Abstract
Context: Carum carvi L., (Umbelliferae) known as caraway, is a famous traditional herbal plant supposed to contain active components with pharmacological properties.
Objective: In this study, the effects of caraway extracts on preventing sepsis induced by oxidative tissue injuries have been investigated by measuring heart and kidney oxidative stress parameters.
Materials and methods: Sepsis was induced in rats (n = 6) by experimental cecal ligation and puncture (CLP) model. Then, either hydroalcoholic extract or essential oils (50 and 100 mg/kg body weight) were injected intraperitonially immediately after CLP operation. Twenty-four hours after CLP, the rats were anesthetized when kidney and heart tissues were removed to analyze the tissue oxidative stress parameters, that is, glutathione (GSH) and lipid peroxidation (LP).
Results: Sepsis induction caused a significant increase in kidney but not heart LP, indicating that kidney was more affected by sepsis induction than heart. Kidney LP and plasma urea/creatinine ratio levels were readily reversed in rats treated with essential oils but not in those treated with hydroalcoholic extract. Unlike LP, the heart and kidney GSH levels were not affected in all treated groups.
Discussion and conclusion: Our data imply that caraway oils probably have a protective role in kidney tissue against oxidative injury in advanced stages of sepsis.
Key words::
Introduction
Septic shock is a severe form of sepsis associated with the development of progressive damage to multiple organs in the late stage. It is also an important cause of mortality in intensive care units (CitationHubbard et al., 2005). During sepsis, reductions of enzymatic and nonenzymatic antioxidants [e.g. glutathione (GSH)] and also over-production of oxygen-free radicals [reactive oxygen species (ROS)] ultimately lead to oxidative stress (CitationPeralta et al., 1993; CitationGutteridge, 1995). Oxidative stress plays an important role in the pathogenesis of sepsis and its complications. Experimental and clinical studies have shown that any harmful tissue events, such as infection, trauma, or anoxia, are perceived by macrophages and monocytes, which in turn secrete cytokines such as interleukin (IL)-1 and tumor necrosis factor-α. These cytokines activate inflammatory cells, such as neutrophils, macrophages or monocytes, platelets, and mastocytes, releasing large amounts of ROS leading to cellular injury via several mechanisms including the peroxidation of membrane lipids and the oxidative damage of proteins and DNA (CitationHubbard et al., 2005; CitationAndrades et al., 2005). These events can eventually cause secondary damages to organs including heart and kidney, which may contribute to organ dysfunction and failure (CitationSinger & Brealey, 1999).
The frequent question for the treatment of sepsis nowadays favors the investigation of oxidative stress and the role of antioxidative defense systems to develop new pharmacological approaches to this life-threatening disease (CitationVictor et al., 2004). There is substantial evidence indicating that natural antioxidant and herbal drugs exert their antioxidant properties by scavenging oxygen-free radicals (CitationHuh et al., 1994; CitationGómez-Zubeldia et al., 2000). Silymarine (a mixture of bioactive flavonoliganans isolated from Asteraceae silybum) can protect APAP (N-acetyl-para-aminophenol OR acetaminophen) -induced damage in rat brain by preventing lipid peroxidation (LP) and replenishing the GSH levels (CitationNencini et al., 2007). A membrane radical scavenger, that is Tempol-attenuated organ dysfunction and injury by reducing the plasma concentration of IL1β and NO (nitric oxide) and decreasing the neutrophil infiltration in the liver and lung tissues (CitationLiaw et al., 2005).
Carum carvi L. (Umbelliferae), known as caraway, is a famous traditional herbal plant and is normally found in northern and central Europe, Siberia, Turkey, Iran, India, and North Africa. In Iran, the seeds of caraway, commonly known as “Zireh Siah,” have been used extensively in food. The seeds have been traditionally used as a medicinal plant in traditional medicine for treating aliments such as flatulence, colic pain, and bronchitis. In addition, caraway has been widely used as stomachic, antispasmodic, antibacterial, antiulcerogenic, and antiproliferative agent (CitationEddouks et al., 2004).
In vitro and in vivo studies indicated that essential oils and aqueous extract of caraway have various physiological and pharmacological properties (CitationDe Carvalho & Da Fonseca, 2006; CitationLemhadri et al., 2006; CitationMazaki et al., 2006). One previous study showed the suppressive effects of caraway extracts on cytochrome P-4501A1, a phase-I xenobiotic metabolizing enzyme, which was over-expressed with dioxane in hepatoma cell line (CitationNaderi-Kalali et al., 2005). More freshly, we proved the in vitro antioxidant and antibacterial properties of caraway oil preparation (Fatemi et al., in press). In addition, we showed that caraway essential oils modulate the parameters related to oxidative liver and lung injuries, that is, myeloperoxidase (MPO) activity, thiobarbituric acid reactive substances (TBARS), and GSH levels in cecal ligation and puncture (CLP) rat model (CitationFatemi et al., 2010a,Citationb).
In regard to our previous studies (CitationFatemi et al., 2010a,Citationb, in press) and also the importance of free radicals in the mortality of septic patients, we decided to consider the effects of caraway oils and hydroalcoholic extract on preventing oxidative heart and kidney injuries in sepsis for the first time. Thus, in this study, after analyzing the composition of caraway extracts and their antioxidant capacities, we considered the effects of these fundamental constitutes on parameters related to oxidative kidney and heart injuries in septic rats.
Materials and methods
Chemicals
Fresh caraway seeds were purchased from a local market in Tehran, Iran. The plants were collected in June 2008 from the Varzaneh town in Isfahan province, Iran. A scientist of botany, Mrs. Bagherzadeh (M.Sc., Institute for Research in Forests and Rangelands, Isfahan, Iran) authenticated the plant materials. Aluminum trichloride (AlCl3), methanol, trichloroacetic acid, ethylenediaminetetraacetic acid, dimethyl sulfoxide (DMSO), and Tris-base were obtained from Merck Co., Germany. Quercetin, 2,2-diphenylpicrylhydrazyl (DPPH), Trolox, thiobarbituric acid (TBA), dithionitrobenzene, and GSH were from Sigma Chemical Co., USA. Serum urea, creatinine, and creatinine kinase-MB (CK-MB) detection kits were from Pars Azmoon Co., Tehran, Iran.
Oil extraction and analysis
Caraway seeds were subjected to oil extraction using a Clevenger-type apparatus. The extraction was carried out for 2 h and the oil was stored in dark glass bottles in a freezer (−20°C) until further use. Gas chromatographic (GC) analysis was performed using GC (9-A-Shimadzu; Japan) equipped with a flame ionization detector. Quantitation was performed using Euro Chrom 2000 software (KNAUER Company, Germany) by the area normalization method. The analysis was carried out using a DB-5 fused-silica column (30 m × 0.25 mm; film thickness, 0.25 µm) using a temperature program of 40–250°C at a rate of 4°C/min, injector temperature of 250°C, detector temperature of 265°C. The carrier gas was helium (99.99%). The GC/MS unit consisted of a Varian-3400 GC coupled to a Saturn II ion trap detector. The type of the column and the conditions used for analysis was similar to those of GC analysis. The constituents were identified by comparing their mass spectra with those of authentic standards. The peaks were further identified by comparing their retention indices with those of authentic standards.
Preparation of hydroalcoholic extract and estimation of total flavonoids
Powdered caraway seeds (30 g) were mixed with distilled water (50 ml) and methanol (50 ml) at 70–80°C and maintained at 60°C for 24 h. Then, the hydroalcoholic extract was filtered through a Whatman filter (#4, with pore size 20–25 µm) to remove particulate matters. The filtrate was then freeze-dried for further use.
Total flavonoid content of the hydroalcoholic extract was determined using Dowd method as adapted by CitationArvouet-Grand et al. (1994). Briefly, 5 ml of the extract solution (0.1 mg/ml) was mixed with an equal volume of 2% aluminum trichloride (AlCl3) prepared in methanol. The absorption was recorded at 415 nm using Shimadzu UV-3100 spectrophotometer after 10 min against a blank sample. The blank sample was prepared by mixing 5 ml hydroalcoholic extract with 5 ml methanol alone. Total flavonoids were determined using a standard curve prepared with quercetin (0–100 mg/l) as the standard. The mean of three readings was used and expressed as milligram quercetin equivalents (QE)/gram of hydroalcoholic extract.
Radical-scavenging capacity (DPPH assay) of the extracts
This spectrophotometric assay uses the stable radical DPPH as a reagent (CitationBurits & Bucar, 2000). The essential oils in methanol (50 µl of 1:5 dilution) were added to 5 ml of DPPH solution (0.004% DPPH in methanol). After 30 min incubation at room temperature, the absorbance was recorded against the blank at 517 nm. Trolox, (1 mM) a stable antioxidant, was used as a synthetic reference. The inhibition of free radical (DPPH) was calculated as percentage (I%) by the following formula:
where Ablank is the absorbance of the control reagent (containing all reagents except the test compound) and Asample is the absorbance of the test compound. All the assays were carried out in triplicate.
The radical scavenging activity of the hydroalcoholic extract was also measured spectrophotometrically using the DPPH radical (CitationBlois et al., 1958). The caraway hydroalcoholic extract (50 µg/ml) was added to 2 ml DPPH solution (125 μM DPPH in methanol) and the final volume was adjusted to 4 ml with water. The mixture was mixed and incubated at 37°C in dark for 30 min. Changes in the absorbance of DPPH were recorded at 517 nm. A parallel experiment was performed in which caraway extract was replaced with vitamin C (5 µg/ml) and considered as a positive control. Percent inhibition was calculated by comparing the absorbance values of the blank and the sample as discussed above.
CLP model
Male albino rats of Wistar strain (150–170 g) were purchased from the Pasteur Institute of Iran. Animal studies were approved by the Medical Ethics Committee of Tarbiat Modares University.
Polymicrobial sepsis in rats was induced by CLP according to the method of CitationHubbard et al. (2005). Briefly, rats were anesthetized by a single injection [intraperitoneal (i.p.)] of ketamine (90 mg/kg) and xylozine (10 mg/kg) mixture. A small midabdominal incision (2–3 cm) was made and the cecum was exposed. A distended portion of the cecum just distal to the ileocecal valve was isolated, filled with fecal content, and tied with a 3-0 silk suture in a manner not to disrupt bowel continuity. The ligated portion of the cecum was punctured twice with a 20-gauge needle. The cecum was then placed in its original position within the abdomen, the abdomen was then closed with a 3-0 suture in two layers, and the animals were allowed to recover. In the sham-operated rat, the cecum was exposed, manipulated, and returned to the peritoneal cavity without being ligated and punctured. After surgery, normal saline [3 ml/100 g body weight (b.w.)] was given subcutaneously to all rats to prevent dehydration.
Animal treatments and tissue processing
As shown in , animals were divided into seven groups. The caraway essential oils, hydroalcoholic extracts (50 and 100 mg/kg b.w.) and also indomethacin (10 mg/kg b.w.) were dissolved in 0.5 ml DMSO and injected immediately after CLP operation. Twenty-four hours after CLP, blood samples were collected and kidney and heart tissues were removed, washed, and processed for biochemical analysis.
Table 1. Treatment groups.
Measurement of LP products in liver
A weighed portion of tissues was homogenized in phosphate buffer (100 mM, pH 7.0) and used to measure the levels of TBARS as indices for LP. The concentration of TBARS was measured spectrophotometrically using TBA reagent based on the procedure described by Buege and Aust (1987).
GSH estimation
GSH was estimated in tissue homogenates according to the procedure of CitationSeldak and Lindsay (1986).
Plasma biomarkers
To confirm the kidney and heart injury, serum urea, creatinine and creatine kinase-MB were determined respectively, using commercial kits on an autoanalyzer (Technicon RA-1000).
Statistical analysis
Data are presented as means ± standard error. The results were subjected to one-way analysis of variance followed by Tukey’s honestly significant differences using SPSS 13.0 software. The significance was considered as P < 0.05.
Results
Analysis of caraway extracts
Based on GC and GC/MS analyses, 17 known compounds were identified in the essential oil samples extracted from Iranian caraway seeds ( and ). The peaks identified as cumin aldehyde (20.90%), γ-terpinene (16.78%), γ-terpinene-7-al (16.69%), and p-cymene (7.23%) constitute the major compounds of the essential oils ().
Table 2. Essential oil analysis prepared from caraway seeds.
Figure 1. MS chromatogram plot. The constituents were identified by comparing their mass spectra with those of authentic standards. The peaks were further identified by comparing their retention indices with those of authentic standards.
The total flavonoid content of hydroalcoholic extract of caraway seeds was found to be 18.5 mg of QE/g hydroalcoholic extract calculated from a standard curve generated by quercetin.
DPPH radical-scavenging activity of caraway extracts
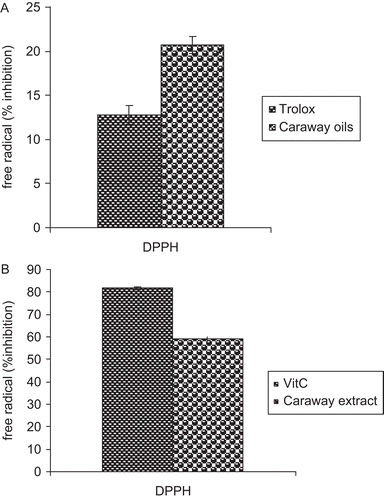
The antioxidant capacities of the essential oils and hydroalcoholic extract prepared from caraway seeds were determined by in vitro DPPH radical-scavenging activity ( and ). The essential oils notably reduced the concentration of DPPH free radical, with an efficacy higher than that of Trolox (). Also, the hydroalcoholic extract reduced the concentration of DPPH free radical, with an efficacy lower than that of vitamin C ().
Figure 2. (A) Free radical-scavenging activity of the caraway seed extracts. The radical scavenging activity of the oil extract was measured spectrophotometrically using the DPPH free radical. Trolox (1 mM) was used as positive control. (B) The radical scavenging activity of the hydroalcoholic extract was measured spectrophotometrically using the DPPH free radical. Vitamin C (5 µg/ml) was used as positive control. Results are mean ± SEM of three analyses carried out on essential oil and hydroalcoholic extract derived from caraway seeds.
The effects of caraway extracts on parameters related to oxidative kidney and heart injuries in CLP rats
Administration (i.p.) of caraway oils (50 and 100 mg/kg b.w.) to septic rats resulted in a significant reduction in kidney MDA levels (). CLP operation in rats without further treatments resulted in increased TBARS level, an index of LP. Treatment of rats with either caraway oil doses (50 and 100 mg/kg b.w.) could reverse the MDA level to normal level (laparatomy group) while hydroalcoholic extracts with similar concentrations failed to alter the TBARS level. The caraway preparations at a dose of 50 or 100 mg/kg b.w. given to rats were comparable with the effects of indomethacin (10 mg/kg b.w.), which is considered as a positive control. As shown in , kidney GSH was not changed in all treated animals (P > 0.05).
Table 3. Effect of caraway extracts on kidney and heart oxidative parameters in sepsis rats.
Under similar conditions of treatments, the levels of heart TBARS and GSH levels were not affected by CLP operation (). Administration of both caraway extracts also caused no significant changes in heart LP and GSH levels (P > 0.05).
Cardiac and renal biomarkers in plasma
The data presented in are consistent with results in , which shows that the ratio of urea/creatinine, the indicator of renal function, was approximately greater than in septic rats as compared with that in control group. Similar to indomethacin, caraway essential oils (50 and 100 mg/kg b.w.) influenced this ratio. In this connection, a marked increase in urea/creatinine ratio due to CLP operation was significantly suppressed in CLP rats treated with caraway oils and also indomethacin (P < 0.05). Nevertheless, caraway hydroalcoholic extracts failed to alter this parameter.
Table 4. Effect of caraway extracts on renal and cardiac biomarkers in plasma of sepsis rats.
Plasma CK-MB (as the indicator of heart injury) was also unaffected in all treated animals (P > 0.05) ().
Discussion
Previously, we reported the suppressive effects of caraway essential oils on colon premalignant lesions induced by dimethylhydrazine (Dadkhah et al., in press). More recently, we proved the in vitro antioxidant and antibacterial properties of caraway oil preparation (Fatemi et al., in press). To continue with this, after indicating the influence of caraway essential oils and hydroalcoholic extract on oxidative liver and lung injury parameters (MPO activity, TBARS, and GSH levels) in CLP rat model (CitationFatemi et al., 2010a,Citationb), attempts were made to find out the mechanism by which the caraway extracts reduce the possible kidney and heart damages that may occur in septic rats.
In the first step, we evaluated the antioxidant strength of caraway efficient extracts in vitro. As shown in this study (), the radical scavenging capacity of caraway seed essential oils (DPPH assay) containing cumin aldehyde (20.90%), γ-terpinene (16.78%), γ-terpinene-7-al (16.69%), and p-cymene (7.23%) () implied that caraway oil is a potent free-radical scavenger and antioxidant. As shown in , the hydroalcoholic extract of caraway seeds containing 18 mg/g quercetin equivalent of flavonoids is also an effective antioxidant.
Our in vivo results () clearly showed that sepsis causes partial oxidative damage in the kidney as demonstrated by the increased LP. These data, together with the biochemical findings (urea/creatinine ratio) confirmed the fractional kidney damage. Other studies also indicated that the oxidative stress in sepsis has a direct relationship with organ injuries in CLP model. Releasing large amounts of ROS in sepsis can cause cellular injury via several mechanisms including the peroxidation of membrane lipids and the oxidative damages to proteins and DNA (CitationGutteridge, 1995; CitationHubbard et al., 2005).
Treatment of septic rats with caraway oils and also indomethacin could significantly reverse the increased LP product (P < 0.05) but conversely, caraway hydroalcoholic extract did not modulate the kidney LP level (P > 0.05). The protective role of caraway oils in kidney was further confirmed by showing that the urea/creatinine ratio increased in septic rats (the indicator of renal function) is reversed in caraway oil and indomethacin-treated groups (). On the other hand, GSH, which is an important constituent of the intracellular protective mechanism against various noxious stimuli (CitationVilla et al., 2002), is not affected in kidney after CLP operation indicating that the injury was not serious enough to cause GSH depletion. So, the GSH level was not changed after treatment of septic rats with both caraway preparations. Quite the opposite of this result, our findings indicate that heart was not severely injured under CLP operation. The oxidative parameters of heart injury, that is GSH, LP, and also plasma CK-MB were not affected in all treated animals indicating that sepsis due to CLP operation caused no damage to heart ().
Our previous results also indicated that hydroalcoholic extract of caraway seeds has no effects in modulating the oxidative stress parameters in lung tissue (CitationFatemi et al., 2010b). In this regard, there are different reasons that can justify the superiority of essential oils over hydroalcoholic fractions in modulating the partially injured kidney. First, the effectiveness of the essential oils in remedy of sepsis is probably due to pharmacokinetic properties and the disposition of the caraway monoterpenes in the body. Considering the physicochemical properties of the terpenes, such as lipophilicity and molecular mass and size, penetration into and permeation through the skin as well as subsequent systemic effects can be expected (CitationCrowell et al., 1992; CitationPhillips et al., 1995; CitationStott et al., 1998; CitationHongratanaworakit & Buchbauer, 2006). The presence of aromatic compound glucosides, alkyl glycosides, glucides, nucleoside, and flavonoids in hydroalcoholic fraction of caraway seeds (CitationMatsumura et al., 2002) offer relatively poor bioavailability to this fraction.
The bioavailability of dietary polyphenols is limited not only by the physiochemical properties of the molecule but also by enzyme and microbial-mediated biotransformation and active efflux (CitationScalbert et al., 2002; CitationLambert & Yang, 2003; CitationManach et al., 2004, Citation2005; CitationYang et al., 2008), which directly undergo phase II metabolism (CitationLi et al., 2001; CitationLambert & Yang, 2003; CitationLu et al., 2003a,Citationb; CitationHu et al., 2003). On the other hand, the more protective role of essential oils, rather than hydroalcoholic extracts in septic rats can partly be due to antimicrobial properties of caraway essential oils. In this connection, our data indicate that caraway essential oils but not hydroalcoholic extracts obtained from caraway seeds possessed antimicrobial activity against some Gram-negative and Gram-positive bacteria (Fatemi et al., in press; data not shown). Other studies also implied the severe antimicrobial activity of essential oils (CitationRasooli et al., 2006, Citation2008; CitationCosta et al., 2008).
On the other hand, despite the fact that polyphenols have antioxidant activities, there are different reports indicating the prooxidant properties of the flavonoids (CitationGalati & O’Brien, 2004; CitationMurzakhmetova et al., 2008; CitationBabich et al., 2008; CitationPanemangalore & Bebe, 2009). CitationRobaszkiewicz et al. (2007) demonstrated concentration-dependent effects of quercetin on A549 cells in vitro, including augmentation of cell proliferation and increase in total antioxidant capacity (TAC) of the cells at low quercetin concentrations and a decrease in cell survival and viability, thiol content, TAC and activities of superoxide dismutase, catalase and GSH S-transferase at higher concentrations of the flavonoid (>50 mM) (CitationRobaszkiewicz et al., 2007). In addition, in the presence of H2O2, catechol B ring of flavonoids is oxidized by MPO to produce phenoxyl radicals responsible for oxidative damage to macromolecules (CitationGalati & O’Brien, 2004). Intracellular accumulations of ROS and protein carbonyls were detected in the HL-60 cells treated with apigenin (a flavonoid) in a dose-dependent manner. When these cells were treated with MPO inhibitors, the ROS level enhanced by apigenin was significantly reduced. The gathered data suggested that oxidation of apigenin by MPO and production of B-ring phenoxyl radicals might be responsible for the prooxidant effects (CitationMiyoshi et al., 2007).
One study also indicated the prooxidant activity of polyphenols arising from their interactions with metals such as iron and copper leading to the production of hydroxyl radicals via fenton-like reaction (CitationPerron & Brumaghim, 2009). Considering this information, we believe that polyphenols have antioxidant activities especially in reduced forms. When these compounds are changed to oxidized types such as phenoxyl or quinone forms, they can act as prooxidant agents. So, in regard to the increased levels of ROS and MPO in sepsis (data not shown; CitationFatemi et al., 2010a), it is assumed that polyphenols and flavonoids present in hydroalcoholic extract are changed to oxidized form and act as the prooxidant resulting from the lack of hydroalcoholic extract effects in improving tissue injury induced by sepsis. Alternatively, it should be considered further to assess the protective effect of caraway hydroalcoholic extract in sepsis.
Our results indicated ineffectiveness of i.p. injection of caraway hydroalcoholic extract. In this connection, using other models of sepsis induction and also evaluating other routes of the administration, that is, oral, intravenous, and subcutaneous, should be considered. In addition, different time of injections, for example, pre-treatments or post-treatments in different time intervals should be examined entirely.
Conclusion
In conclusion, our data implied that i.p. administration of caraway essential oil (but not hydroalcoholic extract) modulated sepsis-induced limited kidney damage in the advanced stages of sepsis. Its protective role is justified by its antioxidant properties, which involve the inhibition of LP and decreasing the plasma urea/creatinine ratio. Of course further in vivo studies are needed to consider the exact mechanism of caraway extract effects in sepsis-induced organ failure.
Declaration of interest
The authors declare no conflict of interest.
References
- Arvouet-Grand A, Vennat B, Pourrat A, Legret P. (1994). [Standardization of propolis extract and identification of principal constituents]. J Pharm Belg, 49, 462–468.
- Andrades M, Ritter C, Moreira JC, Dal-Pizzol F. (2005). Oxidative parameters differences during non-lethal and lethal sepsis development. J Surg Res, 125, 68–72.
- Babich H, Gottesman RT, Liebling EJ, Schuck AG. (2008). Theaflavin-3-gallate and theaflavin-3′-gallate, polyphenols in black tea with prooxidant properties. Basic Clin Pharmacol Toxicol, 103, 66–74.
- Blois MS. (1958). Antioxidant determination by use of a stable free radical. Nature, 29, 1199–1200.
- Buege JA, Aust SD. (1978). Microsomal lipid peroxidation. Meth Enzymol, 52, 302–310.
- Burits M, Bucar F. (2000). Antioxidant activity of Nigella sativa essential oil. Phytother Res, 14, 323–328.
- Crowell PL, Lin S, Vedejs E, Gould MN. (1992). Identification of metabolites of the antitumor agent d-limonene capable of inhibiting protein isoprenylation and cell growth. Cancer Chemother Pharmacol, 31, 205–212.
- Costa EV, Teixeira SD, Marques FA, Duarte MC, Delarmelina C, Pinheiro ML, Trigo JR, Sales Maia BH. (2008). Chemical composition and antimicrobial activity of the essential oils of the Amazon Guatteriopsis species. Phytochemistry, 69, 1895–1899.
- Dadkhah A, Allameh A, Khalafi H, Ashrafi-Helan J. (In Press). Inhibitory effects of dietary caraway essential oils on 1,2-dimethylhydrazine-induced colon carcinogenesis is mediated by liver xenobiotic metabolizing enzymes. Nutr Cancer.
- De Carvalho CCCR, Da Fonseca MMR. (2006). Carvone: Why and how should one bother to produce this terpene. Food Chem, 95, 413–422.
- Eddouks M, Lemhadri A, Michel JB. (2004). Caraway and caper: Potential anti-hyperglycaemic plants in diabetic rats. J Ethnopharmacol, 94, 143–148.
- Fatemi F, Allameh A, Khalafi H, Ashrafihelan J. (2010a). Hepatoprotective effects of gamma-irradiated caraway essential oils in experimental sepsis. Appl Radiat Isot, 68, 280–285.
- Fatemi F, Allameh A, Khalafi H, Rezaei MB, Seyhoon M. (2010b). [The effect of essential oils and hydroalcoholic extract of caraway seed on oxidative stress parameters in rats suffering from acute lung inflammation before and after γ-irradiation]. IJ Med Aroma Plant, 25, 441–455.
- Fatemi F, Allameh A, Khalafi H, Rajaee R, Rezaei MB. (In Press). Biochemical properties of γ-irradiated caraway essential oils. J Food Biochem.
- Galati G, O’Brien PJ. (2004). Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic Biol Med, 37, 287–303.
- Gómez-Zubeldia MA, Hernandez R, Viguera J, Arbues JJ, Aparicio A, Millán JC. (2000). Effect of bilateral ovariectomy and ovarian steroid hormones on the antioxidant systems and plasma malondialdehyde levels in Wistar rats. Endocr Res, 26, 97–107.
- Gutteridge JM. (1995). Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem, 41, 1819–1828.
- Hongratanaworakit T, Buchbauer G. (2006). Relaxing effect of ylang ylang oil on humans after transdermal absorption. Phytother Res, 20, 758–763.
- Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW 3rd, Bland KI, Chaudry IH. (2005). Cecal ligation and puncture. Shock, 24 Suppl 1, 52–57.
- Hu M, Chen J, Lin H. (2003). Metabolism of flavonoids via enteric recycling: mechanistic studies of disposition of apigenin in the Caco-2 cell culture model. J Pharmacol Exp Ther, 307, 314–321.
- Huh K, Shin US, Choi JW, Lee SI. (1994). Effect of sex hormones on lipid peroxidation in rat liver. Arch Pharm Res, 17, 109–114.
- Lambert JD, Yang CS. (2003). Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat Res, 523–524, 201–208.
- Lemhadri A, Hajji L, Michel JB, Eddouks M. (2006). Cholesterol and triglycerides lowering activities of caraway fruits in normal and streptozotocin diabetic rats. J Ethnopharmacol, 106, 321–326.
- Liaw WJ, Chen TH, Lai ZZ, Chen SJ, Chen A, Tzao C, Wu JY, Wu CC. (2005). Effects of a membrane-permeable radical scavenger, Tempol, on intraperitoneal sepsis-induced organ injury in rats. Shock, 23, 88–96.
- Li C, Meng X, Winnik B, Lee MJ, Lu H, Sheng S, Buckley B, Yang CS. (2001). Analysis of urinary metabolites of tea catechins by liquid chromatography/electrospray ionization mass spectrometry. Chem Res Toxicol, 14, 702–707.
- Lu H, Meng X, Yang CS. (2003a). Enzymology of methylation of tea catechins and inhibition of catechol-O-methyltransferase by (-)-epigallocatechin gallate. Drug Metab Dispos, 31, 572–579.
- Lu H, Meng X, Li C, Sang S, Patten C, Sheng S, Hong J, Bai N, Winnik B, Ho CT, Yang CS. (2003b). Glucuronides of tea catechins: Enzymology of biosynthesis and biological activities. Drug Metab Dispos, 31, 452–461.
- Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. (2004). Polyphenols: Food sources and bioavailability. Am J Clin Nutr, 79, 727–747.
- Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. (2005). Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr, 81, 230S–242S.
- Matsumura T, Ishikawa T, Kitajima J. (2002). Water-soluble constituents of caraway: Aromatic compound, aromatic compound glucoside and glucides. Phytochemistry, 61, 455–459.
- Mazaki M, Kataoka K, Kinouchi T, Vinitketkumnuen U, Yamada M, Nohmi T, Kuwahara T, Akimoto S, Ohnishi Y. (2006). Inhibitory effects of caraway (Carum carvi L.) and its component on N-methyl-N’-nitro-N-nitrosoguanidine-induced mutagenicity. J Med Invest, 53, 123–133.
- Miyoshi N, Naniwa K, Yamada T, Osawa T, Nakamura Y. (2007). Dietary flavonoid apigenin is a potential inducer of intracellular oxidative stress: the role in the interruptive apoptotic signal. Arch Biochem Biophys, 466, 274–282.
- Murzakhmetova M, Moldakarimov S, Tancheva L, Abarova S, Serkedjieva J. (2008). Antioxidant and prooxidant properties of a polyphenol-rich extract from Geranium sanguineum L. in vitro and in vivo. Phytother Res, 22, 746–751.
- Naderi-Kalali B, Allameh A, Rasaee MJ, Bach HJ, Behechti A, Doods K, Kettrup A, Schramm KW. (2005). Suppressive effects of caraway (Carum carvi) extracts on 2, 3, 7, 8-tetrachloro-dibenzo-p-dioxin-dependent gene expression of cytochrome P450 1A1 in the rat H4IIE cells. Toxicol in Vitro, 19, 373–377.
- Nencini C, Giorgi G, Micheli L. (2007). Protective effect of silymarin on oxidative stress in rat brain. Phytomedicine, 14, 129–135.
- Panemangalore M, Bebe FN. (2009). Short- and long-term exposure to low levels of pesticide and flavonoid mixtures modify endogenous antioxidants in tissues of rats. J Environ Sci Health b, 44, 357–364.
- Peralta JG, Llesuy S, Evelson P, Carreras MC, Flecha BG, Poderoso JJ. (1993). Oxidative stress in skeletal muscle during sepsis in rats. Circ Shock, 39, 153–159.
- Perron NR, Brumaghim JL. (2009). A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys, 53, 75–100.
- Phillips LR, Malspeis L, Supko JG. (1995). Pharmacokinetics of active drug metabolites after oral administration of perillyl alcohol, an investigational antineoplastic agent, to the dog. Drug Metab Dispos, 23, 676–680.
- Rasooli I, Fakoor MH, Yadegarinia D, Gachkar L, Allameh A, Rezaei MB. (2008). Antimycotoxigenic characteristics of Rosmarinus officinalis and Trachyspermum copticum L. essential oils. Int J Food Microbiol, 122, 135–139.
- Rasooli I, Rezaei MB, Allameh A. (2006). Ultrastructural studies on antimicrobial efficacy of thyme essential oils on Listeria monocytogenes. Int J Infect Dis, 10, 236–241.
- Robaszkiewicz A, Balcerczyk A, Bartosz G. (2007). Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biol Int, 31, 1245–1250.
- Scalbert A, Morand C, Manach C, Rémésy C. (2002). Absorption and metabolism of polyphenols in the gut and impact on health. Biomed Pharmacother, 56, 276–282.
- Seldak J, Lindsay RH. (1986). Estimation of total protein bound and non-protein sulfidryl groups in tissue with Elman’s reagent. Anal Biochem, 25, 192–205.
- Singer M, Brealey D. (1999). Mitochondrial dysfunction in sepsis. Biochem Soc Symp, 66, 149–166.
- Stott PW, Williams AC, Barry BW. (1998). Transdermal delivery from eutectic systems: enhanced permeation of a model drug, ibuprofen. J Control Release, 50, 297–308.
- Victor VM, Rocha M, De la Fuente M. (2004). Immune cells: Free radicals and antioxidants in sepsis. Int Immunopharmacol, 4, 327–347.
- Villa P, Saccani A, Sica A, Ghezzi P. (2002). Glutathione protects mice from lethal sepsis by limiting inflammation and potentiating host defense. J Infect Dis, 185, 1115–1120.
- Yang CS, Sang S, Lambert JD, Lee MJ. (2008). Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res, 52 Suppl 1, S139–S151.