Abstract
Context: Eryngium creticum Lam. (Umbelliferae), Geranium graveolens L.Her.exn Ait (Geraniaceae), Paronychia argentea Lam. (Caryophyllaceae), and Varthemia iphionoides Boiss (Compositae) have traditionally been used as antidiabetic phytomedicines. However, their alleged benefits and mechanisms remain elusive.
Objectives: To evaluate the effect of these plants on in vitro and in vivo enzymatic starch digestion.
Materials and methods: In vitro enzymatic starch digestion with acarbose or (1–50 or 100 mg/ml) plants aqueous extracts was assayed using α-amylase and α-amyloglucosidase. Oral starch tolerance tests and oral glucose tolerance tests were determined for the plant extracts at concentrations 125, 250, and 500 mg/kg body weight. Blood glucose levels in rats treated with plant extracts or drugs (acarbose or metformin and glipizide) were measured at −30, 0, 45, 90, and 135 min.
Results and discussion: In vitro, acarbose, and water extracts of G. graveolens and V. iphionoides exerted significant dose-dependent dual inhibition of α-amylase and α-glucosidase, with respective IC50s of 1.2 μg/ml, 84.7, and 65.2 mg/ml. Comparable in vivo acute postprandial antihyperglycemic efficacies were obtained for G. graveolens and V. iphionoides in starch-fed rats. E. creticum exhibited substantial acute antihyperglycemic activities in starch-treated rats, despite lacking any favorable in vitro effectiveness. However, P. argentea lacked any inhibitory efficacy. None of the plant extracts qualified for improving the glucose tolerance in fasted rats on glucose loading.
Conclusion: G. graveolens and V. iphionoides can be considered as potential candidates for therapeutic modulation of impaired fasting glycemia, impaired glucose tolerance, and type 2 diabetes.
Introduction
The current accepted definition of diabetes mellitus (DM) is characterized by altered metabolism of lipids, proteins, and carbohydrates and an increased risk of complications from vascular disease (CitationWright et al., 2008). DM is the most common metabolic disorder affecting millions of people worldwide. Also, it is one of the leading causes of morbidity and mortality in Jordan. Based on International Diabetes Federation data, Jordan has the ninth highest prevalence of diabetes in Middle Eastern and North Africa countries and stands at 10.1% (IDF, 2009). Several studies indicated that the prevalence of type 2 diabetes (T2D) and impaired fasting glycemia in Jordan is increasing (CitationBulatova et al., 2007; CitationAjlouni et al., 2008; CitationZindah et al., 2008). On the other hand, ethnopharmacological studies and surveys confirmed that there is an appreciable prevalence of herbal use among patients with diabetes in Jordan (CitationHudaib et al., 2008; CitationAl-Aboudi & Afifi, in press; CitationWazaify et al., in press). The medicinal plants-rich flora of Jordan combined with the traditional nature of its habitants contribute to the flourishing of this praxis. Unfortunately, scientific knowledge is very limited or even lacking for many plant species used for the management of chronic diseases such as DM.
The selected plants are Eyngium creticum Lam. (Umbelliferae), Geranium graveolens L.Her.exn Ait (Geraniaceae), Paronychia argentea Lam. (Caryophyllaceae), and Varthemia iphionoides Boiss (Compositae). These plants can be easily collected by the users and also purchased by the herbalists’ shops in a dry form throughout the country. Several ethnopharmacological surveys based on the information from the herbalists and inhabitants classify the selected plants as antidiabetic plants preferably used in form of infusions (CitationAbu-Irmaileh & Afifi, 2000; CitationHudaib et al., 2008). Evidently, E. creticum aqueous decoction was reported for its hypoglycemic effects in normal and diabetic rats (CitationJaghabir, 1991). G. graveolens was recognized for its antidiabetic properties among the Jordan flora (CitationOran & Al-Eisawi, 1998). CitationAbu-Soud et al. (2004) detected significant α-amylase inhibitory activity for P. argentea in 50% methanol in water extract, despite the lack of a corresponding efficacy in vivo (CitationHamdan & Afifi, 2004). Moreover, CitationAfifi et al. (1996a,Citationb) demonstrated the hypoglycemic activity of V. iphionoides in normoglycemic and streptozocin (STZ) hyperglycemic rats.
New approaches to the prevention/modulation of postprandial hyperglycemia may emerge from the therapeutic use of α-amylase and α-glucosidase inhibitors. Hence, the present in vivo and in vitro experiments evaluate the acute (up to 165 min carbohydrate tolerance testing in normal animal models) postprandial antihyperglycemic activity of the selected four plants with claimed antidiabetic activity exploring the enzymatic starch digestion as a possible mode of antidiabetic action.
Materials and methods
Chemicals, kits, and reagents
α-Amylase (from hog pancreas), α-amyloglucosidase, and acarbose were purchased from Sigma-Aldrich, Switzerland, and d (+) glucose was from Riedel-deHaen, Seize, Germany. Glucose GOD-PAP kit was obtained from BioLabo Reagents, France, and Accu-Chek® Active Glucose meter for blood glucose determinations was from Roche Diagnostics GmbH, Mannheim, Germany. For filtration of the water extracts, Whatman No.5 filter paper (Whatman, USA) was used. Metformin was purchased from Merck Santé s.a.s, Lyon, France (Glucophage® 850 mg) while glipizide was kindly donated by Hikma Pharmaceutical, Jordan. Corn starch was obtained from Uni-Chem, North Carolina. In UV determinations, UV–VIS spectrophotometer from SpectroScan 80D, United Kingdom, was used.
Plant material
Fresh aerial parts of E. creticum [8 UMBE-FMJ], V. iphionoides [9 LABI-FMJ], and P. argentea [2 CARY-FMJ], as well as the leaves of G. graveolens [1 GERA-FMJ] were collected from the Greater Amman area in spring 2009. All plants were taxonomically identified by Prof. Barakat Abu Irmaileh, Faculty of Agriculture-University of Jordan. Voucher specimens, as indicated above, were deposited in the Department of Pharmaceutical Sciences, Faculty of Pharmacy-University of Jordan. All collected fresh plant samples were cut into small pieces, air dried at room temperature, and coarsely powdered.
Preparation of plant aqueous extracts
The aqueous extracts (AEs) were prepared by refluxing each 10 g of the dried coarsely powdered plant material with 100-ml tap water for 15 min and keeping the extract overnight. After filtering twice through filter paper, the volume of the filtered solution was increased to 100 ml with tap water to obtain 10% crude aqueous solutions (CitationHamdan & Afifi, 2004).
In vitro enzymatic starch digestion assay
In vitro enzymatic starch digestion was assayed according to CitationGranfeldt (1992) with some modifications. For this purpose, 100-mg corn starch was suspended and gelatinized in 3-ml distilled water with or without plant AE or acarbose. To this starch solution, 4-μg α-amylase was added, vortexed, and subsequently the mixture was incubated for 20 min at 80°C (first incubation). Afterward, the 3 ml of the mixture was diluted to a total volume of 10 ml with distilled water, and from this, 1 ml was removed and added to 2 ml of 0.1 M sodium acetate buffer (pH 4.75). To this 3-ml mixture, 30 μg of α-amyloglucosidase was added and the mixture was incubated for 30 min at 60°C (second incubation). Samples were removed to ice to inhibit the thermophilic enzymes. Later, samples were evaluated for glucose liberation by glucose oxidase method, using double-beam UV–VIS spectrophotometer. The extent of polysaccharide breakdown into glucose was evaluated in a concentration range of plant AE 1, 5, 10, 12.5, 25, 50, and 100 mg/ml. The effects of acarbose at 0, 0.32, 1.6, 8, 40, 200, and 1000 μg/ml concentrations were evaluated as well. Control (tap water only) samples contained neither acarbose nor plant extract.
In vivo confirmatory studies
Experimental animals
The study was conducted in the Experimental Animal Laboratory of the Faculty of Medicine, University of Jordan. All animals were housed, fed, and treated in accordance with the University of Jordan ethical guidelines for animal protection, and experimental approval (registration number 218/2007–2008) was obtained from the Scientific Research Council at the Deanship of Academic Research and the Faculty of Pharmacy. Before the investigations, the rats were kept for 1 week to be acclimatized under the standard laboratory conditions. Throughout the experimentation period, healthy female Sprague-Dawley rats weighing 200–250 g were used. They were housed in single cages and were given proper pellet diet water ad libitum. Rats were fasted for 14 h before in vivo blood glucose determination. Blood glucose levels from cut tail tips were determined using Accu-Chek® Active Glucose meter.
Oral starch tolerance test
In this experiment, rats were divided into five groups per a test plant (n = 5–8 rats per group). At −30 min, fasting blood glucose levels were determined and instantly acarbose [3 mg/kg body weight (b.w.)] or treatment plant in doses 125, 250, and 500 mg/kg b.w. were administered orally via 2-ml intragastric intubations under mild anesthesia. Control untreated animals were given tap water (2 ml/rat). At 0 min, all rats of five groups (plant extracts and acarbose) were given corn starch 3 g/kg b.w. following the fasting blood glucose determination. Later evaluations of glycemia took place at 45, 90, and 135 min from 0 min (CitationMatsuo & Izumori, 2009).
Oral glucose tolerance test
Rats were divided into six groups per a test plant (n = 5–8 rats per group). The oral glucose tolerance test (OGTT) was carried out as the above protocol for oral starch tolerance test (OSTT) except for glucose (3 g/kg b.w.) utilization instead of starch and metformin (300 mg/kg b.w.) and glipizide (600 μg/kg b.w.) administration as positive controls instead of acarbose (CitationAbesundara et al., 2004; CitationShokeen et al., 2008; CitationMatsuo & Izumori, 2009).
Statistical analysis
The values are presented as mean ± SEM. Area under glucose curve (AUC) and one-way analysis of variance (ANOVA) were determined using Graphpad Prism (version 3.02 for windows; GraphPad Software, San Diego, CA). Statistical difference in plasma glucose levels or in in vitro assays glucose samples and AUC between control and different treatment groups were determined using Graphpad Prism ANOVA followed by Newman–Keuls test whenever appropriate. Values were considered significantly different if P < 0.05.
Results
In vitro enzymatic starch digestion
Using acarbose (1 mg/ml) as a positive control, glucose liberation from starch was inhibited by 97.6% highly substantially (P < 0.001, , , , ).
Figure 1. Effects of plants aqueous extracts (AE) concentrations in mg/ml on enzymatic starch digestion. (A) Eryngium creticum, (B) Geranium graveolens, (C) Paronychia argentea, and (D) Varthemia iphionoides. Results are mean ± SEM (n = 3 replicates). Significance of difference from corresponding control incubation values: *P < 0.05 and ***P < 0.001.
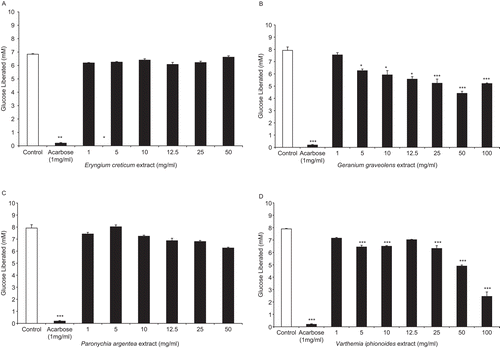
demonstrates the lack of the effect of E. creticum AEs on enzymatic starch digestion. Nevertheless, G. graveolens AEs in concentrations 5–100 mg/ml had highly significant inhibitions of aldohexose release from polymeric cornstarch (P < 0.05–0.001, , ). Similar to E. creticum, P. argentea did not cause any inhibitory activity on polysaccharide digestion (). illustrates the highly significant (P < 0.001) decreases in enzymatic cornstarch hydrolysis by dosage gradient (5, 10, 25–100 mg/ml) of V. iphionoides AEs ().
Table 1. Effects of plants aqueous extracts (AE) concentrations (mg/ml) on % reduction of enzymatic starch digestion.
Confirmatory in vivo studies
OSTT
Acarbose 3 mg/kg b.w. administration at −30-min time point managed to reduce highly significantly the starch induced hyperglycemia at 45, 90, and 135 min post-corn starch load at 0 min, thus evoking highly substantial reduction (P < 0.001) of the overall glycemic excursion compared with controls (, , , ).
Figure 2. Effects of plants aqueous extracts (AE) concentrations in mg/kg b.w. on oral starch tolerance over 165-min period and AUC in normal rats. (A) Eryngium creticum, (B) Geranium graveolens, (C) Paronychia argentea, and (D) Varthemia iphionoides. Results are mean ± SEM (n = 5–8 rats per treatment group). Significance of difference from corresponding control untreated rats values: *P < 0.05, **P < 0.01 and ***P < 0.001.
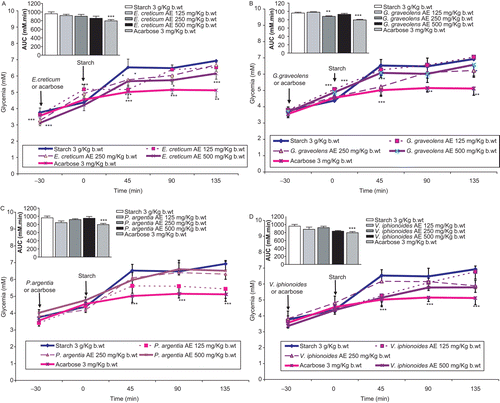
Although E. creticum treatment groups did not demonstrate any decrease (P < 0.001) in overall glycemic excursion () versus the control and drug-treated animals, substantial improvements (P < 0.05–0.001) of glucose handling were evident at 45 min per each plant treatment group. More importantly, the same figure illustrates the significant acute antihyperglycemic effects of E. creticum 500 mg/kg b.w. on effective sugar tolerance, which further extended to 90 min (P < 0.05) and 135 min (P < 0.001) after corn starch ingestion in the same treatment group.
Less effectively than acarbose, G. graveolens at concentration 250 mg/kg b.w. AE enhanced highly markedly (P < 0.01) glucose tolerance AUC (). mirrors the smaller increments in rats’ acute hyperglycemia evoked by G. graveolens 250 mg/kg b.w. dose 45 min (P < 0.001) after the corn starch oral intake, compared with control rats, and comparable with acarbose at the same determination time point.
On the other hand, none of the AEs of P. argentea or V. iphionoides induced any significant reductions in overall glycemic excursions ( and , respectively). Compared with control animals, neither plant treatments evoked enhanced handling of hyperglycemic circulation following corn starch loading to rats at any time determination point ( and ).
OGTT
Compared with control normal rats, 30-min pre-glucose-load treatments with metformin (300 mg/kg b.w.) and glipizide (0.6 mg/kg b.w.) reduced highly significantly (P < 0.001) the overall glycemic excursions in OGTTs (, , , ). These figures demonstrate the highly substantial (P < 0.001) antihyperglycemic efficacies of both oral antidiabetic therapeutics 45, 90, and 135 min after sugar load. shows the drugs-improved glucose tolerance in minimizing blood sugar increments in comparison with control acute hyperglycemia.
Table 2. The effects of plants aqueous extract (AE) concentrations in mg/kg b.w. on % change in oral glucose tolerance test glycemia from basal fasting levels at −30 min, over 165-min period in normal rats.
Figure 3. Effects of plants aqueous extracts (AE) concentrations in mg/kg b.w. on oral glucose tolerance over 165-min period and AUC in normal rats. (A) Eryngium creticum, (B) Geranium graveolens, (C) Paronychia argentea, and (D) Varthemia iphionoides. Results are mean ± SEM (n = 5–8 rats per treatment group). Significance of difference from corresponding control untreated rats values: *P < 0.05 and ***P < 0.001.
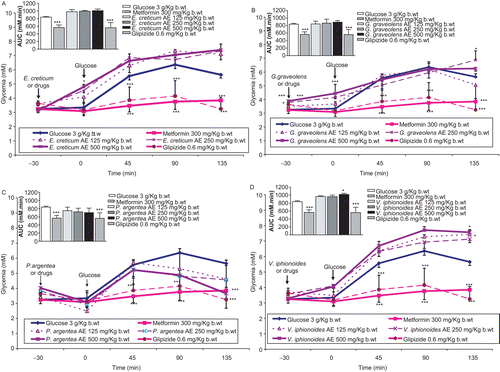
Unlike metformin and glipizide therapeutic efficacies and in comparison with control determinations, oral administration of E. creticum AEs had no marked improvement in glucose tolerance AUCs (). Conversely, when glucose load was ingested by E. creticum rats, the corresponding glycemic incremental curves were not affected during the 165-min time course of acute experiments (). In parallel terms, none of G. graveolens or P. argentea AEs exhibited any acute antihyperglycemic activity in normal rats ( and , respectively). However, illustrates that P. argentea in doses of 250 and 500 mg/kg b.w. decreased significantly (P < 0.05) the plasma sugar increments 90 min after glucose loading. V. iphioniodes AEs, equal to the other plants treatment groups, did not evoke marked enhancements in peripheral plasma glucose concentrations in acute hyperglycemia rats ().
Discussion
It is well established that DM increases the risk of all-cause and cardiovascular disease (CVD) mortality (CitationMorgan et al., 2000; CitationSaydah et al., 2002). Experimental studies have long indicated that abnormal glucose metabolism increases the likelihood of macrovascular diseases via disruption of normal endothelial function, accelerating atherosclerotic plaque formation and contributing to plaque rupture and subsequent thrombosis. Additionally, the risk attributed to other CVD risk factors such as hypertension and hyperlipidemia may be compounded by the presence of abnormal glucose metabolism (CitationWu et al., 1999).
Previous multiple data suggest that a significant proportion of the population with impaired fasting glucose (IFG) or impaired glucose tolerance (IGT) develops DM (CitationUnwin et al., 2002). Moreover, there is new bulk of evidence emphasizing them as strong predictors and genuine independent risk factors of CVD mortality (Nakagami, Citation2004; CitationBarr et al., 2007). In effect, these findings suggest that public health strategies to prevent premature mortality, particularly CVD death, need to be targeted not only to people with DM but also toward people with milder forms of abnormal glucose metabolism (CitationBarr et al., 2007). So, given the strong link between postprandial hyperglycemia and diabetes complications, a number of indigenously diverse herbal extracts were primarily investigated here to verify their efficacy in experimental animals and in vitro bioassays. It might be expected then that the active principles of proven-efficacy plants have antidiabetes pharmacotherapeutic actions.
Inhibition of α-glucosidase and α-amylase should result in delayed carbohydrate digestion and glucose absorption with attenuation of postprandial hyperglycaemic excursions and improvement of IGT (CitationGodbout & Chiasson, 2007). The α-glucosidase and α-amylase inhibitors represent adjuncts to the dietary therapy of diabetes, either as mono-therapy or in combination with insulin or oral hypoglycaemic medications. Acarbose, a standard drug widely prescribed to patients with T2D, exerts dual inhibition of the two enzymes, thereby reducing carbohydrate digestion after meals. This lowers postprandial glucose elevation (CitationGodbout & Chiasson, 2007). Furthermore, acarbose is particularly effective in those with IGT, early diabetes and patients with comorbidities of the metabolic syndrome, thus proven for its substantially CVD benefits (CitationHanefeld & Schaper, 2008). For comparison purposes in our course of screening, it abolished completely the glucose liberation from starch hydrolysis at 1 mg/ml concentration.
When postprandial hyperglycaemia strongly depends on the amount of absorbed monosaccharides and the velocity of the intestinal absorption, strict normalization of postprandial hyperglycaemia should be the target in modern diabetes treatment (CitationHanefeld & Schaper, 2007). Currently, in the effective plant treatment groups, the glucose tolerance was improved post-starch bolus administration, despite the lack of appreciable postprandial antihyperglycemic effect in glucose-loaded rats. This indicates that the reduction of glycemia peaks by efficacious extracts was due to extrapancreatic retardation of intestinal glucose release, rather than pancreatic insulinotropic effect. Additionally, such effectiveness was achieved primarily by restrictive enzymes inhibition, rather than by hindering glucose/Na+ symportation across the intestinal absorptive brush border. Evidently, plant-derived extracts and phytochemicals are potential alternatives to synthetic inhibitors of α-amylase and α-glucosidase (CitationMatsuda et al., 1999; CitationLee & Kim, 2001). The potent AEs in this study (namely G. graveolens and V. iphionoides) proved to be dual inhibitors of the two enzymes tested, which is a significant finding in light of the operatively successive relationship between these intestinal digestive enzymes.
Evidently, in our initial in vivo screening studies, the maximum decrease in postprandial glycemic peak was not observed in a major dose-dependent relationship. Such a phenomenon is common with indigenous plants (CitationRao et al., 2003; CitationSharma et al., 2003; CitationKesari et al., 2005). It is also likely that the higher doses could not produce the expected higher hypoglycemic effect because of the presence of some other substances in the plant extracts, which interfere with the hypoglycemic effect. It has also been reported that high concentrations of Syzygium cumini seed extract may autoinhibit its hypoglycemic action (CitationPrince et al., 1998). The decreased activity at a higher dose of the extract could be due to reduced or no effect of components present in the extract at higher doses (CitationRao et al., 2001a,b; CitationMurthy et al., 2003).
E. creticum is a perennial plant commonly known as eryngo and grows in natural habitats of the Mediterranean region. Eryngium species have been used traditionally for the treatment of rheumatic pain (CitationSezik et al., 2001). E. creticum particularly is used as an antidote for scorpion poison (CitationAfifi et al., 1996a,b). E. creticum has been shown to exhibit antioxidant activity (CitationLjubuncic et al., 2005), whereas other members of the genus Eryngium have been demonstrated to possess anti-inflammatory activity (CitationKüpeli et al., 2006). Furthermore, antimutagenic properties were ascribed to E. creticum (CitationKhader et al., 2010). Previous reports stressed the use of eryngo for its hypoglycaemic effects (CitationTwaij et al., 2002) in rat models. In our studies, a favorable acute antihyperglycemic trend was observed for E. creticum bolus treatment in starch-fed rats. However, the lack of in vitro inhibitory activity does not relate to the in vivo activity. Nevertheless, the intestinal luminal activation of effective entities precursors offers a valid justification for the acute in vivo outcomes. The contradicting effects ascribed to E. creticum does not necessarily role out any other potential pancreatic or extrapancreatic mode of action.
More commonly known as Pelargonium graveolens, G. graveolens attributed less-pronounced health benefits in the literature, except for few reports on its antimicrobial qualities (CitationDorman & Deans, 2000; CitationRosato et al., 2007). Remarkably, G. graveolens-based dual inhibitory efficacy of α-amylase and α-glucosidase in vitro was confirmed by highly significant and potent acute antihyperglycemic trends in starch-fed rats. More importantly, G. graveolens extracts had no improvements in postprandial glycemic responses in fasting rats on glucose loading. Collectively, G. graveolens offers a viable and substantial plant candidate for combination drug therapies of prediabetes and T2D.
Collectively, CitationLev and Amar (2000, Citation2002) and CitationAburjai et al. (2007) classified P. argentea, known as “rejel elhamama,” as popular and most important medicinal plant in the West Bank, Israel, and Jordan for the amelioration of urinary system infections and kidney stones. In Damascus, it is one of the main botanical components of multi-herbal tea “Zahraa,” usually recommended as the after meal tea to promote good health (CitationCarmona et al., 2005). Subjected to scientific scrutiny, P. argentea showed significant α-amylase inhibitory activity in vitro but not a significant hypoglycemic activity in STZ-diabetic rats (CitationHamdan & Afifi, 2004) or alloxan diabetic rabbits (CitationAfifi et al., 2005). CitationBraca (2008) had extensive spectroscopic and spectrometric analysis for P. argentea isolating and characterizing two oleanane saponins, one flavonol glycoside, and six flavonoids. Interestingly, in our in vitro model of enzymatic starch digestion, P. argentea demonstrated no appreciable anti-α-amylase or anti-α-glucosidase effectiveness. Similarly, the plant extracts lacked any acute reductions in glycemic rise following starch bolus in fasting rats.
With moderate antioxidative capacity of V. iphionoides, strongly correlated with its total phenolic content (CitationAl-Dabbas et al., 2006a; CitationAl-Mustafa & Al-Thunibat, 2008), V. iphionoides, generally known as common varthemia, was distinguished as a traditional diabetes remedy. Moreover, it was attributed antiplatelets benefits (CitationAfifi & Aburjai, 2004). Especially important, markedly high-inhibitory activity against porcine pancreas α-amylase was demonstrated by V. iphionoides aqueous and ethanol extracts (CitationAl-Dabbas et al., 2006b). In addition, ethyl acetate and ethanol extracts of this plant exhibited pronounced antibacterial activity (CitationAbu-Hijleh et al., 2009). Our studies are the first to document the highly significant dose-dependent dual anti-α-amylase and anti-α-glucosidase efficacies of V. iphionoides water extracts in vitro. The intestinal luminal inactivation of its effective principles may possibly explain the lack of comparably appreciable activity in vivo in correspondence to the striking in vitro outcomes. As a result, V. iphionoides herbal teas can be recognized as potential nutritious food with diabetes pharmacological modulation properties.
Conclusions
Inhibitors α-amylase and/or α-glucosidase should be considered whenever postprandial hyperglycemia is the dominant metabolic abnormality. Our data indicate that G. graveolens and V. iphionoides can improve glucose homeostasis via delaying carbohydrate digestion significantly. However, further chronic testing is required to validate their clinical implementation as therapeutic agents for improvements in impaired peripheral carbohydrate tolerance. Taken together, G. graveolens and V. iphionoides represent sources for discovery of new orally active antidiabetic therapeutics. Also, they are potentially useful dietary adjuncts for the management/reversal of IFG, IGT, and diabetes.
Acknowledgements
The authors acknowledge Mr. Ismail Abaza for his technical assistance.
Declaration of interest
This research work was supported by a grant from Deanship of Academic Research, University of Jordan. The authors alone are responsible for the content and writing of this manuscript.
References
- Abesundara KJ, Matsui T, Matsumoto K. (2004). alpha-Glucosidase inhibitory activity of some Sri Lanka plant extracts, one of which, Cassia auriculata, exerts a strong antihyperglycemic effect in rats comparable to the therapeutic drug acarbose. J Agric Food Chem, 52, 2541–2545.
- Abu-Irmaileh B, Afifi F (2000): Treatment with medicinal plants in Jordan. Dirasat 27: 53–74.
- Abu-Hijleh A, Jarrar N, Adwan K (2009): Antibacterial activity of common Varthemia, Varthemia iphionoides ethanol extract alone and in combination with cefotaxine. Adv Biol Res 3: 144–147.
- Aburjai T, Hudaib M, Tayyem R, Yousef M, Qishawi M. (2007). Ethnopharmacological survey of medicinal herbs in Jordan, the Ajloun Heights region. J Ethnopharmacol, 110, 294–304.
- Abu-Soud R, Hamdan II, Afifi F (2004): Alpha amylase inhibitory activity of some plant extracts with hypoglycemic activity. Scientia Pharmaceutica 72:25–33.
- Afifi FU, Aburjai T. (2004). Antiplatelet activity of Varthemia iphionoides. Fitoterapia, 75, 629–633.
- Afifi FU, Al-Khalidi B, Khalil E. (2005). Studies on the in vivo hypoglycemic activities of two medicinal plants used in the treatment of diabetes in Jordanian traditional medicine following intranasal administration. J Ethnopharmacol, 100, 314–318.
- Afifi FU, Saket MM, Jaghabir MW, Al-Eisawi D (1996a): Effect of Varthemia iphionoides on blood glucose level of normal rats and rats with streptozocin-induced diabetes mellitus. Cur Therap Res 58: 888–892.
- Afifi FU, Saket MM, Jaghabir MW, Disi MD (1996b): Antagonistic effect of an aqueous root extract of Eryngium creticum on the hyperglycemic response caused by Cerastes cerastes envenomation in rats. Alexandria J Pharm Sci 10:195–199.
- Ajlouni K, Khader YS, Batieha A, Ajlouni H, El-Khateeb M. (2008). An increase in prevalence of diabetes mellitus in Jordan over 10 years. J Diabetes Complicat, 22, 317–324.
- Al-Aboudi A, Afifi FU (2011): Plants used for the treatment of diabetes in Jordan: A review of scientific evidence. Pharm Biol 49: In press.
- Al-Dabbas MM, Suganuma T, Kitahara K, Hou DX, Fujii M. (2006a). Cytotoxic, antioxidant and antibacterial activities of Varthemia iphionoides Boiss. extracts. J Ethnopharmacol, 108, 287–293.
- Al-Dabbas MM, Kitahara K, Suganuma T, Hashimoto F, Tadera K. (2006b). Antioxidant and alpha-amylase inhibitory compounds from aerial parts of Varthemia iphionoides Boiss. Biosci Biotechnol Biochem, 70, 2178–2184.
- Al-Mustafa AH, Al-Thunibat OY. (2008). Antioxidant activity of some Jordanian medicinal plants used traditionally for treatment of diabetes. Pak J Biol Sci, 11, 351–358.
- Barr EL, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, Cameron AJ, Dwyer T, Taylor HR, Tonkin AM, Wong TY, McNeil J, Shaw JE. (2007). Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: The Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation, 116, 151–157.
- Braca A, Bader A, Siciliano T, De Tommasi N. (2008). Secondary metabolites from Paronychia argentea. Magn Reson Chem, 46, 88–93.
- Bulatova NR, Yousef AM, AbuRuz SM. (2007). Antiplatelet therapy for primary and secondary prevention in Jordanian patients with diabetes mellitus. Thromb Res, 121, 43–50.
- Carmona MD, Llorach R, Obon C, Rivera D. (2005). “Zahraa”, a Unani multicomponent herbal tea widely consumed in Syria: Components of drug mixtures and alleged medicinal properties. J Ethnopharmacol, 102, 344–350.
- Dorman HJ, Deans SG. (2000). Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J Appl Microbiol, 88, 308–316.
- Godbout A, Chiasson JL. (2007). Who should benefit from the use of alpha-glucosidase inhibitors? Curr Diab Rep, 7, 333–339.
- Granfeldt Y, Björck I, Drews A, Tovar J. (1992). An in vitro procedure based on chewing to predict metabolic response to starch in cereal and legume products. Eur J Clin Nutr, 46, 649–660.
- Hamdan II, Afifi FU. (2004). Studies on the in vitro and in vivo hypoglycemic activities of some medicinal plants used in treatment of diabetes in Jordanian traditional medicine. j Ethnopharmacol, 93, 117–121.
- Hanefeld M, Schaper F (2007): The role of alpha-glucosidase inhibitors (Acarbose). In: Pharmacotherapy of Diabetes: New Developments Improving life and Prognosis for Diabetic Patients (part 2). Springer US.
- Hanefeld M, Schaper F (2008): Acarbose: Oral antidiabetes drug with additional cardiovascular benefits. Expert Rev Cardiovasc Ther 6: 153–163
- Hudaib M, Mohammad M, Bustanji Y, Tayyem R, Yousef M, Abuirjeie M, Aburjai T. (2008). Ethnopharmacological survey of medicinal plants in Jordan, Mujib Nature Reserve and surrounding area. J Ethnopharmacol, 120, 63–71.
- International Diabetes Federation (IDF) (2009): IDF Diabetes Atlas 4th Edition, Prevalence estimates of diabetes mellitus (DM), 2010 -MENA. IDF [Online]. Available at: http://www.diabetesatlas.org/content/prevalence-estimates-diabetes-mellitus-dm-2010. Accessed on 10 June 2010.
- Jaghabir M. (1991): Hypoglycemic effects of Eryngium creticum. Arch Pharm Res 14: 295–297.
- Kesari AN, Gupta RK, Watal G. (2005). Hypoglycemic effects of Murraya koenigii on normal and alloxan-diabetic rabbits. J Ethnopharmacol, 97, 247–251.
- Khader M, Bresgen N, Eckl PM. (2010). Antimutagenic effects of ethanolic extracts from selected Palestinian medicinal plants. J Ethnopharmacol, 127, 319–324.
- Küpeli E, Kartal M, Aslan S, Yesilada E. (2006). Comparative evaluation of the anti-inflammatory and antinociceptive activity of Turkish Eryngium species. J Ethnopharmacol, 107, 32–37.
- Lee HS, Kim MK (2001): Rat intestinal α-glucosidase and lens aldose reductase inhibitory activities of grain extracts. Food Sci Biotechnol 10: 172–177.
- Lev E, Amar Z. (2000). Ethnopharmacological survey of traditional drugs sold in Israel at the end of the 20th century. J Ethnopharmacol, 72, 191–205.
- Lev E, Amar Z. (2002). Ethnopharmacological survey of traditional drugs sold in the Kingdom of Jordan. J Ethnopharmacol, 82, 131–145.
- Ljubuncic P, Azaizeh H, Portnaya I, Cogan U, Said O, Saleh KA, Bomzon A. (2005). Antioxidant activity and cytotoxicity of eight plants used in traditional Arab medicine in Israel. J Ethnopharmacol, 99, 43–47.
- Matsuda H, Murakami T, Yashiro K, Yamahara J, Yoshikawa M. (1999). Antidiabetic principles of natural medicines. IV. Aldose reductase and qlpha-glucosidase inhibitors from the roots of Salacia oblonga Wall. (Celastraceae): Structure of a new friedelane-type triterpene, kotalagenin 16-acetate. Chem Pharm Bull, 47, 1725–1729.
- Matsuo T, Izumori K. (2009). d-Psicose Inhibits Intestinal alpha-glucosidase and suppresses the glycemic response after ingestion of carbohydrates in rats. J Clin Biochem Nutr, 45, 202–206.
- Morgan CL, Currie CJ, Peters JR. (2000). Relationship between diabetes and mortality: A population study using record linkage. Diabetes Care, 23, 1103–1107.
- Murthy PS, Moorti R, Pugazhenthi S, Babu BV, Prabhu KM, Ratnakar P, Shukla R, Puri D, Dev G, Rusia U, Aggarwal S (2003): Studies with purified orally active compounds from fenugreek seeds, banyan tree bark, bittergourd fruits and garlic bulbs in diabetes mellitus, hypercholesterolemia and tuberclosis. Trends in Clin Biochem Lab Med: 635–639.
- Nakagami T; DECODA Study Group. (2004). Hyperglycaemia and mortality from all causes and from cardiovascular disease in five populations of Asian origin. Diabetologia, 47, 385–394.
- Oran SA, Al-Eisawi DM (1998): Check list of medicinal plants in Jordan. Dirasat 25: 84–112.
- Prince PS, Menon VP, Pari L. (1998). Hypoglycaemic activity of Syzigium cumini seeds: effect on lipid peroxidation in alloxan diabetic rats. J Ethnopharmacol, 61, 1–7.
- Rao KB, Kesavulu MM, Giri R, Rao ChA (2001a): Effect of oral administration of bark extracts of Pterocarpus santalinus L. on blood glucose level in experimental animals. J Ethnopharmacol 74: 69–74.
- Rao BK, Kesavulu MM, Apparao C. (2001b). Antihyperglycemic activity of Momordica cymbalaria in alloxan diabetic rats. J Ethnopharmacol, 78, 67–71.
- Rao BK, Sudarshan PR, Rajasekhar MD, Nagaraju N, Rao ChA (2003): Antidiabetic activity of Terminalia pallida fruit in alloxan-induced diabetic rats. J Ethnopharmacol 85: 169–172.
- Rosato A, Vitali C, De Laurentis N, Armenise D, Antonietta Milillo M. (2007). Antibacterial effect of some essential oils administered alone or in combination with Norfloxacin. Phytomedicine, 14, 727–732.
- Saydah SH, Eberhardt MS, Loria CM, Brancati FL. (2002). Age and the burden of death attributable to diabetes in the United States. Am J Epidemiol, 156, 714–719.
- Sezik E, Yesilada E, Honda G, Takaishi Y, Takeda Y, Tanaka T. (2001). Traditional medicine in Turkey X. Folk medicine in Central Anatolia. J Ethnopharmacol, 75, 95–115.
- Sharma SB, Nasir A, Prabhu KM, Murthy PS, Dev G. (2003). Hypoglycaemic and hypolipidemic effect of ethanolic extract of seeds of Eugenia jambolana in alloxan-induced diabetic rabbits. J Ethnopharmacol, 85, 201–206.
- Shokeen P, Anand P, Murali YK, Tandon V. (2008). Antidiabetic activity of 50% ethanolic extract of Ricinus communis and its purified fractions. Food Chem Toxicol, 46, 3458–3466.
- Twaij H, Abdel-Jalil H, Ibrahim J (2002): Screening methods for the possible hypoglycemic activity of Jordanian medicinal plants (Part II). Jordan J Appl Sci (Nat Sci) 4: 16–21.
- Unwin N, Shaw J, Zimmet P, Alberti KG. (2002). Impaired glucose tolerance and impaired fasting glycaemia: The current status on definition and intervention. Diabet Med, 19, 708–723.
- Wazaify M, Afifi FU, El-Khateeb M, Ajlouni K (2011): Complementary and alternative medicine use among Jordanian diabetes patients. Complement Ther Clin Pract. In press.
- Wright J, Gray AH, Goodey V (2008): Clinical Pharmacy Pocket Companion, 1st edn.: 83–84. Pharmaceutical Press, London
- Wu T, Brooks B, Yue D (1999): Macrovascular disease: The sword of Damocles in diabetes. In: Turtle J, Kaneko T, Osato S, eds. Diabetes in the New Millennium. Sydney, Australia: Endocrinology and Diabetes Research Foundation of the University of Sydney: 403–414.
- Zindah M, Belbeisi A, Walke H, Mokdad AH. (2008). Obesity and diabetes in Jordan: Findings from the behavioral risk factor surveillance system, 2004. Prev Chronic Dis, 5, A17.