Abstract
Objective: Punarnavashtak kwath (PNK) is a classical Ayurvedic formulation, mentioned in Ayurvedic literature Bhaishajya Ratnavali, for hepatic disorders and asthma. This study investigated the hepatoprotective activity of PNK to validate the traditional use of this formulation.
Materials and methods: PNK was prepared in the laboratory according to the method given in Ayurvedic literature. Phytochemical screening was performed to determine the presence of phytoconstituents. Hepatoprotective activity was evaluated against CCl4-induced hepatotoxicity in rats and by its effect on the HepG2 cell line.
Results: Preliminary phytochemical screening revealed the presence of alkaloids, tannins, flavonoids, saponins, and a bitter principle in PNK. Administration of PNK produced significant hepatoprotective effect as demonstrated by decreased levels of serum liver marker enzymes such as aspartate transaminase, serum alanine transaminase, serum alkaline phosphatase, and serum bilirubin and an increase in protein level. Thiopentone-induced sleeping time was also decreased in the PNK-treated animals compared with the CCl4-treated group. It also showed antioxidant activity by increase in activity of glutathione, superoxide dismutase, and catalase and by a decrease in thiobarbituric acid reactive substance level compared with the CCl4-treated group. Results of a histopathological study also support the hepatoprotective activity of PNK. Investigation carried out on the HepG2 cell line depicted significant increase in viability of cells exposed to PNK as compared with CCl4-treated cells.
Discussion and Conclusion: It can be concluded that PNK protects hepatocytes from CCl4-induced liver damages due to its antioxidant effect on hepatocytes. An in vitro study on HepG2 cell lines also supports its protective effect.
Introduction
The liver is an important organ actively involved in many metabolic functions and is a frequent target for a large number of toxicants. Hepatic damage is associated with distortion of these metabolic functions (CitationWolf, 1999). Liver disease is still a worldwide health problem. Unfortunately, conventional or synthetic drugs used in the treatment of liver diseases are inadequate and sometimes can have serious side effects. In the absence of a reliable liver protective drug in modern medicine, there are a number of medicinal preparations in Ayurveda recommended for the treatment of liver disorders (CitationChatterjee 2000).
In the traditional system of Indian medicine, plant formulation and combined extracts of plants are used as the drug of choice rather than individual plant extracts and these herbal formulations are used for the treatment of a wide variety of diseases (CitationAnsarullah et al., 2009). This therapeutic approach is often ignored by many and considered to be an alternative to conventional medicine by others due to lack of scientific validation of efficacy and safety (CitationYuan & Lin, 2000). Hence, there is a requirement for scientific proof (biological assays, animal models, clinical trials, and chemical standardization).
Punarnavashtak kwath (PNK) is an Ayurvedic polyherbal preparation mentioned in Ayurvedic literature Bhaishajya Ratnavali (Vidhyotiny 2004) for hepatic disorders and asthma. It consists of Boerhaavia diffusa Linn. (Nyctaginaceae), Picrorhiza kurroa Royle ex Benth. (Scrophulariaceae), Tinospora cordifolia (Willd.) Miers (Menispermaceae), Zingiber officinalis Rosc. (Zingiberaceae), Berberis aristata DC (Berberidaceae), Terminalia chebula Retz (Combretaceae), Azadirachta indica A. Juss. (Meliaceae), and Tricosanthes dioica Roxb. (Cucurbitaceae) plants. An aqueous extract of thinner roots of B. diffusa exhibited in vivo hepatoprotective activity against hepatic injury in rats (CitationRawat et al., 1997). P. kurroa and its active constituents were effective in preventing liver toxicity caused by numerous toxic agents (CitationSaraswat et al., 1999). T. cordifolia showed significant in vivo hepatoprotective activity in CCl4-induced hepatopathy in goats and in vitro inactivating property against Hepatitis B and E surface antigen (CitationSingh et al., 2003). The aqueous ethanol extract of Z. officinalis showed hepatoprotective effect against acetaminophen-induced acute toxicity due to its direct radical scavenging capacity (CitationAjith et al., 2007). B. aristata and berberine (an alkaloid from B. aristata) were found to be protective against both paracetamol and CCl4-induced liver damage (CitationJanbaz, 1995). T. chebula extract was found to prevent the hepatotoxicity caused by the administration of rifampicin, isoniazid, and pyrazinamide (CitationTasduq et al., 2006). The aqueous extract of A. indica was found to offer protection against paracetamol-induced liver necrosis in rats (CitationBiswas et al., 2002). T. dioica was reported as a hepatoprotective agent in ferrous sulfate (FeSO4)-intoxicated rats (CitationGhaisas et al., 2008). Literature showed that scientific evidence is available for the individual plants but not for this formulation, so in this investigation, PNK has been evaluated for its hepatoprotective action against CCl4-induced hepatotoxicity and its effect on HepG2 cell lines. The hepatotoxin used was CCl4, as CCl4-induced liver dysfunction in rats simulates liver cirrhosis in humans (CitationPérez-Tamayo, 1983; CitationWensing et al., 1990).
At present, one of the plant-derived medicines approved for use in liver cirrhosis and alcoholic liver diseases is silymarin. There are a number of studies that establish the efficacy of silymarin in these conditions (CitationSaller et al., 2001). Therefore, in this study, silymarin was used as a positive control to compare the efficacy of PNK against CCl4-induced hepatotoxicity.
Materials and methods
Materials
Carbon tetrachloride (extra pure) was obtained from Samir Tech-Chem (Baroda, India). Reduced glutathione and thiobarbituric acid were purchased from Kemphasol (Mumbai, India). Tris-HCl was obtained from Loba Chemie (Mumbai, India). All other chemicals were obtained from SD Fine Chemicals (Mumbai, India). HepG2 cell lines were obtained from the National Centre for Cell Sciences (Pune, India), minimum essential medium (MEM) was from Gibco (CA), and fetal calf serum was from Hyclone (UT). Dimethyl sulfoxide (DMSO), trypsin, and EDTA were obtained from Sigma Chemicals (St. Loius, MO). 3-(4,5-Dimethyl thiazole 2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Hi Media (Mumbai, India). Diagnostic kits purchased from Span Diagnostic (Baroda, India) were used for the measurement of biochemical parameters.
Collection of plants and preparation of formulation
B. diffusa root, T. cordifolia stem, T. dioica leaves, and A. indica bark were collected from the medicinal garden of APMC College of Pharmaceutical Education and Research (January, 2008), while other plants, P. kurroa stem, B. aristata stem, T. chebula fruit, and Z. officinalis rhizome, were purchased from the market. All the plants were authenticated by Mukesh Prajapati, botanist, HNSB Ltd. Science College, Himatnagar, and voucher specimens of all plants were kept in the Department of Pharmacognosy, APMC College of Pharmaceutical Education and Research, Himatnagar (APMC 0801 to 0808). PNK (decoction) was prepared by boiling the powdered drugs () in equal quantities in proportion with 16 times the amount of water reduced to one fourth and strained through cloth. The filtrate was evaporated and dried under reduced pressure (CitationVidhyotiny, 2004). Yield of the extract was 10% w/w with respect to air-dried drugs.
Table 1. Composition of punarnavashtak kwath (PNK).
Preliminary phytochemical screening
The dried extract of PNK was subjected to the preliminary phytochemical analysis for the presence of different phytoconstituents (CitationKhandelwal, 2000). Thin layer chromatography (TLC) study of PNK was also done for the presence of different phytoconstituents (CitationWagner & Bladt, 2002).
Preparation of test sample
For the in vivo study, PNK was dissolved in distilled water and was given orally in different doses. Silymarin was suspended in sodium carboxymethyl cellulose (0.3%) in distilled water. The doses are expressed as milligrams of dried extract per kilogram of rat.
For investigations of the HepG2 cell line, dried extract of PNK was dissolved in MEM to obtain a stock solution of 1 mg/mL, which was stored at −20°C before use. Further dilutions were made to get concentrations ranging from 1 to 15 µg/mL with respective media. A suspension of standard powdered silymarin (50 µg/mL) was also prepared. Standard silymarin powder was first dissolved in DMSO and further dilution was done with MEM to get a concentration of 50 µg/mL, as it is not directly soluble in media. All the dilutions were done under aseptic conditions only.
Animals
Healthy untreated Wistar rats of either sex (equal ratio) weighing 180–250 g (16–18 weeks old) were used for hepatoprotective activity and Swiss albino female mice weighing between 25 and 30 g (10–12 weeks old) were used for acute toxicity study. All animals (mice and rats) were collected from the animal house, Zydus Cadila Pharmaceuticals, Ahmedabad. The animals were grouped and housed in polyacrylic cages with not more than two animals per cage and maintained under well-controlled conditions of temperature (27 ± 2°C), humidity (55 ± 5%), and 12/12 h light–dark cycle. Conventional laboratory diet and tap water were provided ad libitum. The protocol of the experiments was approved by the Institutional Animal Ethical Committee as per the guidance of the Committee for the Purpose of Control and Supervision of Experiments on Animals, Ministry of Social Justice and Empowerment, Government of India (Proposal no. 08/02, APMC, Himatnagar).
Acute toxicity study
The animals (mice) were divided into seven groups of six animals each. The control group received normal saline (2 mL/kg body weight p.o.) while other groups received 300, 600, 800, 1000, 2000, and 3000 mg/kg of the test extract. Immediately after dosing, the animals were observed continuously for the first 4 h for any behavioral changes. They were then kept under observation for 14 days after drug administration to find out the mortality if any. Observations were made twice daily, one at 7 a.m. and again at 7 p.m. (OECD 2001).
Hepatoprotective effect of PNK in CCl4-induced liver damage
The animals (rats) were divided into six groups each consisting of six animals. Carbon tetrachloride diluted with liquid paraffin (1:1) was administered in a dose of 1 mL/kg body weight intraperitoneally for 2 days to all animal groups except the control group to induce hepatotoxicity. Groups III, IV, V, and VI received CCl4, and after 30 min of first dose of CCl4, they were treated with the listed dose of PNK or silymarin (CitationRao and Mishra 1998; CitationPorchezhian and Ansari 2005).
Group I: Normal control animals (received vehicle).
Group II: Carbon tetrachloride-treated control animals.
Group III: PNK-treated animals (50 mg/kg bw, p.o. 3 times at 12 h interval).
Group IV: PNK-treated animals (100 mg/kg bw, p.o. 3 times at 12 h interval).
Group V: PNK-treated animals (150 mg/kg bw, p.o. 3 times at 12 h interval).
Group VI: Silymarin-treated animals (50 mg/kg bw, p.o. 3 times at 12 h interval).
After the last dose of PNK, thiopentone sodium was injected (40 mg/kg bw i.p.; CitationGujrati et al. 2007) to all groups to record sleeping time. After 36 h of carbon tetrachloride treatment, blood was collected and serum was separated and analyzed for biochemical parameters. After killing, the liver was isolated and used for biochemical parameters and histopathological analysis.
Biochemical studies
Blood was obtained from the retro-orbital plexus of all animals. Serum was separated by centrifugation at 800g at 30°C for 15 min and used for the estimation of various biochemical parameters, namely serum aspartate transaminase (AST), serum alanine transaminase (ALT) (CitationReitman & Frankel 1957), serum alkaline phosphatase (ALP) (CitationKind & King 1954), serum bilirubin (SBRN) (CitationMalloy & Evelyn 1937), and total protein content (CitationLowry et al., 1951).
Antioxidant activity
For estimating antioxidant activity, the animals were killed by light ether anesthesia and their livers were excised, rinsed in ice-cold normal saline, followed by 0.15 M Tris-HCl (pH 7.4) blotted dry and weighed. A 10% w/v of homogenate was prepared in 0.15 M Tris-HCl buffer and processed for the estimation of thiobarbituric acid reactive substance (TBARS) (CitationOhkawa et al., 1979). After precipitating proteins with trichloroacetic acid, part of the homogenate was used for estimation of glutathione (GSH) (CitationEllman, 1959). The remaining homogenate was centrifuged at 10,500g for 20 min at 4°C. The supernatant thus obtained was used for the estimation of superoxide dismutase (SOD) (CitationMisra & Fridovich, 1972) and catalase (CAT) (CitationAebi, 1984).
Histopathological studies
Paraffin sections (7-µm thick) of buffered formalin-fixed liver samples were stained with hematoxylin-eosin to study the histological structure of control and treated rat liver.
Hepatoprotective activity of PNK on HepG2 cell line
The screening of hepatoprotective activity was based on protection of human liver-derived HepG2 cells against CCl4-induced damage (CitationIra et al., 1997; CitationVijayan et al., 2003) determined by estimating mitochondrial synthesis using tetrazolium assay (CitationKe et al., 1999; CitationBrugisser et al., 2002). HepG2 cells were routinely grown and subcultured as monolayers in MEM supplemented with 10% fetal calf serum. The experiment in this investigation was conducted with the cells that had been initially batch cultured for 10 days. At this stage, the cells were harvested and plated at approximately 2 × 105 cells/well in 96-well microtiter plates (Nunclon, Denmark) and placed in a humidified atmosphere of 5% CO2 at 37°C for a 24 h. After a 24-h period, the cells were incubated for 1 h with 100 µL medium containing 1% CCl4 (toxicant), with or without various concentration of extract (1–15 µg/mL) or the standard (50 µg/mL). Some cells were incubated with fresh medium alone (as normal/control). At the end of the period, the medium was removed and replaced with 100-µL fresh medium (washing step), so that there was no direct interaction between the test extract and MTT (CitationBrugisser et al., 2002). The cytotoxicity was assessed by estimating viability of HepG2 cells by MTT reduction assay (CitationKe et al., 1999; CitationBrugisser et al., 2002). After the washing step, 10 µL MTT (5 mg/mL MTT prepared in MEM without phenol red) was added to each well. The plate was gently shaken and incubated for 3 h at 37°C in a humidified 5% CO2 atmosphere. At the end of the period, 100-µL MTT solubilizing buffer (propanol) was added to each well and the plate was gently shaken to solubilize the blue-colored formazan. The plates were directly read on a microtiter plate reader SPECTRAmax 190 (Molecular Devices, USA) at 540 nm.
Statistical analysis
Results are expressed as mean ± SEM. The statistical difference was analyzed by one-way analysis of variance followed by the Tukey–Kramer multiple comparison test. Significance was calculated as the P value, and P values of less than 0.05 were regarded as statistically significant.
Results
Preliminary phytochemical screening
Preliminary phytochemical screening showed the presence of alkaloids, tannins, carbohydrates flavonoids, and saponins in PNK (). The TLC pattern of PNK showed the presence of tannins, alkaloids, phenolic compounds, saponins, and bitter principle ().
Table 2. Preliminary phytochemical screening of PNK (chemical test).
Table 3. TLC pattern of PNK solvent system: toluene:ethylacetate:methanol:water (3:3:0.2:0.8).
Acute toxicity study
In the acute toxicity study, it was observed that there was no mortality at any of the tested doses (up to 3000 mg/kg) at the end of 14 days of observation, so the LD50 was more than 3 g/kg.
Hepatoprotective effect of PNK in CCl4-induced liver damage
Intoxication of rats treated with CCl4 significantly (P < 0.001) altered the biochemical parameters (ALT, AST, ALP, SBRN, and total protein) when compared with normal control rats, coupled with marked hepatic oxidative stress (). Thiopentone-induced sleeping time was also significantly (P < 0.001) increased in CCl4-treated group (). CCl4 challenge significantly decreased (P < 0.001) the level of SOD and CAT in liver. Intracellular antioxidant GSH level was also significantly depleted (P < 0.001). The level of TBARS, which is produced as a result of lipid peroxidation, was significantly increased (P < 0.001) ().
Table 4. Effect of PNK on different biochemical parameters in CCl4-induced hepatotoxicity in rats.
Table 5. Effect of PNK on thiopentone sodium-induced sleeping time in CCl4-induced hepatotoxicity in rats.
Table 6. Effect of PNK on lipid peroxidation (TBARS), superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH) level in CCl4-induced hepatotoxicity in rats.
Treatment with PNK showed a significant decrease in the levels of marker enzymes, namely ALT, AST, ALP, and SBRN compared with CCl4-intoxicated rats. A dose-dependent significant increase in total protein level was also observed in PNK-treated animals as compared with CCl4-treated groups (). At 100 and 150 mg/kg, PNK showed significant protective effect (P < 0.01). A significant reduction (P < 0.001) in thiopentone-induced sleeping time was observed in PNK (100 and 150 mg/kg)-treated animals compared with the CCl4-treated group ().
Treatment with PNK at doses of 100 and 150 mg/kg significantly prevented the increase in TBARS level (P < 0.001) and brought them near a normal level, and GSH, SOD and CAT activities were significantly increased (P < 0.05) in PNK-treated groups (). The effects of PNK were compared with the standard reference drug silymarin.
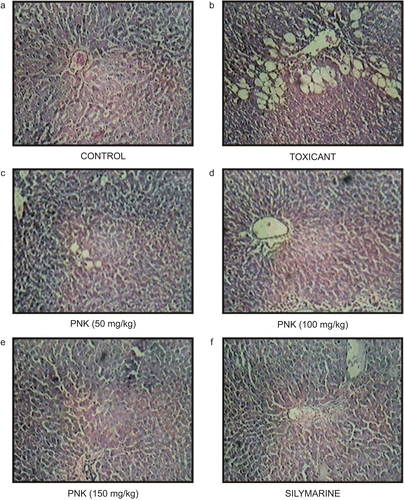
In the histopathological studies, normal animals showed central vein normal liver parenchymal cells (). CCl4-intoxicated animals showed extensive necrosis, inflammation, and infiltration by lymphocytes (). In the treated groups, at the lowest dose, necrosis, sparse lymphocyte infiltration, and occasional regenerating parenchymal cells were seen (). With 100 and 150 mg/kg, there was a greater amount of regeneration with mild inflammation and some lymphocytic infiltration in the necrotic area ( and ). Standard silymarin showed a greater number of regenerating liver cells around the necrotic area (). Thus, the histopathology studies also support the protective effect of PNK.
Figure 1. Photomicrograph of liver section taken from rats against CCl4-induced hepatotoxicity. (A) Normal control rat. Section of liver showing hepatic cells with nuclei, cytoplasm, central vein, and portal triad. (B) Liquid paraffin: CCl4-treated rats. Section of liver showing marked necrosis, inflammation, lymphocytic infiltration severe. (C) PNK (50 mg/kg)-treated rats. Section of liver showing necrosis, lymphocytic infiltration and occasional regenerating parenchymal cells. (D–F) PNK (100 and 150 mg/kg) and silymarin-treated rats. Section of liver showing mild inflammatory changes and greater area of regeneration.
Hepatoprotective effects on HepG2 cell line
The CCl4-exposed HepG2 cells showed 25.37% viability. These exposed cells when treated with different concentrations of PNK showed a significant dose-dependent increase in the percentage viability when compared with the CCl4-exposed cells. The percentage viability ranged between 30 and 88% at 1–15 µg/mL concentration of PNK (). The increase in the percentage cell viability of HepG2 cells treated with PNK at 15 µg/mL concentration was the same as that produced by standard silymarin at 50 µg/mL, from which we can infer that PNK required a lower dose than silymarin in the HepG2 cell line study.
Table 7. Effect of PNK on CCl4 intoxicated HepG2 cell lines.
Discussion
It is well established that the cleavage of a carbon–chloride bond (C–Cl bond) of carbon tetrachloride leads the formation of trichloromethyl free radical, which further reacts with O2 and is converted into a peroxy radical, which is involved in the pathogenesis of liver injury (CitationCheeseman et al., 1985). The abnormal higher level of serum AST, ALT, ALP, and SBRN observed in our study () is the consequence of carbon tetrachloride-induced liver dysfunction and denotes the damage to the hepatic cells (CitationSingh et al., 1999). Feeding of PNK (100 and 150 mg/kg) to CCl4-treated rats significantly reduced the increased level of serum ALT, AST, ALP, and SBRN, and it seemed to offer protection and maintain the functional integrity of hepatic cells. In hepatotoxicity, a depression in total protein is also observed due to the defect in protein biosynthesis (CitationClauson, 1989; CitationDubey et al., 1999). This is due to the disruptions and disassociation of polyribosome from endoplasmic reticulum after CCl4 administration. Treatment with PNK exhibited significant restoration of the altered biochemical parameters toward normal in CCl4-intoxicated rats. This may be due to the promotion of the assembly of ribosomes in the endoplasmic reticulum to facilitate uninterrupted protein biosynthesis.
Barbiturates are a class of xenobiotics that are extensively metabolized in the liver. Deranged liver function leads to delay in the clearance of barbiturates, resulting in a longer duration of hypnotic effect (CitationKulkarni, 1999). In this study, administration of thiopentone sodium to rats treated with an acute dose of CCl4 resulted in an increased duration of thiopentone sleep time. Treatment with PNK decreased the thiopentone-induced sleep time, which is the indirect evidence of hepatoprotective activity (). These findings can be further corroborated by histopathological studies.
Enhanced lipid peroxidation expressed in terms of TBARS and reduced activities of SOD and CAT observed in CCl4-treated rats in our study () confirms the hepatic damage to the rats (CitationKaplowitz et al., 1986). Oral administration of PNK caused a significant decrease in TBARS level indicating the anti-lipid peroxidative and/or adaptive nature of the system brought by PNK against the damaging effects of free radicals produced by the carbon tetrachloride.
Glutathione is one of the most abundant tripeptide, nonenzymatic biological antioxidants present in the liver. Its functions are concerned with the removal of free-radical species such as hydrogen peroxide, superoxide radicals, alkoxy radicals and maintenance of membrane protein thiols, and as a substrate for glutathione peroxidase and glutathione transferase (CitationPrakash et al., 2001). In this study, the decreased level of GSH has been associated with an enhanced lipid peroxidation in CCl4-treated rats. Administration of PNK significantly increased the level of glutathione.
SOD has been reported as one of the most important enzymes in the enzymatic antioxidant defense system (CitationCurtis et al., 1972). It scavenges the superoxide anion to form hydrogen peroxide, hence diminishing the toxic effect caused by this radical. In this study, it was observed that PNK significantly (P < 0.05) increased hepatic SOD activity in CCl4-induced liver damage in rats. This showed that PNK can reduce reactive free radicals, thereby reducing oxidative damage to the tissues besides improving activity of hepatic antioxidant enzymes.
CAT is an enzymatic antioxidant widely distributed in all animal tissues, and the highest activity is found in the red blood cells and liver. CAT decomposes hydrogen peroxide and protects the tissue from highly reactive hydroxyl radicals (CitationChance et al., 1952). Therefore, reduction in the activity of these enzymes may result in a number of deleterious effects due to the accumulation of superoxide radicals and hydrogen peroxide. Administration of PNK increases the activity of CAT in CCl4-induced liver damage in rats to prevent the accumulation of excessive free radicals, protecting the liver from CCl4 intoxication.
HepG2 cells, a human hepatoma cell line, are considered a good model to study in vitro xenobiotic metabolism and toxicity to the liver, because they retain many of the specialized functions that characterize normal human hepatocytes (CitationKnasmüller et al., 1998). The investigation carried out on human liver-derived HepG2 cells against CCl4-induced damage also confirmed the hepatoprotective nature of the PNK.
The results from this study indicate a good correlation between in vivo and in vitro studies. The effects were compared with those of the standard drug silymarin. Our findings support the reported therapeutic use of this formulation in Ayurvedic literature for liver ailments. This may be due to synergistic effects between different herbs in a formulation (CitationWilliamson, 2001). Significant effect of PNK on antioxidant enzyme levels showed hepatoprotective activity by its antioxidant effect. Further study is in progress for the effect of PNK in other toxicant (paracetamol, ethanol)-induced hepatotoxicity for mechanism of action. Clinical study is also in progress to support traditional use of this formulation in jaundice and other hepatic disorders. The study has been registered in the clinical trial registry of India (CTRI/2009/091/000719, 22-09-2009).
Acknowledgments
The authors thank Mukul Jain (Vice President and Head), Prabodha Swain (Principal Scientist), and Gaurav Pandya (Research Associate) of the Pharmacology Department, Zydus Research Centre, Ahmedabad, for providing facilities to work on HepG2 cell lines.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Aebi H. (1984). Catalase in vitro. Meth Enzymol, 105, 121–126.
- Ajith TA, Hema U, Aswathy MS. (2007). Zingiber officinale Roscoe prevents acetaminophen-induced acute hepatotoxicity by enhancing hepatic antioxidant status. Food Chem Toxicol, 45, 2267–2272.
- Ansarullah Jadeja, RN, Thounaojam MC, Patel V, Devkar RV, Ramachandran AV. (2009). Antihyperlipidemic potential of a polyherbal preparation on triton WR 1339 (Tyloxapol) induced hyperlipidemia: A comparison with lovastatin. Int J Green Pharmacy, 3, 119–124.
- Biswas K, Chattopadhyay I, Banerjee R, Bandyopadhyay U. (2002). Biological activities and medicinal properties of Neem (Azadirachta indica). Curr Sci, 82, 1336–1345.
- Brugisser R, Daeniken KV, Jundt G, Schaffner W, Reinsrt HT. (2002). Interference of plant extract, phytoestrogen and antioxidants with the MTT tetrazolium assay. Planta Med, 68, 445–448.
- Chance B, Greenstein DS, Roughton FJ. (1952). The mechanism of catalase action. I. Steady-state analysis. Arch Biochem, 37, 301–321.
- Chatterjee TK. (2000). Medicinal plants with hepatoprotective properties. Herbal Options. Calcutta, India: Books & Allied, 143.
- Cheeseman KH, Albano EF, Tomasi A, Slater TF. (1985). Biochemical studies on the metabolic activation of halogenated alkanes. Environ Health Perspect, 64, 85–101.
- Clauson GA. (1989). Mechanism of carbon tetrachloride hepatotoxicity. Pathol Immunopathol Res, 8, 104–112.
- Curtis SJ, Moritz M, Snodgrass PJ. (1972). Serum enzymes derived from liver cell fractions. I. The response to carbon tetrachloride intoxication in rats. Gastroenterology, 62, 84–92.
- Dubey GP, Agrawal A, Dixit SP. (1999). Effect of Liv-52 on different biochemical parameters in alcoholic cirrhosis. Antiseptic, 91, 205–208.
- Ellman GL. (1959). Tissue sulfhydryl groups. Arch Biochem Biophys, 82, 70–77.
- Ghaisas MM, Tanwar MB, Ninave PB, Navghare VV, Takawale AR, Zope VS, Deshpande AD. (2008). Hepatoprotective activity of aqueous and ethanolic extract of Trichosanthes dioica Roxb. in ferrous sulphate-induced liver injury. Pharmacology Online, 3, 127–135.
- Gujrati V, Patel N, Rao VN, Nandakumar K, Gouda TS, Shalam M, Shanta Kumar SM. (2007). Hepatoprotective activity of alcoholic and aqueous extracts of leaves of Tylophora indica (Linn.) in rats. Indian J Pharmacol, 39, 43–47.
- Ira TM, Hughes RD, McFarlane IG. (1997). Screening of hepatoprotective plant components using HepG2 cell cytotoxicity assay. J Ethnopharmacol, 49, 1132–1135.
- Janbaz KH. (1995). Investigation of hepatoprotective activity of herbal constituents. PhD thesis, Department of Pharmacology, Faculty of Pharmacy, University of Karachi, p. 130.
- Kaplowitz N, Aw TY, Simon FR, Stolz A. (1986). Drug-induced hepatotoxicity. Ann Intern Med, 104, 826–839.
- Ke H, Hisayoshi K, Aijiun D, Yongkui J, Shigeo I, Xinsheng Y. (1999). Antineoplastic agents III: Steroidal glycoside from Solanum nigrum. Planta Med, 65, 35–38.
- Khandelwal KR. (2000). Practical Pharmacognosy Techniques and Experiments. Pune, India: Nirali Prakashan, 149–156.
- Kind PR, King EJ. (1954). Estimation of plasma phosphatase by determination of hydrolysed phenol with amino-antipyrine. J Clin Pathol, 7, 322–326.
- Knasmüller S, Parzefall W, Sanyal R, Ecker S, Schwab C, Uhl M, Mersch-Sundermann V, Williamson G, Hietsch G, Langer T, Darroudi F, Natarajan AT. (1998). Use of metabolically competent human hepatoma cells for the detection of mutagens and antimutagens. Mutat Res, 402, 185–202.
- Kulkarni SK. (1999). Handbook of Experimental Pharmacology, 3rd edition. New Delhi, India: Vallabh Prakashan, 135.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem, 193, 265–275.
- Malloy HJ, Evelyn KA. (1937). The determination of SBRN with the photoelectric colorimeter. J Biol Chem, 11, 481–490.
- Misra HP, Fridovich I. (1972). The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem, 247, 3170–3175.
- OECD. (2001). Guideline for Testing of Chemicals, No. 420. Acute Oral Toxicity – Fixed Dose Procedure. Paris: Organization for Economic Cooperation and Development.
- Ohkawa H, Ohishi N, Yagi K. (1979). Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem, 95, 351–358.
- Pérez Tamayo R. (1983). Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology, 3, 112–120.
- Porchezhian E, Ansari SH. (2005). Hepatoprotective activity of Abutilon indicum on experimental liver damage in rats. Phytomedicine, 12, 62–64.
- Prakash J, Gupta SK, Kochupillai V, Singh N, Gupta YK, Joshi S. (2001). Chemopreventive activity of Withania somnifera in experimentally induced fibrosarcoma tumours in Swiss albino mice. Phytother Res, 15, 240–244.
- Rao KS, Mishra SH. (1998). Anti-inflammatory and hepatoprotective activities of fruits of Moringa pterygosperma Gaertn. Ind J Nat Prod, 14, 3–6.
- Rawat AK, Mehrotra S, Tripathi SC, Shome U. (1997). Hepatoprotective activity of Boerhaavia diffusa L. roots–a popular Indian ethnomedicine. J Ethnopharmacol, 56, 61–66.
- Reitman S, Frankel S. (1957). A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol, 28, 56–63.
- Saller R, Meier R, Brignoli R. (2001). The use of silymarin in the treatment of liver diseases. Drugs, 61, 2035–2063.
- Saraswat B, Visen PK, Patnaik GK, Dhawan BN. (1999). Ex vivo and in vivo investigations of picroliv from Picrorhiza kurroa in an alcohol intoxication model in rats. J Ethnopharmacol, 66, 263–269.
- Singh K, Khanna AK, Chander R. (1999). Hepatoprotective activity of ellagic acid against carbon tetrachloride induced hepatotoxicity in rats. Indian J Exp Biol, 37, 1025–1026.
- Singh SS, Pandey SC, Srivastava S, Gupta VS, Patro B, Ghosh AC. (2003). Chemistry and medicinal properties of Tinospora cordifolia (Guduchi). Indian J Pharmacol, 35, 83–91.
- Tasduq SA, Singh K, Satti NK, Gupta DK, Suri KA, Johri RK. (2006). Terminalia chebula (fruit) prevents liver toxicity caused by sub-chronic administration of rifampicin, isoniazid and pyrazinamide in combination. Hum Exp Toxicol, 25, 111–118.
- Vidhyotiny. (2004). Bhaishyajyaratnavali, Udarrogchikitsa, Chaukhamba Sanskrut Sansthan, Varanasi 40, 432.
- Vijayan P, Prashant HC, Vijayraj P, Dhanaraj SA, Badami S, Suresh B. (2003). Hepatoprotective effect of the total alkaloid fraction of Solanum pseudocapsicum leaves. Pharm Biol, 41, 443–448.
- Wagner H, Bladt S. (2002). Screening of unknown commercial drugs. Plant Drug Analysis (A thin layer chromatography Atlas, second edition, India:Thomson, 349–354.
- Wensing G, Sabra R, Branch RA. (1990). Renal and systemic hemodynamics in experimental cirrhosis in rats: relation to hepatic function. Hepatology, 12, 13–19.
- Williamson EM. (2001). Synergy and other interactions in phytomedicines. Phytomedicine, 8, 401–409.
- Wolf PL. (1999). Biochemical diagnosis of liver diseases. Indian J Clin Biochem, 14, 59–90.
- Yuan R, Lin Y. (2000). Traditional Chinese medicine: an approach to scientific proof and clinical validation. Pharmacol Ther, 86, 191–198.