Abstract
Context: In folk medicine in China, Desmodium caudatum (Thunb.) DC (Leguminosae) has been used to treat febrile diseases, rheumatic arthritis, and bacillary dysentery; nevertheless, there have been no reports on the analgesic, antipyretic, and anti-inflammatory effects of this plant in animals.
Objective: To investigate the analgesic, anti-inflammatory, and antipyretic activities of D. caudatum extract (DCE) in animals.
Materials and methods: The analgesic effect of DCE was measured in mice using the acetic acid-induced writhing test and the hot-plate test. The anti-inflammatory activity was assessed using the carrageenan-induced rat paw edema model and the dimethylbenzene-induced mouse inflammation model. The antipyretic effect was estimated using the lipopolysaccharide (LPS)-induced rat fever model. In addition, the acute oral toxicity of DCE was studied.
Results: DCE significantly and dose-dependently inhibited the writhing responses in mice, increased reaction time in mice in the hot-plate test, reduced carrageenan-induced paw edema in rats and the dimethylbenzene-induced ear edema in mice, and attenuated LPS-induced fever in rats. Furthermore, no death was observed when mice were orally administered DCE up to 40 g/kg.
Discussion and conclusion: DCE possesses evident analgesic, anti-inflammatory, and antipyretic activities, and has a favorable safety, which supports the use of D. caudatum as an analgesic, anti-inflammatory and antipyretic drug in folk medicine.
Introduction
Desmodium caudatum (Thunb.) DC (Leguminosae), a type of shrub, is widely found in China, India, Japan, Philippines, and other East African countries. According to the ancient Chinese literature, the whole plant is used in the treatment of febrile diseases, rheumatic arthritis, and bacillary dysentery in folk medicine. To the best of our knowledge, there have been no reports on the analgesic, antipyretic, and anti-inflammatory effects of this plant in animals so far. In this study, the above activities of the ethanol extract of D. caudatum were evaluated in animals. The results show that the D. caudatum extract (DCE) significantly and dose-dependently inhibited the writhing responses in mice in the acetic acid writhing reflex test, increased reaction time in mice in the hot-plate test, and reduced carrageenan-induced paw edema in rats and xylene-induced ear edema in mice. It also evidently attenuated rat pyrexia induced by lipopolysaccharide (LPS). In addition, we determined the acute oral toxicity of the extract, which exhibited a favorable safety.
Materials and methods
Plant material and extraction
The whole plant of D. caudatum was collected from Tianmu mountain in Zhejiang Province in July 2009. The plant was identified by Lu-Ping Qin, a pharmacognosist from the Department of Pharmacognosy, School of Pharmacy, Second Military Medical University (Shanghai, China). A voucher specimen of D. caudatum was deposited with the number SY3246 in the Herbarium of the Department of Pharmacognosy, School of Pharmacy, Second Military Medical University (Shanghai, China). The dried plant (500 g) was pulverized in a motor-driven grinder to prepare the extract. After refluxing extraction with 8 L 85% (v/v) aqueous ethanol at 55°C four times for 0.5 h each time, the extract was filtered and then the solvent was evaporated to dryness under reduced pressure in a rotary evaporator. The extractum was heat-dried at 50°C for in vivo evaluation. The yield of DCE was 15.13%.
Animals
ICR mice (20–25 g), male Sprague-Dawley (SD) rats (200– 220 g), and male Wistar rats (200–220 g), obtained from the Experimental Animal Center of the Second Military Medical University (Shanghai, China), were housed in a regulated environment (20 ± 2°C), with a 12-h light/dark cycle (08:00–20:00, light). The animals were deprived of food for 15 h before the experiment, with free access to drinking water. Each animal was used only once in the experiment. All animal treatments were strictly in accordance with international ethical guidelines concerning the care and use of laboratory animals, and all the experiments were carried out under the approval of the Committee of Experimental Animal Administration of the University. Each experimental group consisted of 10 animals.
Drugs and reagents
The following reagents and drugs were used: ethanol (AR), dimethylbenzene (AR), and acetic acid (AR) (Sinopharm Chemical Reagent, Shanghai, China), aspirin, ibuprofen, indomethacin, dexamethasone, paracetamol (Chengdu Pharmaceutical Factory, Chengdu, China).
Treatment
DCE, aspirin, ibuprofen, indomethacin, dexamethasone, and paracetamol were each dissolved in distilled water prior to administration. Three groups of animals (n = 10) were orally administered 100, 200, and 300 mg/kg DCE, respectively, by intubation. The positive control group of animals (n = 10) were given aspirin (100 mg/kg), ibuprofen (200 mg/kg), indomethacin (5 mg/kg), dexamethasone (5 mg/kg), and paracetamol (100 mg/kg), respectively. Another group of animals (negative control group, n = 10) were given distilled water, and was run concurrently with the drug-treated groups, all of which were given in a volume of 10 mL/kg body weight irrespective of dose. One hour after oral administration, DCE and positive drugs were evaluated.
Analgesic test
The peripheral analgesic activity of DCE was evaluated in male mice using the acetic acid-induced writhing test (CitationGarcía et al., 2004), whereas central analgesic activity of DCE against thermal stimuli was studied in female mice using the hot-plate test (CitationFranzotti et al., 2000).
In the writhing test, male mice (n = 10) were orally administered DCE or aspirin (100 mg/kg), 1 h before intraperitoneal administration of acetic acid (1%, 10 mL/kg). The number of writhing reflexes was counted during the following 15 min and the experiment was repeated twice.
In the hot-plate test, the latency period was recorded when female mice were put on the hot plate maintained at 55 ± 0.5°C. After pretreatment latencies were determined, only mice that showed initial nociceptive responses between 5 and 30 sec were selected for the experiment. The posttreatment reaction time of each animal was recorded after 30, 60, 90, and 120 min of administration of DCE or ibuprofen (200 mg/kg).
Anti-inflammatory test
The anti-inflammatory activity of DCE was evaluated using the carrageenan-induced rat paw edema model (CitationWinter et al., 1962) and dimethylbenzene-induced mouse inflammation model (CitationZheng et al., 2009).
In the rat paw edema test, male Wistar rats were used and acute inflammation was produced by subplantar injection of 0.1 mL of freshly prepared 1% (w/v) carrageenan in normal saline into the right hind paws of the rats. Paw volume was measured plethysmometrically using a paw edema detector (YLS-7A; Shandong Academy of Medical Science Device Station, Shandong, China) at 0, 0.5, 1, 2, 3, 4, and 6 h after carrageenan injection. Animals were orally pre-medicated with either DCE or indomethacin (5 mg/kg) before 0.5 h of injection. Mean increase in paw volume was measured and inhibitory percentage was calculated. The edema rate of rats was calculated as follows:
where V0 is the volume before carrageenan injection (mL) and Vt is the volume at t h after carrageenan injection (mL).
In the dimethylbenzene-induced ear edema test, DCE or dexamethasone (5 mg/kg) was orally administered before 1 h of topical application of dimethylbenzene (40 μL/ear) to the right ears of mice. The ear swelling was measured by subtracting the weight of the left ear from that of the right after 1 h of dimethylbenzene treatment as reported in the literature (CitationZheng et al., 2009).
Antipyretic test
The antipyretic activity of DCE was evaluated according to the method previously reported (CitationSantos et al., 1998). Rat fever can be induced by intraperitoneal injection of LPS (1 mg/kg). SD rats were pretreated with LPS after 0.5 h of the oral administration of DCE or paracetamol (100 mg/kg). The temperature in the external auditory meatus was measured in a temperature-controlled room (ambient temperature 20 ± 2°C) using an electronic temperature recorder (DT-IDB; Shanghai Medical Instruments Factory, Shanghai, China). The temperature was measured every 30 min from 9 a.m. to 5 p.m.
Acute toxicity
The acute toxicity test for DCE evaluated any possible toxicity. ICR mice of either sex were tested by orally administering different doses of the extract by increasing or decreasing the dose according to the responses of animals (CitationBruce, 1985). The given maximum dose was 40 g/kg, while the control group only received distilled water. All animals were observed for any gross effect or mortality within 7 days.
Statistical analysis
The data were analyzed using the SPSS 11.0 statistical package. Data for multiple comparisons were performed by one-way ANOVA followed by least significant difference t-test. A value of P < 0.05 was considered statistically significant and all results are presented as mean ± SD.
Results
Effects of DCE on the writhing reflex of mice
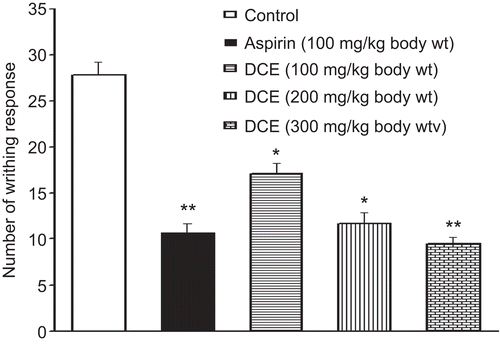
In the writhing test, intraperitoneal injection of acetic acid evidently resulted in writhing reflexes of mice. In contrast, DCE significantly inhibited the number of writhing responses in a dose-dependent manner within 15 min of injection of acetic acid. The number of writhings of the mice given a high dose of DCE (300 mg/kg) was even lower than that of the mice receiving aspirin ().
Figure 1. Effects of Desmodium caudatum extract (DCE) on writhing reflex of mice in the writhing test. When mice were given acetic acid (1% 10 mL/kg) intraperitoneally, the writhing number was counted immediately for 15 min. The experiment was repeated twice. Data are presented as mean ± SD, n = 10. *P < 0.05, **P < 0.01, significance versus control.
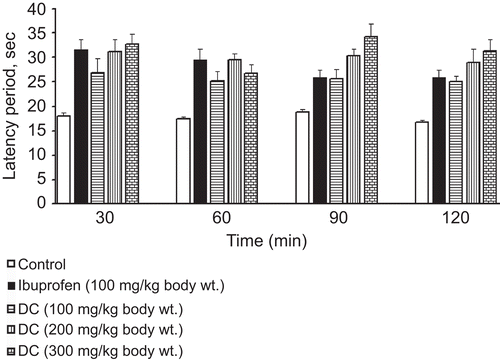
Effects of DCE in heat-stimulated mice
In the hot-plate test, the antinociceptive activity of DCE was also significantly revealed, for which the duration of action was >120 min. DCE markedly prolonged the latency period of mice when compared with the negative group of animals given distilled water. The antinociceptive effect of DCE at high dose was even more than that of ibuprofen 90 min after administering drugs ().
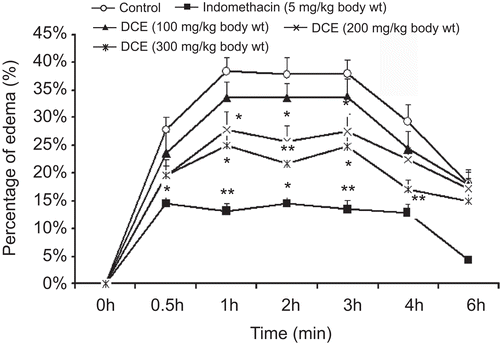
Effects of DCE on carrageenan-induced paw edema in rats
As shown in , subplantar injection of carrageenan noticeably induced paw edema in rats, which persisted for over 6 h. However, the percentage of paw edema in rats was significantly reduced by the administration of DCE, which lasted for 3 h, when compared with the model group of rats given distilled water.
Figure 3. Effects of Desmodium caudatum extract (DCE) on carrageenan-induced paw edema in rats. When 0.1 mL of freshly prepared 1% (w/v) carrageenan was injected subplantarly into the right hind paws of male rats, acute inflammation was induced. Data are presented as mean ± SD, n = 10. *P < 0.05, **P < 0.01, significance versus control.
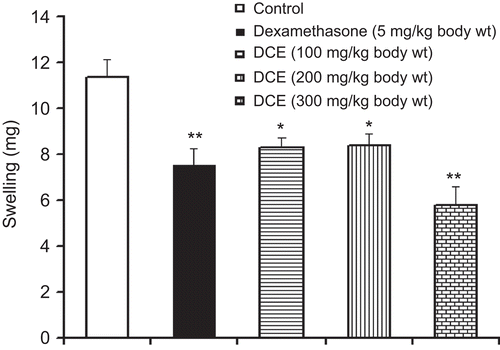
Effects of DCE on dimethylbenzene-induced ear edema in mice
Effects of pretreatment with DCE on mouse ear edema induced by dimethylbenzene are shown in . Dimethylbenzene evidently increased ear edema in mice, but DCE significantly inhibited ear edema compared with the control group. These results revealed that DCE possesses a good activity against acute inflammation induced by dimethylbenzene.
Figure 4. Effects of Desmodium caudatum extract (DCE) on dimethylbenzene-induced ear edema in mice. Mice were orally given DCE or dexamethasone before 1 h of topical application of dimethylbenzene (20 μL/ear) to the right ears of mice. Data are presented as mean ± SD, n = 10. *P < 0.05, **P < 0.01, significance versus control.
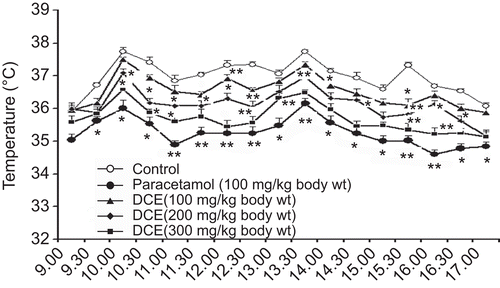
Effects of DCE on fever in rats
LPS evidently elevated the temperature in rats between 9 a.m. and 5 p.m., as shown in . In contrast to the control group of animals given distilled water, the temperature in the rats receiving DCE significantly decreased in a time- and dose-dependent manner from 9 a.m. to 5 p.m. These results exhibited that oral administration of DCE inhibited pyrexia induced by LPS.
Figure 5. Time course of the effects of Desmodium caudatum extract (DCE) on the change of the temperature in external auditory meatus of rats. The rats were injected with lipopolysaccharides (LPS) after 0.5 h of the oral administration of DCE or paracetamol. The temperature was measured every 30 min from 9 a.m. to 5 p.m. Data are presented as mean ± SD, n = 10. *P < 0.05, **P < 0.01, significance versus control.
Acute toxicity
Although the mice were given orally up to 40 g/kg of DCE, no mortality was observed during the assessment period (7 days). So, we can conclude that oral administration of DCE is safe at this dosage.
Discussion
In the present study, the DCE inhibited the writhing responses in mice in the acetic acid writhing reflex test, increased reaction time in mice in the hot-plate test, reduced carrageenan-induced paw edema in rats and xylene-induced ear edema in mice, and attenuated rat pyrexia induced by LPS. The acute oral toxicity test exhibited good security of the extract, which did not result in any death of the mice even up to 40 g/kg. These results suggest that DCE probably inhibits the central synthesis of prostaglandins, which can elicit a hyperalgesic response, including pain, pyrexia, and inflammation.
The acetic acid writhing mice is known as a nonselective antinociceptive screening model. When animals are intraperitoneally injected with acetic acid, a painful reaction and acute inflammation emerge in the peritoneal area. The stimulation of peritoneal nociceptors is indirect and occurs with the release of endogenous substances, which stimulate nervous endings (CitationGyires & Torma, 1984). After acetic acid injection a great increase occurs in prostaglandin E2 and F2α levels in peritoneal fluid, and the analgesic effect of DCE may be due to inhibition of the local level of prostaglandins. However, the result of this writhing test alone does not substantiate whether the antinociceptive effect is associated with central analgesia substances. The hot-plate test, when associated with the writhing test, can usually distinguish the central from peripheral effects (CitationSrinivasan et al., 2003). Analgesic drugs such as aspirin and paracetamol usually have little effect in the hot-plate test, indicating their peripheral analgesic effects. Our results exhibited that DCE significantly prolonged latency period of mice in the hot-plate test, suggesting that this analgesic effect is carried out via the participation of the central nervous system.
The carrageenan rat paw edema test is suitable for evaluation of anti-inflammatory drugs, which has been used frequently to assess the anti-edematous effect of natural products (CitationBasu & Chaudhuri, 1991). As we know, prostaglandins play an important role in pain progress in chemical nociception models (CitationSantos et al., 1998; CitationZheng et al., 2009) and are the target of action of commonly used anti-inflammatory drugs. Several inflammatory mediators, such as sympathomimetic amines, tumor necrosis factor-α (TNF-α), interleukin-1b (IL-1b), and IL-8, are also involved in the nociceptive response to chemical stimulus in mice (CitationSantos et al., 1998; CitationRibeiro et al., 2000). shows DCE at doses of 200 and 300 mg/kg significantly inhibited every phase of edema in rats, suggesting that the extract has a nonselective inhibitory effect on the release or actions of these mediators. In order to further evaluate the anti-inflammatory activity of the extract, the dimethylbenzene-induced ear edema test was employed. As results, both of the tests showed the extract possessed significant anti-inflammatory activity. The preliminary phytochemical analysis indicated that the flavonoids could exert antinociceptive and anti-inflammatory effects.
LPS can stimulate myeloid cells, which further synthesize many cytokines such as IL-1, IL-6, and TNF-α, inducing a general homeostatic reaction, serving as the organism’s first line of defense against infection, and finally causing fever (CitationHart, 1988). In the present study, the rat hyperthermia induced by LPS was employed to investigate the antipyretic activity of DCE. In the present study, experimental results exhibited that DCE attenuated rat pyrexia induced by LPS. We presume that DCE most likely inhibits the prostaglandin synthesis of the heat regulation center in the hypothalamus, and the body has no response to these cytokines. In addition, we also evaluated the acute oral toxicity of DCE, which did not cause any death of mice at the dose of 40 g/kg, showing a favorable safety.
In conclusion, our findings demonstrate that DCE has favorable antinociceptive, anti-inflammatory, and antipyretic activities, which are involved in possible inhibition of the central synthesis of prostaglandins, and affirm the claim by traditional medicine practitioners that DCE can be used to treat febrile diseases, rheumatic arthritis, and bacillary dysentery. However, further studies are necessary to fully elucidate the mechanism of action of the plant.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Basu A, Chaudhuri AK. (1991). Preliminary studies on the antiinflammatory and analgesic activities of Calotropis procera root extract. J Ethnopharmacol 31:319–324.
- Bruce RD. (1985). An up-and-down procedure for acute toxicity testing. Fundam Appl Toxicol 5:151–157.
- Franzotti EM, Santos CV, Rodrigues HM, Mourão RH, Andrade MR, Antoniolli AR. (2000). Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L. (Malva-branca). J Ethnopharmacol 72:273–277.
- García MD, Fernández MA, Alvarez A, Saenz MT. (2004). Antinociceptive and anti-inflammatory effect of the aqueous extract from leaves of Pimenta racemosa var. ozua (Mirtaceae). J Ethnopharmacol 91:69–73.
- Gyires K, Torma Z. (1984). The use of the writhing test in mice for screening different types of analgesics. Arch Int Pharmacodyn Ther 267:131–140.
- Hart BL. (1988). Biological basis of the behavior of sick animals. Neurosci Biobehav Rev 12:123–137.
- Ribeiro RA, Vale ML, Thomazzi SM, Paschoalato AB, Poole S, Ferreira SH, Cunha FQ. (2000). Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur J Pharmacol 387:111–118.
- Santos AR, Vedana EM, De Freitas GA. (1998). Antinociceptive effect of meloxicam, in neurogenic and inflammatory nociceptive models in mice. Inflamm Res 47:302–307.
- Srinivasan K, Muruganandan S, Lal J, Chandra S, Tandan SK, Raviprakash V, Kumar D. (2003). Antinociceptive and antipyretic activities of Pongamia pinnata leaves. Phytother Res 17:259–264.
- Wei F, Ma LY, Jin WT, Ma SC, Han GZ, Khan IA, Lin RC. (2004). Antiinflammatory triterpenoid saponins from the seeds of Aesculus chinensis. Chem Pharm Bull 52:1246–1248.
- Winter CA, Risley EA, Nuss GW. (1962). Carrageenin-induced edema in hind paw of the rat as an assay for antiiflammatory drugs. Proc Soc Exp Biol Med 111:544–547.
- Zheng CJ, Tang WZ, Huang BK, Han T, Zhang QY, Zhang H, Qin LP. (2009). Bioactivity-guided fractionation for analgesic properties and constituents of Vitex negundo L. seeds. Phytomedicine 16:560–567.