimalarial, antipyretic, and laxative agent (CitationVyas et al. 1979). It is incorporated in an antidiabetic herbomineral preparation (CitationBabu and Prince 2004). The aerial parts have been reported to show hypolipidemic effect in p-dimethylaminobenzene-induced hepatotoxic animals (CitationGopal et al. 2004; CitationVasu et al. 2005). Various Ayurvedic formulations containing E. hyssopifolium have produced anti-hyperglycemic activity in hyperglycemic rat models (CitationGupta et al. 1962). This plant has been used as folk medicine for the treatment of diabetes mellitus in western and southern India (CitationGupta et al. 1962). Hot aqueous extract of E. hyssopifolium has been used by tribal inhabitants for the treatment of diabetes, fever, stomachache, dyspepsia, and malaria in interior of Gujarat state, India (CitationMurali et al. 2002). This herb is also known for its anti-inflammatory (CitationSadique et al. 1987) and anticancer properties (CitationKavimani and Manisenthlkumar 2000). Crude plant extracts have been tested in clinical trials for treatment of diabetes (CitationUpadhyay and Goyal 2004). The plant is reported to contain phenols, tannins, flavonoids, glycosides, anthraquinones, sterols (CitationVasu et al. 2005), flavonoids (apigenin, genkwanin, isovitexin, swertisin, saponarin, 5-O-glucosylswertisin, and 5-O-glucosylisoswertisin) (CitationGhosal and Jaiswal 1980), monoterpene alkaloids (enicoflavin and gentiocrucine) (CitationGhosal et al. 1974; CitationChaudhuri et al. 1975), catechins, saponins, steroids (CitationRetnam and DeBritt 1988), a triterpene sapogenin, betulin (CitationRai and Thakar 1966; CitationDesai et al. 1966), an alkaloid, gentianin (CitationGovindachari et al. 1966), a secoiridoid glycoside, swertiamarin (CitationRai and Thakar 1966; CitationDesai et al. 1966), phenolic acids (vanillic acid, syringic acid, p-hydroxy benzoic acid, protocatechuic acid, p-coumaric acid, and ferulic acid) (CitationDaniel and Sabnis 1978), amino acids (l-glutamic acid, tryptophan, alanine, serine, aspartic acid, l-proline, l-tyrosine, threonine, phenyl alanine, l-histidine monohydrochloride, methionine, isoleucine, l-arginine monohydrochloride, DOPA, l-glycine, 2-amino butyric acid, and valine) (CitationRetnam and DeBritt 1988).
Although several types of compounds have hypoglycemic properties (CitationLamba et al. 2000), the mechanisms for achieving this effect are different. There is a growing body of evidence that flavonoids, although active in several systems, are responsible for modulation of glucose disposal and insulin release (CitationAttele et al. 2002). For example, the crude methanol extract of E. hyssopifolium showed hypoglycemic and hypolipidemic effects in rats (CitationSrinivasan et al. 2005). However, the active principles responsible for hypoglycemia are yet to be discovered.
In this study, we demonstrated that swertisin, a C-glycosyl flavonoid of E. hyssopifolium, stimulated insulin secretion from rat insulinoma cell line and also acted via other mechanisms by virtue of which the plant exhibited hypoglycemic activity.
Materials and methods
Reagents
Reduced nicotinamide adenine dinucleotide phosphate (NADPH), lithium sulfate, d-xylose, quercetin, sucrose, maltose, RPMI-1640, l-glutamine, sodium bicarbonate, glucose, 4-(-2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), sodium pyruvate, fetal bovine serum, streptomycin, penicillin, and 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazoliumbromide (MTT) was procured from Hi Media, Mumbai, India. Dexamethasone (DEX), streptozotocin (STZ) and 8-(4-chlorophenylthio) adenosine 3′, 5′-cyclic monophosphate sodium salt (pCPT-cAMP) was purchased from Sigma-Aldrich Corporation, Bangalore, India. Voglibose (Abbott India, Goa, India) was received as a gift sample.
Extraction and fractionation
The flowering plants were collected fresh in the month of August 2007 from the village nearby Junagadh district, Gujarat, India, and were authenticated as E. hyssopifolium Verdoon (Gentianaceae) by Dr. K.S. Rajput, at the Botany Department, M.S. University of Baroda, Gujarat. Voucher specimens (Pharmacy/EH I/Samp 1) have been deposited in our laboratory for future reference. Aerial parts, including leaves, stem, and flowers, were separated, shade-dried, and powdered in a pulverizer to obtain gauge 10 powder. E. hyssopifolium fraction (EHFRA) was prepared from methanol extract (obtained by hot continuous extraction in a Soxhlet apparatus, yield 26.58% w/w) by suspending the methanol extract (50 g) in distilled water (500 mL) and partitioned with hexane (3 × 200 mL) and then with n-butanol (3 × 200 mL). Butanol was allowed to evaporate at room temperature. The dried fraction (3.5 g) was stored at 2–8°C.
Purification
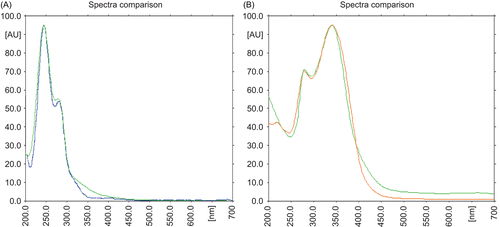
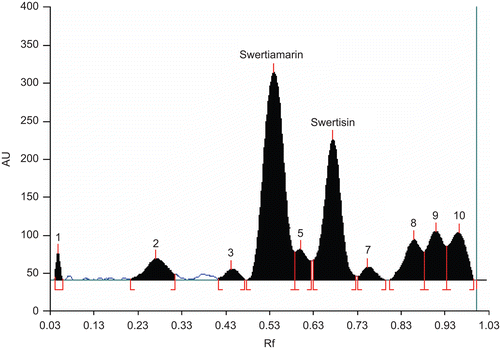
Thin-layer chromatographic studies on precoated high-performance thin-layer chromatography (HPTLC) plates (Merck, Germany) confirm the presence of two major components in EHFRA (). Compound 1 (Rf 0.57) and compound 2 (Rf 0.71) were identified and isolated from EHFRA. It was solubilized in methanol and chromatographed by column chromatography over silica gel (60–120). Elution was done first with petroleum ether (60–80°C), then with gradual gradient of ethyl acetate (5–100%), followed by gradient of methanol (0–10%). Fractions (25 mL each) were monitored by thin layer chromatography (TLC). Fractions showing presence of compounds 1 and 2 were pooled together and volume was reduced under vacuum. Compounds were then further purified by repetitive preparative TLC using silica gel HF254 as stationary phase and ethyl acetate:methanol:water (77:15:8 v/v/v) as mobile phase. Compounds 1 and 2 were identified under UV light (254 nm) and eluted with methanol. Methanol was evaporated on a water bath (70°C) and compounds were collected after repeated crystallization from cold methanol.
Figure 1. HPTLC chromatogram of EHFRA, mobile phase—ethylacetate:methanol:water (77:15:8), scanned at 254 nm.
AR inhibition
Inhibitory effect on rat lens aldose reductase (AR) activity was measured according to the method of CitationHayman and Kinoshita (1965) with slight modifications. Briefly, the reaction mixture was prepared at 25°C, with a total volume of 3 mL, containing 50 mM Na phosphate buffer (pH 6.2), 0.125 mM NADPH, 400 mM LiSO4, 0.3 mL of enzyme preparation and 10 mM d-xylose as a substrate with or without sample. The reaction was initiated by addition of NADPH and continued for 5 min. Absorbance readings were conducted in a double beam spectrophotometer at 340 nm, at every 30 s intervals for 5 min. Quercetin; a known AR inhibitor was used as positive control to compare the extracted compounds’ inhibitory activity. Samples were reconstituted in phosphate-buffered saline (PBS) to prepare stock solutions. Percentage inhibition of AR activity and IC50 value was obtained from a dose–response curve (DRC) calculated by plotting dose concentration versus percentage inhibition.
Alpha glucosidase inhibition
The assay was performed as per the method of CitationDahlqvist (1964). The inhibitory activity was determined by incubating a solution of pancreatic alpha glucosidase (Sigma, St. Louis, MO) containing 0.588 IU of enzyme with 80 mM sodium phosphate buffer, pH 7 (700 μL) containing various concentrations of the samples at 37°C for 10 min. Sucrose or maltose was used as substrate. Voglibose was used as positive control.
Cytoprotective effect
Cell culture
Rat insulinoma RINm5F cell line was obtained from the National Centre for Cell Sciences, Pune, India. RINm5F cells were selected based on their established use to study cytoprotective effects (CitationKang et al. 2007). Cells were cultured and incubated at 37°C in an atmosphere of 5% CO2 and 95% air. Growth media of RPMI-1640 (Hi Media) supplemented with 2 mM l-glutamine adjusted to contain 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM HEPES, and 1 mM sodium pyruvate, 90%; fetal bovine serum 10% (Hi Media) was added with streptomycin (100 μg/mL) and penicillin (100 units/mL). Cells were sub-cultured when 80% confluency was reached. Cells were plated in 96-well plates (1 × 105 cells/well) in 200 μL of media without test sample and allowed to reach a 70–80% confluent state. Media was removed and cells were dosed with toxicant (10 μg STZ) and different concentrations of test samples (10–100 μg). The cytoprotective action of samples was evaluated after 24 h incubation. The plates were submitted to the MTT viability assay at their designated time of 24 h. To assess cell viability, 10 μL of a 10 mg/mL solution of MTT was added directly to cells in the 96-well plates and incubated for 4 h. After 4 h, media was removed and 200 μL of dimethylsulfoxide was added, the resulting intracellular purple formazan was quantified by spectrophotometric means at a wavelength of 540 nm using ELISA reader.
Insulin release study in vitro
The cells were grown and allowed to reach a 70–80% confluent state. Culture medium was then replaced with PBS (pH 7.2) followed by 40 min incubation in fresh Krebs–Ringer Balanced Buffer containing NaCl (115 mM/L), KCl (4.7 mM/L), CaCl2 (1.28 mM/L), MgSO4.7H2O (1.2 mM/L), KH2PO4 (1.2 mM/L), NaHCO3 (10 mM/L), and HEPES (25 mM/L), supplemented with glucose and bovine serum albumin (0.5%), pH 7.4. The cells were incubated (37°C, 5% CO2) with different concentrations of test and the standard compounds, for 30 min. Effect of test and standard was evaluated in the presence of 16.7 mM and 1.1 mM of glucose separately. The supernatant was collected and the insulin amount was measured by enzyme-linked immunosorbent assay using commercial rat insulin ELISA Kit (Linco Research, St. charles, MO).
Hepatic gluconeogenesis
Hepatocytes were isolated by the collagenase perfusion technique (CitationSarkar and Sil 2006). Viability was measured by trypan blue cell exclusion method, 1.3 × 105 cells/well were seeded in 24-well plates. Cells were incubated in medium containing 500 nM of DEX and 0.1 mM of 8-(4-chlorophenylthio) adenosine 3′, 5′-cyclic monophosphate sodium salt (pCPT-cAMP). In the treatment groups, the test samples were added to the DEX/pCPT-cAMP supplemented media with sample. Insulin (10 nM) was added to the cells as a positive control (CitationWaltner-Law et al. 2002). After 5 h incubation, media were removed and cells were washed three times with PBS, incubated with 2 mL of glucose production buffer containing 5 mM sodium pyruvate and 50 mM sodium lactate in Dulbecco’s modified essential medium (without glucose and phenol red, pH 7.4) for 3 h. The glucose concentration in the glucose production buffer was determined by an enzymatic-spectrophotometric glucose oxidase/peroxidase assay kit (Beacon Diagnostics, Gujarat, India). The protein concentration of each sample was determined by the method described by CitationLowry et al. (1951). The amount of glucose production was normalized by protein concentration. Results were expressed as the percentage of glucose production of negative control, which was the glucose production from the cells without test sample treatment.
In vivo studies
Wistar rats of either sex (180 ± 35 g) obtained from Zydus Research Centre, Ahmedabad, Gujarat, were used. The rats were housed in an air conditioned room (25 ± 5°C, 60–65% relative humidity) with a lighting schedule of 12 h light and 12 h darkness. Animals had free access to a standard pellet diet and tap water. The study was conducted after obtaining institutional animal experimentation committee clearance. The in vivo insulin secreting and glucose lowering properties of compounds 1 and 2 were evaluated. Two days prior to the study the animals were randomized and divided into four groups (n = 5), i.e., negative control group, two test groups and positive control group. The animals were fasted overnight and no food was allowed on the day of experiment, though water was available ad libitum. An i.p. glucose tolerance test was used to study the in vivo effect. In rats fasted overnight, vehicle (normal saline)/test/standard compounds were administered i.p. on a body weight basis 90 min before the i.p. injection of glucose (2 g/kg body weight). The control group was given only saline i.p. soon after the administration of glucose (0 min) and the positive control group was administered tolbutamide (10 mg/kg) i.p., one of the test groups was administered swertiamarin (10 mg/kg), and another test group was administered swertisin (10 mg/kg) i.p. Blood was collected via the retro-orbital route under light ether anesthesia, and the samples were marked. A subsequent blood collection was done at 60 min. Blood samples were centrifuged and the separated serum was subjected to glucose estimation. Serum for insulin estimation was stored at −70°C until used for the insulin estimation. The glucose estimation was conducted with GOD/POD method. Furthermore, the insulin estimation was conducted using a rat insulin ELISA kit.
Statistical analysis
In all sets of experiments, the differences between the control and experimental groups were compared using one-way analysis of variance followed by Bonferroni’s multiple comparison test for mean ± SEM of observations. Any difference with p values less than 0.05 were considered as statistically significant.
Results
Fractionation, purification and identification
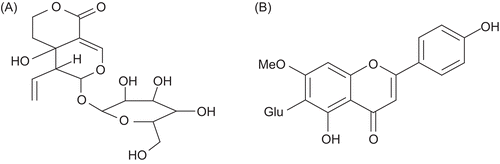
HPTLC fingerprint of EHFRA was obtained by densitometric scanning (Scanner 3, Camag, Switzerland). Compound 1 was identified as swertiamarin by spectral comparison with the reference substance procured from Wako, Osaka, Japan. Compound 2 was identified as C-glycosyl flavone (6-β-d-glucopyaranosy; 4′-5-dihydroxy, 7-methoxy flavone), swertisin. It is reported in the literature as one of the major components of E. hyssopifolium (CitationGhosal and Jaiswal 1980). Identification was done on the basis of phytochemical, elemental, IR and UV spectrum analysis. Content of swertiamarin (19.54%) and swertisin (15.82%) in EHFRA was estimated using HPTLC.
Swertisin (C22H22O10): yellow crystalline powder, m.p. 242°C (decomp.), color test: FeCl3, brown, MgHCl, yellow, ZnHCl, pink. Rf value on paper, 0.54 (butanol:acetic acid:water – 4:2:1), UV λmax (methanol) nm: 272, 332. UV λmax (methanol + AlCl3) nm: 280, 300, 344. UV λmax (methanol+ AlCl3 + HCl) nm: 280, 300, 345. UV λmax (methanol + sodium methoxide) nm: 273, 390. UV λmax (methanol + sodium acetate) nm: 271, 335. UV λmax (methanol + sodium acetate + boric acid) nm: 271, 333. CH percentage calculated for C22H22O10: C, 59.19; H, 4.97. Found: C, 59.56; H, 5.04. The IR spectrum was also found to be superimposed with that of swertisin reported in the literature (CitationKomatsu et al. 1967).
Aerial parts of E. hyssopifolium were found to contain 0.499% w/w swertiamarin and 0.088% w/w of swertisin when estimated using HPTLC.
AR inhibition
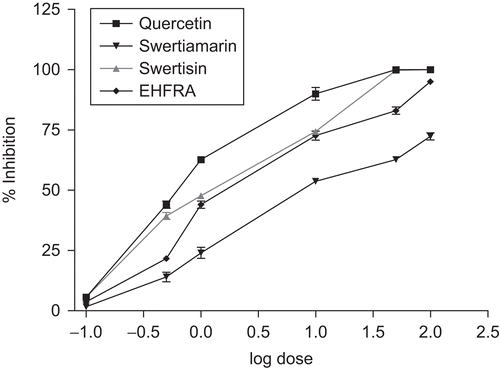
The EHFRA and two isolated compounds were found to inhibit lens AR to various extents. Their inhibitory potencies and IC50 values on the AR enzyme were estimated (). It is evident from the DRC that swertisin offered excellent AR inhibiting activity with IC50 value 1.23 μg/mL. Swertiamarin exhibited comparatively less activity (IC50 value 7.59 μg/mL). At 10 μg/mL concentration, swertisin showed AR inhibition of 82.33%. The activity of EHFRA, swertiamarin and swertisin are presented in .
Table 1. Aldose reductase inhibitory activity of fraction and isolated compounds of Enicostemma hyssopifolium.
Alpha glucosidase inhibition
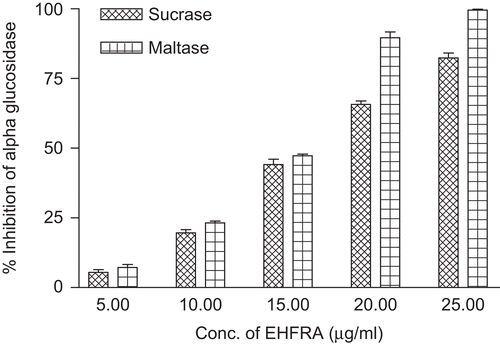
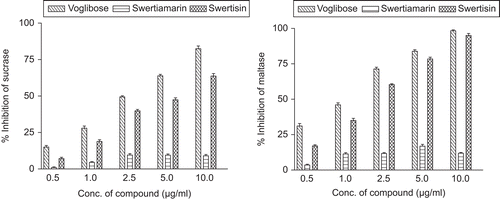
The activity of EHFRA against sucrase and maltase are presented in . illustrates the sucrase and maltase inhibition by isolated compounds. Swertiamarin, a bitter principle, did not inhibit either maltase or sucrase. The activity of EHFRA obtained was probably only due to presence of swertisin, as swertisin alone produced 95.02 and 63.74% inhibition of maltase and sucrase activity, respectively, at the concentration of 10 µg/mL. represents IC50 values of fraction and compounds.
Table 2. Alpha glucosidase inhibitory effect of fraction and compounds.
Cytoprotective effect
The EC50 value for the bioactive fraction and compound was calculated by plotting the dose versus cell viability curve. Dose-dependent cell viability data is summarized in . Study confirms the cytoprotective activity of EHFRA with EC50 63.1 μg (data not shown). Swertisin possesses potent cytoprotective activity against STZ-induced damage in RINm5F cell line. Swertiamarin, a major bitter iridoid glycoside, showed no effect as a protective agent. Cytoprotective activity of EHFRA is due to the presence of swertisin. EC50 value of swertisin was determined to be 9.2 μg.
Table 3. Cytoprotective effect of isolated compounds on RINm5F cells.
Insulin release study in vitro
The effect of EHFRA at 100 µg/mL was tested. At 1.1 mM glucose concentration, no significant rise in insulin level was observed as compared with respective control. The fraction was found to evoke insulin secretion from RINm5F cells in hyperglycemic condition (16.7 mM glucose) as compared to control (without fraction). Positive standard tolbutamide (10 µg/mL) was found to raise insulin secretion significantly (p < 0.001) in all studied concentrations and conditions, i.e., 1.1 mM and 16.7 mm glucose. Swertiamarin was found to possess insulin secreting activity (p < 0.01) at both glucose concentrations, whereas swertisin (100 µg/mL) significantly (p < 0.001) raised insulin levels only in hyperglycemic environment ().
Table 4. Effects of isolated compounds on insulin secretion from RINm5F cells.
Hepatic gluconeogenesis
Prolonged and uncontrolled diabetes is presumed to be predominated by an increase in gluconeogenesis and overproduction of glucose from the liver; therefore it would be of interest to find out the effect of compounds on this process. Results of glucose production assay performed on isolated rat hepatocyte are presented as percentages relative to the glucose produced by DEX/pCPT-cAMP-treated hepatocytes (100%). A significant (p < 0.001) inhibition of glucose production was observed in hepatocytes after treatment with EHFRA at the concentration of 100 µg/mL (). Insulin clearly inhibits the gluconeogenesis. Although studying the effect of swertiamarin and swertisin in a dose-dependent manner (10, 50 and 100 µg/mL) swertiamarin produced 76.5 and 40.4% glucose as compared to control at 50 and 100 µg/mL doses, respectively, whereas swertisin produced 52% glucose as compared to control at 100 µg/mL concentration.
Table 5. Inhibition of gluconeogenesis in rat hepatocytes.
In vivo studies
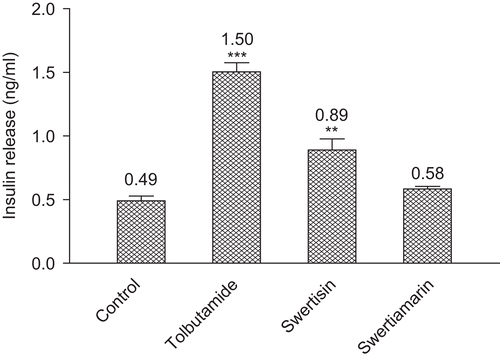
Results shown in and present the effect of the isolated compounds on the serum glucose levels and insulin release of rats, respectively. The dose level of tolbutamide, swertisin and swertiamarin were kept to 10 mg/kg body weight. Swertisin restored normoglycemic conditions 60 min after glucose administration. It also produced a significant rise in serum insulin level. The activity was not comparable to the positive standard tolbutamide at the same dose, i.e., 10 mg/kg, but it was a promising anti-hyperglycemic response along with insulin secreting activity (p < 0.01). Swertiamarin showed a decrease in serum glucose level but it was inferior to the response produced by swertisin. Similarly, the insulin secreting activity of swertiamarin was also not significant as compared to the control group.
Table 6. Hypoglycemic effect of isolated compounds in rat.
Table 7. Insulin secretogogue action of isolated compounds in rat.
Discussion
C-Glucoflavones have been recorded in 78 species in nine genera of Gentianaceae (CitationJensen and Schripsema 2002). Swertisin, a C-glucoflavone, one of the major C-glucoflavones present in aerial parts of E. hyssopifolium (CitationGhosal and Jaiswal 1980), has been found to have mild antioxidant and α-glucosidase inhibitory properties (CitationShibano et al. 2008). Swertisin isolated from E. hyssopifolium was studied for its mechanism of action as an antidiabetic agent. It was found to possess excellent inhibitory activity on AR which is the key enzyme of polyol pathway, to catalyze the conversion of excess glucose into fructose which in turn leads to one of the long-term diabetic complications, cataract. The study confirms that AR inhibitory activity of EHFRA is mainly due to the presence of swertisin. Swertiamarin showed very weak action with a higher IC50 value (7.59 μg/mL).
An anti-hyperglycemic effect may be mediated by the regulation of glucose uptake from the intestinal lumen, through the inhibition of carbohydrate digestion or absorption. This could be possible by retarding the postprandial glucose levels by inhibition of intestinal α-glucosidase complex (CitationShim et al. 2003). In order to corroborate the hypothesis, EHFRA was examined to determine its ability to inhibit the pancreatic α-glucosidase activity. The fraction inhibited α-glucosidase activity in vitro in a concentration-dependent manner (IC50 of 15.31 μg/mL for maltase and 16.38 μg/mL for sucrase). Swertisin showed promising inhibition whereas swertiamarin did not alter the enzyme kinetics in remarkable quantity. Further, kinetic study has been performed to understand the nature of inhibition of α-glucosidase by swertisin. The inhibitory effect was checked on both sucrase and maltase activity of α-glucosidase using sucrose and maltose as substrate, respectively. Michaelis–Menten constant (Km) remained unchanged in the presence of the swertisin. Decreased Vmax with both substrates and constant Km indicate that swertisin inhibit AR in non-competitive manner.
Oxidative stress is suggested as a mechanism underlying the complications of diabetes (CitationHalliwell and Gutteridge 1989). Reactive oxygen species (ROS) have been implicated in the pathology of various disease states, including diabetes mellitus and it is well known that superoxide anion is the radical formed by the reduction of molecular oxygen that may lead to ROS such as hydrogen peroxide and hydroxyl radicals (CitationBaynes 1991). In the STZ-induced rat diabetes model an activated oxygen species was thought to be formed, and involved in the death of the β-cells (CitationSchmezer et al. 1994). STZ, a potent DNA methylating agent acts as a free radical donor in the pancreas where the β-cells are particularly sensitive to damage from free radicals because of a low level of free radical scavenging enzymes (CitationLukic et al. 1998; CitationSpinas 1999). Medicinal plants are currently being investigated for their pharmacological properties in the regulation of blood glucose and apoptosis induced by oxidative stress, a process which is pivotal in the pathology of diabetes mellitus (CitationKinloch et al. 1999; CitationLatha et al. 2004). Investigation for the protective effects of plant extracts, fractions and isolated phytoconstituents has been carried out on the pancreatic β-cell line (RINm5F). Cytoprotection of rat insulinoma cells against toxicity induced by STZ was observed only in presence of swertisin (EC50 9.2 μg).
Dose-dependent insulin-releasing activities at 1.1 and 16.7 mM glucose levels were determined. Both the isolated compounds were found to stimulate insulin release from, RINm5F. Swertiamarin potentiated insulin release in vitro at 1.1 mM and 16.7 mM glucose. Interestingly, β-cell sensitivity to swertisin is higher at 16.7 mM than at 1.1 mM glucose. In vivo study further supports the insulin secreting property of swertisin; however, swertiamarin did not contribute significantly to insulin secretion in vivo. Glucose is the principal physiological stimulator of insulin secretion, entering the pancreatic β-cell through membrane-bound glucose transporters (GLUT2 in rodents, GLUT1 in humans). This hexose sugar then undergoes rapid phosphorylation and metabolism by glucokinase to generate adenosine triphosphate (ATP), and the increase in intracellular ATP:ADP (adenosine diphosphate) ratio causes closure of KATP channels (CitationTarasov et al. 2004), resulting in membrane depolarization, opening of voltage-dependent calcium channels, a rapid rise in intracellular Ca2+, and ultimately insulin release (CitationFlatt and Lenzen 2004; CitationWiederkehr and Wollheim 2006). The circulating insulin levels of tolbutamide and swertisin treated rats were significantly higher when compared to the control group. The increase in insulin levels in rats was attributed to the stimulation of the beta cells, which in turn exerts an anti-hyperglycemic action. Reports are available to show that antidiabetic plants are known to increase circulating insulin levels (CitationLamela et al. 1985). Thus, it can be suggested that swertisin induces the release of insulin and may be thought to act via increasing the glucose uptake by pancreatic β-cells.
In conclusion, swertisin, a C-glycosyl flavonoid from EH, contributed as one of the major constituents which acted via different mechanism to achieve normoglycemic status. It can be considered as a biomarker of EH plant or extract and quantified for the purpose of standardization of EH herb.
Declaration of interest
Mayurkumar Patel would like to thank the All India Council for Technical Education, New Delhi, for providing the financial assistance (National Doctoral Fellowship) to carry out this work. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Attele AS, Zhou YP, Xie JT, Wu JA, Zhang L, Dey L, Pugh W, Rue PA, Polonsky KS, Yuan CS. (2002). Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes, 51, 1851–1858.
- Babu PS, Stanely Mainzen Prince P. (2004). Antihyperglycaemic and antioxidant effect of hyponidd, an ayurvedic herbomineral formulation in streptozotocin-induced diabetic rats. J Pharm Pharmacol, 56, 1435–1442.
- Baynes JW. (1991). Role of oxidative stress in development of complications in diabetes. Diabetes, 40, 405–412.
- Chaudhuri RK, Singh AK, Ghosal S. (1975). Chemical constituents of Gentianaceae. XVIII. Structure of enicoflavine. Monoterpene alkaloid from Enecostimma hyssopifolium. Chemical Industry (London) 3, 127–128.
- Dahlqvist A. (1964). Method for assay of intestinal disaccharidases. Anal Biochem, 7, 18–25.
- Daniel M, Sabnis SD. (1978). Chemical systematics of family Gentianaceae. Curr Sci, 47, 109–111.
- Desai PD, Ganguly AK, Govindachari TR, Joshi BS, Kamat VN, Manmade AH, Mohamed PA, Nagle SK, Nayak RH, Saksena AK, Sathe SS, Vishwanathan N. (1966). Chemical investigation of some Indian plants: Part II. Indian J Chem, 4, 457–459.
- Flatt PR, Lenzen S. (2004). Frontiers of Insulin Secretion and Pancreatic β-Cell Research. London: Smith-Gordon.
- Ghosal S, Jaiswal DK. (1980). Chemical constituents of Gentianaceae XXVIII: Flavonoids of Enicostemma hyssopifolium (Willd.) Verd. J Pharm Sci, 69, 53–56.
- Ghosal S, Singh AK, Sharma PV, Chaudhuri RK. (1974). Chemical constituents of Gentianaceae. IX. Natural occurrence of erythrocentaurin in Enicostemma hyssopifolium and Swertia lawii. J Pharm Sci, 63, 944–945.
- Gopal R, Gnanamani A, Udayakumar R, Sadulla S. (2004). Enicostemma littorale Blume – A potential hypolipidemic plant. Natural Product Radiance, 3, 401–405.
- Govindachari TR, Sathe SS, Vishwanathan N. (1966). Gentianine, an artifact in Enicostemma littorale. Indian J Chem, 4, 201–202.
- Gupta SS, Seth CB, Variyar MC. (1962). Experimental studies on pituitary-diabetes. I. Inhibitory effect of a Ayurvedic anti-diabetic remedies on anterior pituitary extract induced hyperglycaemia in albino rats. Indian J Med Res, 50, 73–81.
- Halliwell B, Gutteridge JMC. (1989). Free Radicals in Biology and Medicine, second edition. Oxford: Oxford University Press.
- Hayman S, Kinoshita JH. (1965). Isolation and properties of lens aldose reductase. J Biol Chem, 240, 877–882.
- Jensen SR, Schripsema J. (2002). In: Struwe L, & Albert V, eds. Gentianaceae – Systematics and Natural History. Cambridge: Cambridge University Press, 573–631.
- Kang KA, Lee KH, Kim SY, Kim HS, Kim JS, Hyun JW. (2007). Cytoprotective effects of KIOM-79 on streptozotocin induced cell damage by inhibiting ERK and AP-1. Biol Pharm Bull, 30, 852–858.
- Kavimani S, Manisenthlkumar KT. (2000). Effect of methanolic extract of Enicostemma littorale on Dalton’s ascitic lymphoma. J Ethnopharmacol, 71, 349–352.
- Kinloch RA, Treherne JM, Furness LM, Hajimohamadreza I. (1999). The pharmacology of apoptosis. Trends Pharmacol Sci, 20, 35–42.
- Komatsu M, Tomimori T, Ito M. (1967). Studies on the constituents of Swertia japonica. I. On the structures of swertisin and isoswertisin. Chem Pharm Bull, 15, 263–269.
- Lamba SS, Buch KY, Lewis HI, Lamba J. (2000). Phytochemicals as potential hypoglycemic agents. Stud Nat Prod Chem, 21, 457–496.
- Lamela M, Cadavid I, Gato A, Calleja JM. (1985). Effects of Lythrum salicaria in normoglycemic rats. J Ethnopharmacol, 14, 83–91.
- Latha M, Pari L, Sitasawad S, Bhonde R. (2004). Scoparia dulcis, a traditional antidiabetic plant, protects against streptozotocin induced oxidative stress and apoptosis in vitro and in vivo. J Biochem Mol Toxicol, 18, 261–272.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem, 193, 265–275.
- Lukic ML, Stosic-Grujicic S, Shahin A. (1998). Effector mechanisms in low-dose streptozotocin-induced diabetes. Dev Immunol, 6, 119–128.
- Maroo J, Vasu VT, Aalinkeel R, Gupta S. (2002). Glucose lowering effect of aqueous extract of Enicostemma littorale Blume in diabetes: a possible mechanism of action. J Ethnopharmacol, 81, 317–320.
- Murali B, Upadhyaya UM, Goyal RK. (2002). Effect of chronic treatment with Enicostemma littorale in non-insulin-dependent diabetic (NIDDM) rats. J Ethnopharmacol, 81, 199–204.
- Rai J, Thakar KA. (1966). Chemical investigation of Enicostemma littorale Blume. Curr Sci, 35, 148–149.
- Retnam KR, DeBritt AJ. (1988). Preliminary phytochemical screening of three medicinal plants of tirunelveli hills. J Econ Texas Botany, 22, 677–681.
- Sadique J, Chandra T, Thenmozhi V, Elango V. (1987). The anti-inflammatory activity of Enicostemma littorale and Mollugo cerviana. Biochem Med Metab Biol, 37, 167–176.
- Sarkar K, Sil PC. (2006). A 43 kDa protein from the herb Cajanus indicus L. protects thioacetamide induced cytotoxicity in hepatocytes. Toxicol in Vitro, 20, 634–640.
- Schmezer P, Eckert C, Liegibel UM. (1994). Tissue-specific induction of mutations by streptozotocin in vivo. Mutat Res, 307, 495–499.
- Shibano M, Kakutani K, Taniguchi M, Yasuda M, Baba K. (2008). Antioxidant constituents in the dayflower (Commelina communis L.) and their alpha-glucosidase-inhibitory activity. J Nat Med, 62, 349–353.
- Shim YJ, Doo HK, Ahn SY, Kim YS, Seong JK, Park IS, Min BH. (2003). Inhibitory effect of aqueous extract from the gall of Rhus chinensis on alpha-glucosidase activity and postprandial blood glucose. J Ethnopharmacol, 85, 283–287.
- Spinas GA. (1999). The dual role of nitric oxide in islet beta-cells. News Physiol Sci, 14, 49–54.
- Srinivasan M, Padmanabhan M, Prince PS. (2005). Effect of aqueous Enicostemma littorale Blume extract on key carbohydrate metabolic enzymes, lipid peroxides and antioxidants in alloxan-induced diabetic rats. J Pharm Pharmacol, 57, 497–503.
- Tarasov A, Dusonchet J, Ashcroft F. (2004). Metabolic regulation of the pancreatic beta-cell ATP-sensitive K+ channel: a pas de deux. Diabetes, 53 Suppl 3, S113–S122.
- Upadhyay UM, Goyal RK. (2004). Efficacy of Enicostemma littorale in Type 2 diabetic patients. Phytother Res, 18, 233–235.
- Vasu VT, Ashwinikumar C, Maroo J, Gupta S, Gupta S. (2003). Antidiabetic effect of Enicostemma littorale Blume aqueous extract in newly diagnosed non-insulin-dependent diabetes mellitus patients (NIDDM): A preliminary investigation. Orient Pharm Exper Med, 3, 84–89.
- Vasu VT, Modi H, Thaikoottathil JV, Gupta S. (2005). Hypolipidaemic and antioxidant effect of Enicostemma littorale Blume aqueous extract in cholesterol fed rats. J Ethnopharmacol, 101, 277–282.
- Vijayvargia R, Kumar M, Gupta S. (2000). Hypoglycemic effect of aqueous extract of Enicostemma littorale Blume (chhota chirayata) on alloxan induced diabetes mellitus in rats. Indian J Exp Biol, 38, 781–784.
- Vyas DS, Sharma VN, Sharma HK, Khanna NK. (1979). Preliminary study of antidiabetic properties of Aegle marmelos and Enicostemma littorale. J Res Indian Med, 14, 2–4.
- Waltner-Law ME, Wang XL, Law BK, Hall RK, Nawano M, Granner DK. (2002). Epigallocatechin gallate, a constituent of green tea, represses hepatic glucose production. J Biol Chem, 277, 34933–34940.
- Wiederkehr A, Wollheim CB. (2006). Minireview: implication of mitochondria in insulin secretion and action. Endocrinology, 147, 2643–2649.