Abstract
Context: Hypertrophic scarring, a common proliferative disorder of dermal fibroblasts, results from an overproduction of collagen and excessive deposition of extracellular matrix. Although the treatment with surgical excisions or steroid hormones can modify the symptoms, numerous treatment-related complications have also been established.
Objective: To investigate the effects of essential oil (EO) from rhizomes of Ligusticum chuanxiong Hort. (Umbelliferae) on hypertrophic scarring in a rabbit ear model.
Materials and methods: A rabbit ear model of hypertrophic scarring was established. EO (5, 10, and 20%) was applied once daily to the scars for 22 days. After 28 days of post-wounding, excision of scars was respectively performed for both histological examination and assays of the levels of collagen I, collagen III, matrix metalloproteinase-1 (MMP-1), and transforming growth factor beta 1 (TGF-β1). The scar elevation index (SEI) was also determined.
Results: After 22 days of treatment with indicated concentrations of EO, hypertrophic scarring was significantly inhibited in the rabbit ears. The levels of TGF-β1, collagen I, and collagen III evidently decreased and MMP-1 level markedly increased in the scar tissue. SEI was also significantly reduced. Immunohistochemical findings exhibited significant amelioration of the scar tissue.
Discussion and conclusion: EO suppresses hypertrophic scarring in the rabbit ear model and is a probably effective cure for human hypertrophic scarring.
Keywords::
Introduction
Hypertrophic scarring, a common proliferative disorder of dermal fibroblasts, occurs as a consequence of an overproduction of collagen and excessive deposition of extracellular matrix (ECM) and may result from wound healing following deep burn, inflammatory, and trauma (CitationSu et al., 1998; CitationScott et al., 2000; CitationO’Leary et al., 2002). Hypertrophic scarring is characteristically red, raised, rigid, painful, and pruritic and often associated with contractures. Patients with hypertrophic scars frequently complain about itching and pain, and experience serious functional and cosmetic problems, which are caused by a myriad of complications, including compression, stiffness sensation, loss of joint mobility, and anatomical deformities (CitationBock et al., 2006; CitationMazharinia et al., 2007).
When an exuberant scar occurs, there are many treatment choices. The therapeutic methods include both conservative procedures, such as corticosteroid injections, radiation therapy, silicone occlusive therapy, pressure therapy, splinting, and serial casting, and nonconservative procedures such as surgical excisions. In addition, there are some new methods developed such as interferon, 5-fluorouracil. Unfortunately, there is no universal consensus about the therapeutic mode causing complete and permanent improvement of scars with few side effects (CitationReish & Eriksson, 2008; CitationBloemen et al., 2009). Therefore, it is necessary to develop a new, effective, safe method or drug to deal with this problem.
It has been generally recognized that traditional Chinese herbal medicines play a unique therapeutic role in the treatment of many diseases (Zhang et al., Citation2008a; CitationDu et al., 2009; CitationWu et al., 2009; CitationXing et al., 2009). Particularly, essential oils (EO) from many plant species have become popular in recent years, and the investigations on their bioactivities and mechanisms of action have been carried out for human health (CitationZhang et al., 2007, 2008b; CitationSamarth et al., 2008).
Rhizoma Chuanxiong (RCX), the dry rhizome of Ligusticum chuanxiong Hort. (Umbelliferae), is one of the well-known traditional Chinese medicines and possesses efficacy in promoting the circulation of blood and qi, expelling wind, and alleviating pain. The volatile compounds in this herbal drug are considered an important part of its pharmacological effects. In previous investigations, we observed that the EO extracted from RCX markedly inhibited the viability of human hypertrophic scar fibroblasts (HSFs) and elicited cell apoptosis (CitationWu et al., 2010). The present study was designed to substantiate whether EO could inhibit hypertrophic scarring in the rabbit ear model and to explore the possible mechanism of action.
Materials and methods
Drug preparation
RCX was collected in May 2008, from Pengzhou, Sichuan, China, and identified as the rhizome of L. chuanxiong by Professor Lu-Ping Qin, a pharmacognosist from the Department of Pharmacognosy, School of Pharmacy, Second Military Medical University (Shanghai, China). The EO was extracted and the GC-MS analysis was performed according to our previous report (CitationWu et al., 2010). The yield of EO was 0.25% (v/w). Dozens of compounds were detected from EO and the major components were ligustilide and butylidenephthalide with relative contents of 67.027 and 5.831%, respectively.
After dissolving in appropriate amount of DMSO, EO was mixed with pure vaseline and liquid paraffin at the ratios of 2:8:0, 1:8:1, and 0.5:8:1.5 (w:v:v), respectively. Basic ointment containing pure vaseline and liquid paraffin (the ratio of 8:2) was used as placebo.
Hypertrophic scar rabbit model
A hypertrophic scar rabbit model previously reported (CitationKryger et al., 2007) was used in this study. Female New Zealand white rabbits, obtained from Shanghai Si-Lai-Ke Experimental Animal Co., Ltd. (Shanghai, China), with an initial body weight of 2.0 ± 0.2 kg, were used. Animals were housed in a regulated environment (22 ± 2°C), with a 12-h dark/light cycle (08:00–20:00, light). Food and water were given ad libitum throughout the experiment. All animal treatments were strictly in accordance with international ethical guidelines and the National Institutes of Health Guide concerning the Care and Use of Laboratory Animals, and the experiments were carried out with the approval of the Committee of Experimental Animal Administration of the University.
The animals were intravenously anesthetized with sodium pentobarbital (30 mg/kg). Under sterile conditions, six full-thickness wounds were created down to bare cartilage on each ear using a 7-mm biopsy punch. Each ear received six round wounds placed in a random order on the ear and each wound was 7 mm in diameter. The epidermis, dermis, and perichondrium in each wound were thoroughly removed, and then the wounds were covered with sterile gauze for 1 day. After recovery from anesthesia, the rabbits were returned to their cages.
Grouping and administration
On the seventh postoperative day and afterward, scars were randomly divided into five groups (n = 12): one control group, three groups treated with EO (5, 10, and 20%), and one group treated with contractubex (Merz Pharma GmbH, Germany). One unwounded rabbit that had full-thickness skin on its ears was used as a normal group without treatment. The scars in the control group were thinly coated with basic ointment once a day. EO was applied once daily to three treatment groups of scars and contractubex to the contractubex group of scars.
Detection of collagen I, collagen III, matrix metalloproteinase-1, and transforming growth factor beta 1
Collagen I, collagen III, matrix metalloproteinase-1 (MMP-1), and transforming growth factor beta 1 (TGF-β1) were measured according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). Animals were killed on postoperative 28 days, the scars were separated, and the cartilages were eliminated. The weighed scar was diced, rapidly frozen in liquid nitrogen, maintained at 4°C after melting, added 1 mL of PBS (pH 7.4), homogenized, centrifuged at 3000 g 4°C for 20 min, and then the supernatant was collected for assay.
In order to determine the amount of collagen I present within the wound, a solid-phase binding assay was performed. In brief, both 40 μL of sample dilution and 10 μL of the supernatant were added to a testing sample well, gently mixed, and incubated for 30 min at 37°C after closing the microplate with membrane. The membrane was uncovered, liquid in the well was discarded, washing buffer was added to the well for 30 sec, and then drained. This procedure was repeated five times. Fifty microliters of HRP-conjugate reagent was added to each well, incubated for 30 min at 37°C, followed by washing five times with washing buffer. Each well was incubated with chromogen solution A (50 μL) and chromogen solution B (50 μL) for 15 min at 37°C, and then stop solution (50 μL) was added to each well to terminate the reaction. The optical density was measured at 450 nm using an ELx-800 universal microplate reader (Bio-Tek, Winooski, VT). The same assay was used to assess the levels of collagen III, MMP-1, and TGF-β1 in the scars using corresponding ELISA kits.
Histological examination
Scar tissue was fixed with 10% buffered formalin for 3 days, embedded in paraffin, sectioned with a dermatome, and stained using hematoxylin–eosin (H&E). Light microscopy was used to examine the wound sections for degree of epithelialization, cellularity, granulation tissue, collagen content, and degree of scar hypertrophy. The degree of scar hypertrophy was expressed as the scar elevation index (SEI).
Determination of SEI
SEI represents the ratio of total scar connective tissue area to the area of underlying dermis. The height of the underlying dermis is determined based on the height of the surrounding unwounded dermis.
Analysis of immunohistochemistry
The production of types I and III collagens was determined by using the ABC method as previously described (CitationChen et al., 2005). In brief, 5-µm sections were deparaffinized, washed with 0.05% Tris-buffered saline solution (TBS), processed for 15 min in blocking buffer, and incubated for 30 min with 0.3% hydrogen peroxidase in methyl alcohol to eliminate endogenous peroxidase activity within the tissue. The sections were incubated for 30 min with 0.3% TBS to increase cell permeability and washed for 15 min in a 1% bovine serum albumin (BSA) in the buffer. Next, they were incubated for 24 h at 4°C with a primary anti-type I collagen or anti-type III collagen antibody (Cambridge, MA) diluted with 1% BSA. After being washed with 0.05% TBS (3 × 5 min), the sections were processed with the secondary antibody (Cambridge, MA) for 2 h, rinsed in TBS (3 × 5 min), followed by streptavidin–biotin complex (SABC Universal Kit), and washed with 0.05% TBS (3 × 5 min). 3,3′-Diaminobenzidine (Sigma, St. Louis, MO) was used as the peroxidase substrate to develop color, yielding a brown reaction product.
Immunostained areas of types I and III collagens on the sections were quantified using computer-assisted morphometry (CitationKunjathoor et al., 2002). The extent of immunostained areas was determined using a selection of threshold for gray scale, brightness, and contrast. The immunostained pixels and total pixels for five randomly selected high-power fields (HPFs) in each specimen were converted to units of squared micrometer. The types I and III collagen densities of each specimen were expressed as mean percent of immunostained areas to HPFs.
Statistical analysis
All results were presented as the mean ± SD. Data were analyzed using a SPSS 13.0 statistical package. Data for multiple comparisons were performed by one-way ANOVA followed by Dunnett’s test. A value of P < 0.05 was considered statistically significant.
Results
Effects on collagen I, collagen III, MMP-1, and TGF-β1
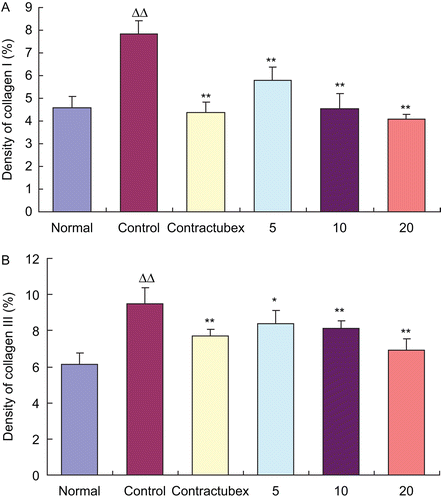
Collagens, including collagen I and collagen III, are the most important ingredients of the ECM. As shown in , the levels of collagens I and III in the control group significantly increased when compared with the normal group. In contrast, after 22 days of the treatment with EO, the levels of collagens I and III decreased dramatically and dose-dependently. The level of TGF-β1 markedly ascended in the control group in comparison with the normal group, but evidently descended in the groups treated with doses of 10 and 20% EO when compared with the control group. MMP-1 plays an important role in the formation of scars. Twenty-eight days after wounding, the MMP-1 level markedly decreased in the dermal tissue of the rabbit ears. However, it significantly increased when the wounds were treated with doses of 5, 10, and 20% EO for 22 days.
Table 1. Effects of essential oil (EO) on the levels of collagen I, collagen III, matrix metalloproteinase-1 (MMP-1), and transforming growth factor beta 1 (TGF-β1).
Histological and immunohistochemical findings
HE staining of the scar tissues was carried out on Day 28 post-wounding. Light microscopic examination revealed typical features of scar tissue in the control group (data not shown). These features include sheets of disorganized collagen covered by an irregular epithelial layer, with fibroblasts scattered throughout the scar. The collagen bundles were thicker and more abundant in the deep dermal portion of the lesion. In contrast, collagen fibers, fibroblasts, and thickness of the scar significantly decreased in the treatment groups, which showed dose-dependent changes. Immunohistochemical analysis showed the similar results (). The EO groups exhibited fewer areas and lower densities of types I and III collagens than the control group (data for groups given doses of 5 and 10% EO not shown). Quantitative analysis indicated that the densities of collagens I and III significantly increased in the control group, but dose-dependently decreased in the EO groups ().
Figure 1. Immunohistochemical findings. (A) Type I collagen. (B) Type III collagen. Immunohistochemical reactivities of collagens I and III were found in the rabbit ear scars. The control group showed the more areas and higher densities of collagens I and III, and 20% essential oil (EO) group exhibited the fewer areas and lower densities than the control group. Figures for groups given doses of 5 and 10% EO were not shown.
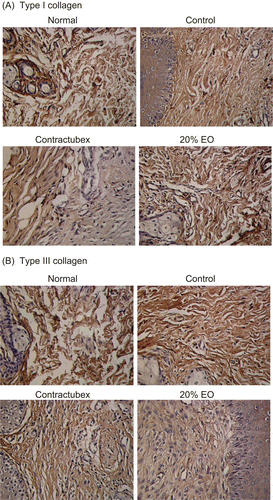
Figure 2. Effects of essential oil (EO) on densities of collagens I and III. The figure quantitatively showed the densities of collagens I (A) and III (B) in the scars. The densities of collagens I and III evidently increased in the control group, but decreased significantly and dose-dependently in the EO groups. ΔΔP < 0.01 compared with the normal group; *P < 0.05, **P < 0.01 compared with the control group. Data are expressed as the mean ± SD. n = 12.
Scar elevation index
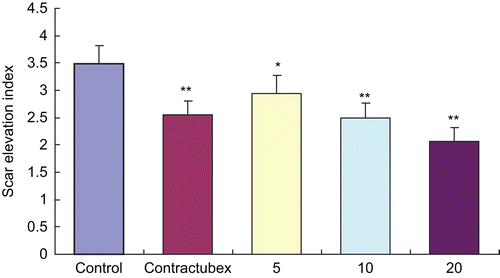
On Day 28 post-wounding, there was significant hypertrophic scarring in the control group with mean SEI of 3.48 ± 0.34. As shown in , after 22 days of successive administration of EO, the hypertrophic scarring was markedly inhibited in a dose-dependent manner in the treatment groups with the mean SEI of 2.95 ± 0.33, 2.49 ± 0.27, and 2.07 ± 0.25, respectively.
Figure 3. Effects of essential oil (EO) on scar elevation index (SEI). The degree of scar hypertrophy is reflected by SEI, which represents the ratio of total scar connective tissue area to the area of underlying dermis. After treatment with EO for 22 days, hypertrophic scarring was alleviated in a dose-dependent manner. *P < 0.05.**P < 0.01 versus the control group. Data are expressed as the mean ± SD. n = 12.
Discussion
Hypertrophic scarring is characterized by excessive deposition of ECM, which consists of collagens, glycoproteins, glycoaminoglycans, and proteoglycans. Collagen is the main component of ECM and is secreted mainly by fibroblasts. Collagen dysmetabolism is the pathological basis for hypertrophic scarring (CitationClark et al., 1996; CitationBeldon, 2000) and is also a common cause of fibrotic diseases. Normal fibroblast synthesizes both types I and III collagens, but this synthesis becomes imbalanced with changes in the surrounding environment. The predominant collagen type in skin is the mature and mechanically stable type I collagen, whereas the immature and instable type III collagen is typically seen during the early phase of wound healing (CitationSchäffer & Becker, 1999). During granulation tissue formation, type III collagen expression increases more than the type I expression (CitationHayakawa et al., 1979). Our results also showed that the amount of type III collagen was more than that of type I collagen in the hypertrophic scar tissue of the model rabbit ears. ELISA analysis indicated that EO, particularly at a concentration of 20.0%, dramatically suppressed both types I and III collagen protein synthesis in the hypertrophic scar tissue of the rabbit ears. The immunohistochemical analysis showed similar results that the areas and the densities of types I and III collagens in the scar were markedly decreased by EO.
Wound repair involves cell migration, proliferation, and tissue remodeling. These ordered and regulated processes are facilitated by matrix-degrading proteases. Collagenase (MMP-1) is the only known enzyme able to initiate the breakdown of the interstitial collagens: types I, II, and III. MMP-1 plays a key role in the remodeling that occurs constantly in both normal and diseased conditions (CitationDasu et al., 2004) and is the key enzyme in the degradation of types I and III collagens in scar. In this study, 28 days after wounding, the MMP-1 level markedly decreased in the dermal tissue of the rabbit ears, but was significantly up-regulated by EO.
There is considerable evidence demonstrating that several cytokines are important components in the process of wound healing and scar formation. One of the most intensively investigated molecules associated with many types of fibrosis is TGF-β1, which stimulates infiltration of inflammatory cells and fibroblasts and induces fibroblast proliferation, angiogenesis, and synthesis of ECM. While, a persistent autocrine loop of TGF-β1 contributes to hypertrophic scar formation (CitationSchmid et al., 1998). In the present study, the level of TGF-β1 markedly ascended in the control group in comparison with the normal group. After treatment with EO for 22 days, the level of TGF-β1 evidently descended compared with the control group.
The abnormal biological behavior of fibroblasts plays a central role in hypertrophic scar formation and development (CitationTuan & Nichter, 1998). Fibroblasts synthesize both types I and III collagens, which are the main components of the ECM. At the same time, they also excrete TGF-β1 and collagenase MMP-1, both of which regulate the synthesis and degradation of collagen. Overexpression of TGF-β1 can diminish types I and III collagen degradation by inhibiting the expression of collagenase (MMP-1). In previous study, we observed that EO significantly suppressed the growth of cultured HSFs and induced cell apoptosis. Therefore, we deduced that EO probably inhibited the activities of scar fibroblasts, decreased the TGF-β1 excretion, up-regulated the collagenase MMP-1 level, diminished the amount of types I and III collagens, and then suppressed hypertrophic scar formation.
In conclusion, EO can inhibit hypertrophic scarring in the rabbit ear model. The present study suggests that EO probably becomes an effective cure for human hypertrophic scarring.
Declaration of interest
This work was supported by a grant from the Key Programs for Basic Research of Shanghai Committee of Science and Technology (Grant No. 08JC1405700).
References
- Beldon P. (2000). Abnormal scar formation in wound healing. Nurs Times 96:44–45.
- Bloemen MC, van der Veer WM, Ulrich MM, van Zuijlen PP, Niessen FB, Middelkoop E. (2009). Prevention and curative management of hypertrophic scar formation. Burns 35:463–475.
- Bock O, Schmid-Ott G, Malewski P, Mrowietz U. (2006). Quality of life of patients with keloid and hypertrophic scarring. Arch Dermatol Res 297:433–438.
- Chen J, Jia-Han W, Hong-Xing Z. (2005). Inhibitory effects of local pretreated epidermis on wound scarring: A feasible method to minimize surgical scars. Burns 31:758–764.
- Clark JA, Cheng JC, Leung KS. (1996). Mechanical properties of normal skin and hypertrophic scars. Burns 22:443–446.
- Dasu MR, Hawkins HK, Barrow RE, Xue H, Herndon DN. (2004). Gene expression profiles from hypertrophic scar fibroblasts before and after IL-6 stimulation. J Pathol 202:476–485.
- Du J, Sun LN, Xing WW, Huang BK, Jia M, Wu JZ, Zhang H, Qin LP. (2009). Lipid-lowering effects of polydatin from Polygonum cuspidatum in hyperlipidemic hamsters. Phytomedicine 16:652–658.
- Hayakawa T, Hashimoto Y, Myokei Y, Aoyama H, Izawa Y. (1979). Changes in type of collagen during the development of human post-burn hypertrophic scars. Clin Chim Acta 93:119–125.
- Kryger ZB, Sisco M, Roy NK, Lu L, Rosenberg D, Mustoe TA. (2007). Temporal expression of the transforming growth factor-beta pathway in the rabbit ear model of wound healing and scarring. J Am Coll Surg 205:78–88.
- Kunjathoor VV, Chiu DS, O’Brien KD, LeBoeuf RC. (2002). Accumulation of biglycan and perlecan, but not versican, in lesions of murine models of atherosclerosis. Arterioscler Thromb Vasc Biol 22:462–469.
- Mazharinia N, Aghaei S, Shayan Z. (2007). Dermatology Life Quality Index (DLQI) scores in burn victims after revival. J Burn Care Res 28:312–317.
- O’Leary R, Wood EJ, Guillou PJ. (2002). Pathological scarring: Strategic interventions. Eur J Surg 168:523–534.
- Reish RG, Eriksson E. (2008). Scar treatments: Preclinical and clinical studies. J Am Coll Surg 206:719–730.
- Samarth RM, Panwar M, Kumar M, Soni A, Kumar M, Kumar A. (2008). Evaluation of antioxidant and radical-scavenging activities of certain radioprotective plant extracts. Food Chem 106:868–873.
- Schäffer M, Becker HD. (1999). [Immune regulation of wound healing]. Chirurg 70:897–908.
- Schmid P, Itin P, Cherry G, Bi C, Cox DA. (1998). Enhanced expression of transforming growth factor-beta type I and type II receptors in wound granulation tissue and hypertrophic scar. Am J Pathol 152:485–493.
- Scott PG, Ghahary A, Tredget EE. (2000). Molecular and cellular aspects of fibrosis following thermal injury. Hand Clin 16:271–287.
- Su CW, Alizadeh K, Boddie A, Lee RC. (1998). The problem scar. Clin Plast Surg 25:451–465.
- Tuan TL, Nichter LS. (1998). The molecular basis of keloid and hypertrophic scar formation. Mol Med Today 4:19–24.
- Wu JG, Ma L, Zhang SY, Zhu ZZ, Zhang H, Qin LP, Wei YJ. (2010). Essential oil from rhizomes of Ligusticum chuanxiong induces apoptosis in hypertrophic scar fibroblasts. Pharm Biol. Posted online on September 3, 2010.
- Wu JG, Wu JZ, Sun LN, Han T, Du J, Ye Q, Zhang H, Zhang YG. (2009). Ameliorative effects of arctiin from Arctium lappa on experimental glomerulonephritis in rats. Phytomedicine 16:1033–1041.
- Xing WW, Wu JZ, Jia M, Du J, Zhang H, Qin LP. (2009). Effects of polydatin from Polygonum cuspidatum on lipid profile in hyperlipidemic rabbits. Biomed Pharmacother 63:457–462.
- Zhang H, Han T, Yu CH, Rahman K, Qin LP, Peng C. (2007). Ameliorating effects of essential oil from Acori graminei rhizoma on learning and memory in aged rats and mice. J Pharm Pharmacol 59:301–309.
- Zhang H, Han T, Zhang L, Yu CH, Wan DG, Rahman K, Qin LP, Peng C. (2008a). Effects of tenuifolin extracted from Radix polygalae on learning and memory: A behavioral and biochemical study on aged and amnesic mice. Phytomedicine 15:587–594.
- Zhang H, Xing WW, Li YS, Zhu Z, Wu JZ, Zhang QY, Zhang W, Qin LP. (2008b). Effects of a traditional Chinese herbal preparation on osteoblasts and osteoclasts. Maturitas 61:334–339.