Abstract
Context: Psoralens are naturally occurring furanocumarins used in photochemotherapy of several skin diseases. They are obtained from dried ripe fruits of Psoralea corylifolia Linn. (Fabaceae). However, little research has been done to study the melanogenic activity of P. corylifolia seeds and their active ingredients on the pigment cells, the melanophores taking account of their cholinergic activity.
Objective: The present work was carried out to determine the effects of lyophilized seed extracts of P. corylifolia, along with pure psoralen on the isolated scale melanophores of Channa punctatus Bloch. (Channidae), which are a disguised type of smooth muscle cells and offer excellent in vitro opportunities for studying the effects of drugs.
Materials and methods: Effects of lyophilized extracts of P. corylifolia and pure psoralen were studied on the isolated scale melanophores of C. punctatus as per the modified method of Citation.
Results: The lyophilized extract of P. corylifolia and its active ingredient psoralen caused significant melanin dispersal responses leading to darkening of the fish scale melanophores, which were completely antagonized by atropine and hyoscine. These melanin dispersal effects were also found to be markedly potentiated by neostigmine, an anticholinesterase agent.
Discussion: In the present study, the lyophilized extract of P. corylifolia seeds and standard psoralen in different dose ranges induced powerful melanin dispersal effects of the previously adrenaline-aggregated isolated scale melanophores of C. punctatus. Comparatively, psoralen caused a more sustained and powerful melanin dispersal within the isolated fish melanophores and interestingly the concentrations required to achieve maximal dispersion of melanophore were 10 times less than that of lyophilized seed extract of P. corylifolia. The physiologically significant dose-related melanin dispersion effects of lyophilized P. corylifolia seeds and synthetic psoralen per se were found to be completely abolished by atropine and hyoscine, which are specific cholino-muscarinic receptor blockers. These data strongly indicate that in the fish C. punctatus, the dispersion of melanin granules within the scale melanophores is mediated by choline receptors of muscarinic nature.
Conclusion: It appears that the melanin dispersal effects of the extracts of P. corylifolia and pure psoralen leading to skin darkening are mediated by cholino-muscarinic- or cholino-psoralen-like receptors having similar properties.
Introduction
Melanophores, the pigmented cells, are a disguised type of smooth muscle cell, which give a black color to vertebrates including humans, a fascinating phenomenon throughout the animal kingdom. Dysfunction of these pigment cells leads to either hypopigmentation or hyperpigmentation, which has been extensively studied in the past and plant extract-based treatment regimes have been suggested (CitationGupta & Anderson, 1987; CitationThody, 1993; CitationAspengren et al., 2003, Citation2009; CitationSolano et al., 2006). Psoralens obtained from Psoralea corylifolia Linn. (Fabaceae) are naturally occurring furanocumarins. The dried ripe fruits are reported to be more potent than other furanocumarins such as xanthotoxin and bergapten (CitationCardoso et al., 2002). These have been used clinically in photochemotherapy of some skin diseases such as psoriasis, mycosis fungoides, vitiligo, and eczema by Indians and Egyptians dating back to 1400 and 1500 bc (CitationGupta & Anderson, 1987). Despite the extensive use of various crude and lyophilized plant extracts for the treatment of hypopigmentation earlier, and later on in combination with ultraviolet radiation (CitationLerner et al., 1953; CitationSharma & Singh, 1979; CitationFitzpatrick & Pathak, 1984; CitationCardosa et al., 2000), literature on dysfunction of melanogenesis leading to hypopigmentation in vertebrates including humans is, however, inconclusive, and several factors such as genetic, immunologic, allergic reactions to chemicals, trace elements have been incorporated in hypopigmentation (CitationBriganti et al., 2003).
Recently, there has been a more concentrated focus on the effects of several plant extracts and their active ingredients on melanocytes (CitationMarwan et al., 1990; CitationLin et al., 1999; CitationMatsuda et al., 2005; CitationHanamura et al., 2008; CitationJeon et al., 2009); however, it is still not clear whether the extracts or their active ingredients affect the melanocytes directly, through the biochemical pathways or cellular receptors present on them. It is not known whether any cellular receptors at the pigment cell membrane are involved in the melanogenetic responses leading to skin darkening of the affected areas in response to the plant extracts or their active bioconstituents. The present work determined the effects of lyophilized seed extracts of P. corylifolia along with pure psoralen on the isolated scale melanophores of Channa punctatus Bloch. (Channidae), which are disguised types of smooth muscle cells and thus may offer further excellent in vitro opportunities for studying the effects of pharmacological and pharmaceutical agents of plant origin. There are several reports in the literature where effects of various hormonal and pharmacological agents have been studied on melanophores of vertebrates such as fish, amphibians, and reptiles, demonstrating the involvement of cellular receptors such as adrenergic, cholinergic, histaminergic, and serotonergic (CitationFujii & Miyashita, 1976; CitationFujii et al., 1982; CitationKasukawa et al., 1985; CitationPeter et al., 1996; CitationAli et al., 1998; CitationRyan et al., 2002). However, this is the first report dealing with the effects of lyophilized extracts of P. corylifolia along with psoralen on the isolated melanophores of the fish and we have demonstrated that lyophilized extracts of P. corylifolia and its active ingredient, the pure psoralen, mimic the melanophore dispersal effects of acetylcholine via cholinergic receptor stimulation leading to skin darkening.
Materials and methods
Preparation of lyophilized P. corylifolia seed extracts and standard psoralen
P. corylifolia seeds were obtained from the Minor Forest Processing and Research Centre, Government of Madhya Pradesh, Bhopal, in March 2008 and authenticated by Shayam Biswas of the Botanical Survey of India, Howrah and West Bengal, recognized by the Government of India. The lyophilized extract was prepared according to the slightly modified method of CitationZhao et al. (2007), where the dried seeds were crushed and homogenized (homogenizer model number 0210, Calton Instruments, New Delhi). The crushed aggregate was diluted with double glass distilled water (weight:volume = 1:10) and heated at 100°C for 1 h. The supernatant was collected and centrifuged (2000 g, at 25°C) for 10 min using a REMI tabletop centrifuge model number R24 (REMI Instruments, Mumbai, India) to remove any water-insoluble materials. The supernatant was lyophilized in a freeze-dryer. The dried powder was redissolved in distilled water (for in vitro studies) using different concentrations expressed in g/mL. High-performance thin-layer chromatography (HPTLC) analysis of the lyophilized extract of P. corylifolia showed that it contains 1.9531 g/L of psoralen. The linear furocoumarin psoralen (containing 5-methoxypsoralen 99%) CAT No. P8399 (molecular weight, 186.17) from Sigma-Aldrich, St. Louis, MO, was used in the present study. A stock solution of standard psoralen (0.01g/mL) was prepared in 0.2% dimethyl sulfoxide (DMSO) and used in further dilutions.
In vitro fish scale preparation
The fish, C. punctatus 12–15 cm in body length of either sex, were purchased from the local fish market and transported to the laboratory alive and they were kept in glass aquaria containing 100 L of dechlorinated tap water. Experiments were performed in the laboratory conditions having ambient temperature of 30.5 ± 1.5°C, the temperature of the aquarium water ranged 24 ± 1°C with a pH of 7.2 to 7.4. Prior to the experiments, the fish were allowed to acclimatize to laboratory conditions for 3 days. Diseased, injured, or lethargic fish were removed and only active, uniformly colored fish were used. For the in vitro studies, the fish scales were removed in accordance with the method of CitationSpaeth (1913), which included the removal of 20–25 scales from the dorsolateral region of live C. punctatus kept in a wet cloth, held loosely. The scales were removed by forceps from the dorsal lateral pigmented area. These were immediately placed in 0.7% normal saline, containing 700 mg of sodium chloride in 100 mL of double distilled water. They were equilibrated in saline medium for 7–10 min with frequent shaking. The responses of control as well as of those melanophores that were incubated in 10 mL 0.7% fish saline containing various concentrations starting from 1 × 10−6 to 6.4 × 10−5 g/mL of lyophilized seed extracts of P. corylifolia and pure psoralen along with their specific antagonists (4 × 10−6 g/mL) were measured in accordance with the method of CitationBhattacharya et al. (1976) based on CitationHogben and Slome (1931). In this method, actual diameter (length × breadth with the processes) of 10 randomly selected melanophores from each scale was measured using a Leitz Occulometer calibrated previously with stage micrometer. The value was then multiplied by the unit of the micrometer 15 µm. Thereafter, the arithmetical mean was calculated and this value was then divided by 100 to obtain the values. This was the mean melanophore size index (MMSI).
Statistical analysis
Statistical data analyses are presented as mean ± standard error of the mean (SEM) and n = 7, which represents the number of individual experiments conducted with equal numbers of animals. Comparisons were made between treated and control groups by use of Student’s t-test. All data were analyzed using GraphPad Prism software (UK). P < 0.05 indicates statistically significant difference.
Results
In a series of experiments, effects of lyophilized P. corylifolia seeds and its synthetically prepared active ingredient psoralen, along with their specific blockers and potentiators in varying concentrations, have been studied on the isolated scale melanophores of the freshwater fish C. punctatus. The concentration used for the agonist ranged from 1 × 10−6 to 6.4 × 10−5 g/mL, that of antagonist was 4 × 10−6 g/mL and the concentration range of pure psoralen was 1 × 10−7 to 6.4 × 10−6 g/mL.
Effect of lyophilized extracts of P. corylifolia seeds per se on the isolated scale melanophores of C. punctatus
It was found that lyophilized extract of P. corylifolia alone induced a dose-dependent melanin dispersal response in the isolated scale melanophores of C. punctatus. The initial three concentrations of lyophilized extracts of P. corylifolia (1 × 10−6, 2 × 10−6, and 4 × 10−6 g/mL) highly dispersed the previously adrenalized scale melanophores, where the MMSI increased from a control (fish saline + adrenaline (2 × 10−8 g/mL) incubated melanophores) value of 4.61 ± 0.41 to 14.4 ± 1.9 (P = 0.0001). In response to the maximal concentration of 6.4 × 10−5 g/mL of P. corylifolia, the MMSI decreased to 9.61 ± 1.51 that was still significantly (P = 0.0063) different from the control (). After repeated washings and re-immersion (RI) of the P. corylifolia-treated melanophores in normal saline, it was found that the powerful melanin dispersal effects of P. corylifolia completely vanished, as the MMSI was found to be 4.49 ± 0.62 and was not significantly different from the control values of 4.61 ± 0.41 ().
Figure 1. The dose–response curve for the melanophore dispersal effect of lyophilized Psoralea corylifolia seed extract (♦, closed diamonds) on the adrenalized melanophores of C. punctatus. The complete blocking effects of specific antagonists atropine 4 × 10−6 g/mL (○) and hyoscine 4 × 10−6 μg/mL (▴) against P. corylifolia seed extract dispersed melanophores are also shown. RI signifies the mean melanophore size index (MMSI) after the re-immersion of scales in normal fish saline after repeated washings. Abscissae: Doses of P. corylifolia and antagonists in molar concentration. Ordinate: responses of melanophores (MMSI). Vertical bars represent the standard error of mean; P signifies the level of significance.
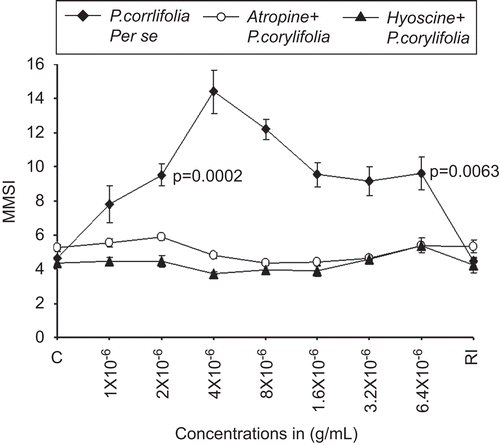
Effect of P. corylifolia on atropine and hyoscine pretreated isolated scale melanophores of C. punctatus
In a series of pilot experiments, the specific cholinergic muscarinic blockers atropine and hyoscine alone in a concentration range of 1 × 10−6 to 6.4 × 10−5 g/mL caused dispersion of the fish melanophores; however, the concentration of 4 × 10−6 g/mL did not cause any effect on the melanophores as they were found to be neither aggregated nor dispersed; hence, this particular concentration of 4 × 10−6 g/mL was selected for both antagonists in studying the involvement of cholinergic system. It was found that the powerful melanophore dispersal effects of lyophilized extracts of P. corylifolia were completely blocked by 4 × 10−6 g/mL of atropine as well as hyoscine (). In response to the maximal concentration of 6.4 × 10−5 μg/mL of lyophilized extract of seeds of P. corylifolia, in the presence of both the antagonists, the MMSI was found to be 5.39 ± 0.41.
Effect of neostigmine and P. corylifolia on the isolated scale melanophores of C. punctatus
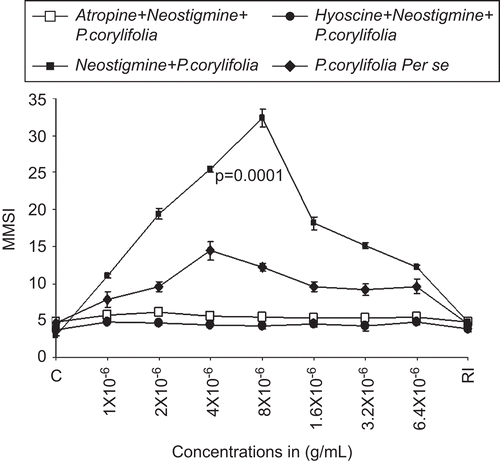
In order to further substantiate the role of cholinergic muscarinic receptors in causing powerful melanin dispersion within the scale melanophores of C. punctatus by lyophilized seeds extracts of P. corylifolia, we used a specific cholinergic potentiator, neostigmine. We treated the adrenalized scale melanophores of C. punctatus with a constant dose of neostigmine (4 × 10−6 g/mL), in combination with increasing concentrations of lyophilized extracts of P. corylifolia from 1 × 10−6 to 6.4 × 10−5 g/mL. It was found that the per se melanin dispersal effects of lyophilized extracts of P. corylifolia were highly potentiated by neostigmine (4 × 10−6 g/mL). The MMSI value of 12.2 ± 0.36 (P = 0.0001) was obtained in comparison with per se treatment of the scale melanophores with lyophilized extracts of P. corylifolia where the MMSI was 9.61 ± 1.51, P = 0.0063. There was a 21.22% potentiation of the melanophore dispersal effects of lyophilized extracts of P. corylifolia by neostigmine. Furthermore, it was also found that the potentiated melanophore dispersal effects of lyophilized extracts of P. corylifolia by neostigmine were completely blocked by the same concentration (4 × 10−6 g/mL) of atropine and hyoscine ().
Figure 2. The dose–response curve for the melanophores dispersal effect of lyophilized extract of seeds of Psoralea corylifolia (alone) (♦, closed diamonds). Closed squares (▪) show the effect of neostigmine (4 × 10−6 g/mL) on the dose–response curve lyophilized extract P. corylifolia seeds. RI signifies the mean melanophore size index (MMSI) after the re-immersion of scales in normal fish saline after repeated washings. Abscissae: doses in g/mL. Ordinate: responses of melanophores (MMSI). Note the potentiation of the dispersal response of Psoralea corylifolia by neostigmine and the same blocked by 4 × 10−6 g/mL of atropine (□), as well as hyoscine 4 × 10−6 g/mL (•).Vertical bars represent the standard error of mean; P signifies the level of significance.
Effect of psoralen alone and its antagonists on the isolated scale melanophores of C. punctatus
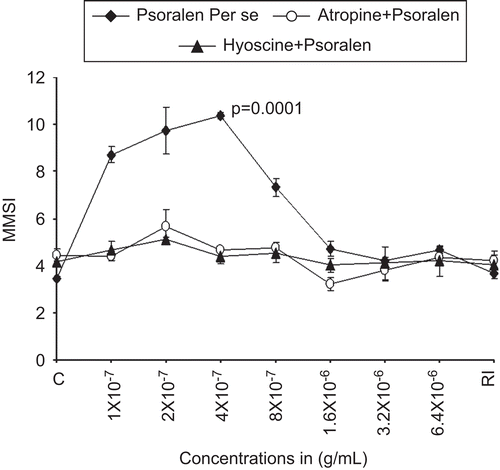
In order to further validate the exact role of P. corylifolia seed extract and its active ingredients in inducing melanin dispersion leading to darkening of isolated scale melanophores of C. punctatus, the melanophores pretreated with 2 × 10−8 g/mL of adrenaline were incubated with psoralen in concentrations ranging from 1 × 10−7 to 6.4 × 10−6 g/mL. It was observed that psoralen induced physiologically significant melanophore dispersion in all concentrations. The highest degree of dispersion from the control value of 3.46 ± 0.07 to 10.37 ± 1.42 (P = 0.0001) was induced by 4 × 10−7 g/mL of psoralen alone (). The powerful dispersal effects of psoralen were also found to be completely blocked by atropine and hyoscine, specific cholinergic muscarinic antagonists in preselected concentration of 4 × 10−6 g/mL ().
Figure 3. The dose–response curve for the melanophore dispersal effect of lyophilized psoralen (psoralen, alone) (♦, closed diamonds) on the adrenalized melanophores of C. punctatus. The complete blocking effects of specific antagonists atropine 4 × 10−6 μg/mL (○) and hyoscine 4 × 10−6 g/mL (▴) against psoralen dispersed melanophores are also shown. RI signifies the mean melanophore size index (MMSI) after the re-immersion of scales in normal fish saline after repeated washings. Abscissae: Doses of P. corylifolia in g/mL. Ordinate: responses of melanophores (MMSI). Vertical bars represent the standard error of mean; P signifies the level of significance.
Effect of psoralen and neostigmine on the isolated scale melanophores of C. punctatus
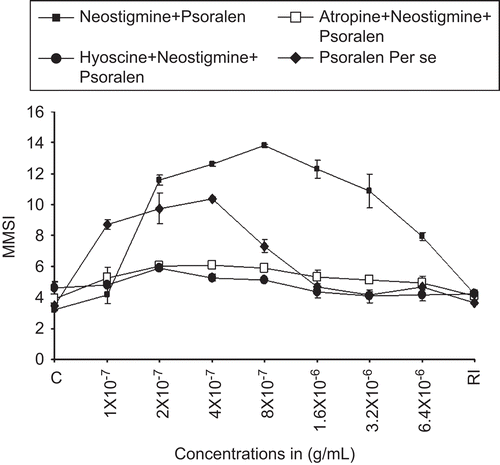
To substantiate the observations that cholinergic receptors may be involved in melanin dispersion caused by psoralen, neostigmine, a potentiator of cholinergic neurotransmitter, was used prior to the treatment of the fish scale melanophores. It was found that neostigmine significantly potentiated the powerful melanin dispersal effects of psoralen by 24.85%. Thus it was found that neostigmine potentiated the pretreated melanin dispersal effects of both the lyophilized extract of P. corylifolia and psoralen. In another experiment, it was found that the combined melanophore dispersal effects of psoralen and neostigmine were completely blocked by atropine and hyoscine at the concentration of 4 × 10−6 g/mL (). The order of potency of the various extracts and their agonists in causing melanophore dispersion is as follows: neostigmine + psoralen > neostigmine + lyophilized seed extract of P. corylifolia > psoralen > lyophilized seed extract of P. corylifolia.
Figure 4. Dose–response curve for the melanophores dispersal effect of psoralen (alone) (♦, closed diamonds). Closed squares show (▪) the effect of neostigmine (4 × 10−6 g/mL) on the dose–response curve psoralen. RI signifies the mean melanophore size index (MMSI) after the re-immersion of scales in normal fish saline after repeated washings. Abscissae: doses in g/mL. Ordinate: responses of melanophores (MMSI). Note the potentiation of the dispersal response of psoralen by neostigmine and the same blocked by 4 × 10−6 g/mL of atropine (□), as well as hyoscine 4 × 10−6 g/mL (•). Vertical bars represent the standard error of mean.
Discussion
In the present study, the lyophilized extract of P. corylifolia seeds and standard psoralen in dose ranges of 1 × 10−6 to 6.4 × 10−5 μg/mL and 1 × 10−7 to 6.4 × 10−6 g/mL, respectively, induced powerful melanin dispersal effects of the previously adrenaline-aggregated isolated scale melanophores of C. punctatus. Comparatively, psoralen caused a more sustained and powerful melanin dispersal within the isolated fish melanophores, and interestingly the concentrations required to achieve maximal dispersion of melanophore were 10 times less than that of lyophilized seed extract of P. corylifolia. The physiologically significant dose-related melanin dispersion effects of lyophilized P. corylifolia seeds and synthetic psoralen alone were found to be completely abolished by atropine and hyoscine, which are specific cholino-muscarinic receptor blockers. These data strongly indicate that in the fish, C. punctatus, the dispersion of melanin granules within the scale melanophores is mediated by choline receptors of muscarinic nature. These data are similar to the earlier findings of CitationAli et al. (1995) where melanin dispersion in amphibians, Hoplobatrachus tigerinus Daudin (Ranidae) (Rana tigerina) and Duttaphrynus melanostictus Schneider (Bufonidae) (Bufo melanostictus), was found to be mediated by the dominantly present cholinergic muscarinic receptors. In some fishes, such as Krytopterus bicirrhi and Corydoras paleatus, acetylcholine, which has been strong candidate for cholinergic transmission, has been found to induce melanin aggregation rather than dispersion, which was effectively blocked by cholinergic antagonists (CitationFujii et al., 1982; CitationKasukawa et al., 1985).
In the present study, neostigmine, an anticholinesterase agent potentiated the melanin dispersal effects of lyophilized seed extract of P. corylifolia and psoralen, which further lends support to the view that cholinergic neurotransmitter substance acetylcholine via initiation of its receptors has a positive role in melanophore dispersion leading to skin darkening. These observations are in concurrence with the historic and traditional use of P. corylifolia seed extracts and 8-methoxypsoralen in the treatment of hypopigmentation via melanin relocation/regeneration/dispersion as reported by CitationFowlks (1959) and CitationPathak et al. (1962).
As far as is known, this is the first report of its kind where lyophilized extracts of any part of P. corylifolia and synthetic psoralen have both been found to stimulate the dominantly present cholinergic muscarinic receptors in the pigment cell system of fish, leading to skin darkening.
There are no reports in the literature showing the activation of muscarinic cholinergic receptors by plant extracts, particularly P. corylifolia and its active ingredients the psoralens, in any of the pigmented cells, the melanophores. In this regard, the work of CitationLaskin et al. (1985) and Mohamed et al. (1990) are worth mentioning as they have demonstrated that psoralen has a direct action on mouse melanocytes, the homologous kin of the melanophores. These workers have cited data demonstrating the existence of specific binding sites for psoralen on a variety of mammalian cell types in culture. CitationLaskin et al. (1985) have indicated that specific binding sites for psoralen receptors may be involved in clinical action of psoralen in the skin. In view of this earlier piece of work, if the present data are interpreted then it seems that both lyophilized extracts of P. corylifolia and the synthetic psoralen stimulate the cholinergic receptors similarly to those of psoralen receptors. It may be mentioned that Fructus Psoralea, dry fruit of P. corylifolia, via M3 cholinergic receptor stimulation had caused enhanced motility of isolated gall bladder strips from guinea pigs (CitationJin et al., 2006). Thus, these findings open new vistas showing the possibility of the presence of psoralen receptors that have characteristics similar to those of muscarinic cholinergic ones with regard to their mode of action by P. corylifolia active ingredients. The present study also signifies the evolutionary aspect of the receptors of the lower vertebrate melanophores, their phylogenetic development, which is homologous to melanocytes in a more evolved mammalian–melanocyte receptor system.
Conclusion
It is concluded that lyophilized extract of P. corylifolia and its active ingredient psoralen induced powerful dose-dependent physiologically significant melanin dispersal effects in the isolated scale melanophores of C. punctatus, which were completely blocked by atropine as well as hyoscine. The per se melanin dispersal effects of lyophilized extracts of P. corylifolia and its active ingredient psoralen got highly potentiated by neostigmine. It appears that the melanin dispersal effects of the extracts of P. corylifolia and pure psoralen leading to skin darkening are mediated by cholino-muscarinic- or cholino-psoralen-like receptors having similar properties.
Acknowledgement
The authors are grateful to the Principal and Secretary Saifia College of Science, Bhopal, affiliated to Barkatullah University Bhopal, for providing necessary facilities.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ali SA, Peter J, Ali AS. (1998). Histamine receptors in the skin melanophores of Indian bullfrog Rana tigerina. Comp Biochem Physiol, Part A Mol Integr Physiol 121:229–234.
- Ali AS, Peter J, Ali SA. (1995). Role of cholinergic receptors in melanophore responses of amphibians. Acta Biol Hung 46:61–73.
- Aspengren S, Hedberg D, Sköld HN, Wallin M. (2009). New insights into melanosome transport in vertebrate pigment cells. Int Rev Cell Mol Biol 272:245–302.
- Aspengren S, Sköld HN, Quiroga G, Mårtensson L, Wallin M. (2003). Noradrenaline- and melatonin-mediated regulation of pigment aggregation in fish melanophores. Pigment Cell Res 16:59–64.
- Bhattacharya SK, Parikh AK, Das PK. (1976). Effect of catecholamines on melanophores of Rana tigrina. Indian J Exp Biol 14:486–488.
- Briganti S, Camera E, Picardo M. (2003). Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res 16:101–110.
- Cardosa CAL, Vilegis W, Honda NKJ. (2000). Rapid determination of furanocoumarins in creams and pomades using SPE and GC. J Pharm Biomed Anal 22:203–214.
- Cardoso CA, Honda NK, Barison A. (2002). Simple and rapid determination of psoralens in topic solutions using liquid chromatography. J Pharm Biomed Anal 27:217–224.
- Fitzpatrick TB, Pathak MA. (1984). Research and development of oral psoralen and longwave radiation photochemotherapy: 2000 B.C.–1982 A.D. Natl Cancer Inst Monogr 66:3–11.
- Fowlks WL. (1959). The chemistry of the psoralens. J Invest Dermatol 32:249–254.
- Fuji R, Miyashita Y. (1976). Beta adrenoceptors, cyclic AMP and melanosome dispersion in guppy melanophores. Pigment Cell Res 3:336–344.
- Fujii R, Miyashita Y, Fujii Y. (1982). Muscarinic cholinoceptors mediate neurally evoked pigment aggregation in glass catfish melanophores. J Neural Transm 54:29–39.
- Gupta AK, Anderson TF. (1987). Psoralen photochemotherapy. J Am Acad Dermatol 17:703–734.
- Hanamura T, Uchida E, Aoki H. (2008). Skin-lightening effect of a polyphenol extract from acerola (Malpighia emarginata DC.) fruit on UV-induced pigmentation. Biosci Biotechnol Biochem 72:3211–3218.
- Hogben LT, Slome D. (1931). Pigmentary effector system VI: The dual character of endocrine co-ordination in amphibian colour change. Proc R Soc Lond B 108:10–53.
- Jeon S, Kim NH, Koo BS, Kim JY, Lee AY. (2009). Lotus (Nelumbo nuficera) flower essential oil increased melanogenesis in normal human melanocytes. Exp Mol Med 41:517–525.
- Jin S, Li M, Lin ML, Ding YH, Qu SY, Li W, Zheng TZ. (2006). Effect of Fructus Psoraleae on motility of gallbladder isolated smooth muscle strips from guinea pigs. World J Gastroenterol 12:5214–5218.
- Kasukawa H, Fujii R. (1985). Receptor mechanisms in fish chromatophores—VII. Muscarinic cholinoceptors and alpha adrenoceptors, both mediating pigment aggregation, strangely coexist in Corydoras melanophores. Comp Biochem Physiol C, Comp Pharmacol Toxicol 80:211–215.
- Laskin JD, Lee E, Yurkow EJ, Laskin DL, Gallo MA. (1985). A possible mechanism of psoralen phototoxicity not involving direct interaction with DNA. Proc Natl Acad Sci USA 82:6158–6162.
- Lerner AB, Denton CR, Fitzpatrick TB. (1953). Clinical and experimental studies with 8-methoxypsoralen in vitiligo. J Invest Dermatol 20:299–314.
- Lin ZX, Hoult JR, Raman A. (1999). Sulphorhodamine B assay for measuring proliferation of a pigmented melanocyte cell line and its application to the evaluation of crude drugs used in the treatment of vitiligo. J Ethnopharmacol 66:141–150.
- Marwan MM, Jiang JW, Castrucci AM, Hadley ME. (1990). Psoralens stimulate mouse melanocyte and melanoma tyrosinase activity in the absence of ultraviolet light. Pigment Cell Res 3:214–221.
- Matsuda H, Hirata N, Kawaguchi Y, Yamazaki M, Naruto S, Shibano M, Taniguchi M, Baba K, Kubo M. (2005). Melanogenesis stimulation in murine b16 melanoma cells by umberiferae plant extracts and their coumarin constituents. Biol Pharm Bull 28:1229–1233.
- Pathak MA, Daniels F Jr, Fitzpatrick TB. (1962). The presently known fistrixuhion of furocoumarins (psoralens) in plants. J Invest Dermatol 39:225–239.
- Peter J, Ali AS, Ali SA. (1996). Effect of histaminergic drugs on integumental melanophores of adult Bufo melanostictus. Indian J Exp Biol 34:427–430.
- Ryan RW, Post JI, Solc M, Hodson PV, Ross GM. (2002). Catecholaminergic neuronal degeneration in rainbow trout assessed by skin color change: A model system for identification of environmental risk factors. Neurotoxicology 23:545–551.
- Sharma SK, Singh VP. (1979). The antifungal activity of some essential oils. Ind Drugs Pharm 14:3–6.
- Solano F, Briganti S, Picardo M, Ghanem G. (2006). Hypopigmenting agents: an updated review on biological, chemical and clinical aspects. Pigment Cell Res 19:550–571.
- Spaeth RA. (1913). The physiology of the chromatophores of fishes. J Exptl Zool 15:527.
- Thody AJ. (1993). Skin pigmentation and its regulation. In: Priestley GC, ed., Molecular Aspects of Dermatology. Chichester: John Wiley, p. 55.
- Zhao G, Li S, Qin GW, Fei J, Guo LH. (2007). Inhibitive effects of Fructus Psoraleae extract on dopamine transporter and noradrenaline transporter. J Ethnopharmacol 112:498–506.