Abstract
Context: In the past few years, an increasing interest in the volatile secondary metabolites of Hypericum perforatum L. (Guttiferae) has been arising.
Objective: The present study is a contribution to better understand the relationship between the morphological variations and volatile composition during the phenological cycle.
Materials and methods: Leaves at the stages of vegetative, floral budding, flowering and green capsule, buds, full opened flowers and green capsules were assayed for essential oil (EO) components by gas chromatography-flame ionization detector (GC-FID) and GC-mass spectrometry (MS).
Results: Significant amounts of sesquiterpenes (oxygenated 26–50% and hydrocarbons 20–40%) and oxygenated hydrocarbons (13–38%) characterized the all analyzed samples showing peculiar fluctuations during the seven phenological stages. Although monoterpenes were present in much lower amounts (monoterpene hydrocarbons 0.4–6%; oxygenated monoterpenes 0.8–6%) they were considered also important discrimination for several stages. The green capsules and the full opened flowers collected at flowering stage were clearly distinguished in terms of EO compositions from the other samples.
Discussion: For the first time, the EO composition of Turkish wild Hypericum perforatum was monitored by the hydrodistillation of different plant organs collected at different seven stages in order to point out the modification of target volatiles related to each phenological step.
Conclusions: Based on the EO composition monitored during these seven morphological stages by GC-MS, principal component analysis and cluster analysis, significant metabolite modifications were observed during the phenological cycle which involved the levels of specific volatile target compounds belonging to the chemical classes of hydrocarbons, monoterpenes and sesquiterpenes.
Introduction
Hypericum perforatum L. (Guttiferae) (St. John’s wort) is a well-known traditional medicinal plant that has been used for centuries for the treatment of several diseases, such as skin lesions, eczema, burns, and microbial, inflammatory, and psychological disorders (CitationSánchez-Mateo et al., 2002; CitationToker et al., 2006). The crude extract of H. perforatum is now widely used in Europe as a drug for the treatment of depression (CitationGreeson et al., 2001). Proven photodynamic, antiviral, antiretroviral, and antitumoral activities of Hypericum extracts also suggests using of this plant in acquired immune deficiency syndrome and cancer treatments (CitationVlietinck et al., 1998; CitationGuedes and Eriksson, 2005).
H. perforatum contains a number of secondary metabolites from different classes namely naphthodianthrones, phloroglucinols, flavonoids, phenylpropanes, essential oils (EOs), amino acids, xanthones, tannins, procyanidins and other water-soluble components (CitationGreeson et al., 2001). Furthermore, the species is especially rich, as are other members of the genus Hypericum, in volatile compounds (CitationMathis and Ourisson, 1964; CitationMimica-Dukic et al., 1998; CitationGudzic et al., 2001; CitationPetrakis et al., 2005; CitationSaroglou et al., 2007).
Although the ecological importance of volatile compounds in the direct interaction with the habitat, and the contribution of EO to the bioactivities of Hypericum spp. have been studied (CitationCouladis et al., 2003; CitationCakir et al., 2005), only a few studies on the volatile chemistry of H. perforatum have been undertaken due to the low amount of EO (CitationRadusiene et al., 2005). In addition, it should be noted that consistent results have not been reported (CitationGudzic et al., 2001; CitationSchwob et al., 2002; CitationAzizi, 2008; CitationCirak et al., 2010). In fact, the plant material studied in previous reports were from various locations where very different ecological conditions are prevalent and in most of those studies, the plant parts used and the phenological stages in which sampling was performed, were not clearly specified. Although ontogenetic changes in EO composition in wild (CitationSchwob et al., 2004) and field grown (CitationAzizi, 2008) H. perforatum plants were previously reported, to our knowledge, there is no report on the organ-dependence of this variation.
As few and variable data have been reported on the EOs variations related to the plant organ-development cycle, the present study aims to give a contribution to determine the links between EO composition and different plant organs considering the course of ontogenesis in H. perforatum var. perforatum of Turkish origin.
Materials and methods
Brief description of plant material
The plant material was described in our previous study (CitationCirak et al., 2010). The species were identified by Dr. Hasan Korkmaz, Faculty of Science and Art, Department of Biology, University of 19 Mayis, Samsun, Turkey. Voucher specimen was deposited in the herbarium of Ondokuz Mayis University Faculty of Agriculture (OMUZF # 61/10).
Experimental procedures
The plant material was collected in a dry grassland within the Çakallı district of Samsun, Turkey (41°04’ N; 36°01’ E; 470 m above sea level) between April and October of 2007 at different stages of plant development. The mean temperature during the sampling period was 18.5°C, and the precipitation sum 450 mm. The sampling site was not grazed or mown during the plant gathering period. The material represented 30 randomly gathered plants in four phenological stages: vegetative, floral budding, full flowering and fresh fruiting. Newly emerged shoots (4–6 weeks old-age) with leaves were harvested at the vegetative stage. For the floral budding stage, only shoots with floral buds were selected. At the full flowering stage, only shoots with full opened flowers were harvested. At the fresh fruiting stage, the shoots which had green capsules were harvested. The top ⅔ of the plant was harvested between 12:00 am and 13:00 pm. After collected, plant materials were dissected into floral, leaf and stem tissues, dried at room temperature (20 ± 2°C) and subsequently assayed for volatile constituents.
Sample preparation
Air-dried plant samples (200 g) were hydrodistilled by Clevenger apparatus for 2 h. The EO were diluted in n-hexane (HPLC solvent grade, 10%) and injected in GC-FID (injection volume 1 μl) and GC-mass spectrometry (MS) (injection volume 0.1 μl).
Gas chromatography-FID analysis
GC analyses were accomplished by HP-5890 Series II instrument equipped with HP-WAX and HP-5 capillary columns (30 m × 0.25 mm, 0.25 μm film thickness), working with the following temperature program: 60°C for 10 min, ramp of 5°C/min up to 220°C; injector and detector temperatures 250°C; carrier gas nitrogen (2 ml/min); detector dual FID; split ratio 1:30; injection of 0.5 μl; The identification of the components was performed, for both the columns, by the comparison of their retention times with those of pure authentic samples and by mean of their linear retention indexes (LRI) relative to a series of n-hydrocarbons.
Gas chromatography-MS analysis
GC/EIMS analyses were performed with a Varian CP-3800 gas-chromatograph equipped with a DB-5 capillary column (30 m × 0.25 mm; coating thickness 0.25 μm) and a Varian Saturn 2000 ion trap mass detector. Analytical conditions: injector and transfer line temperatures 220 and 240°C respectively; oven temperature programmed from 60 to 240°C at 3°C/min; carrier gas helium at 1 ml/min; triplicate injections of 0.2 ml (10% hexane solution); split ratio 1:30. Identification of the constituents was based on the comparison of the retention times with those of authentic samples, comparing their LRI relative to a series of n-hydrocarbons and computer matching by both two commercial data base (NIST 98 and ADAMS) and a home-made library mass spectra built up from pure substances, components of known oils and MS literature data (CitationStenhagen et al., 1974; CitationMassada, 1976; CitationSwigar and Silverstein, 1981; CitationConnolly and Hill, 1991; CitationAdams 1995). Moreover, the molecular weights of the all identified substances were confirmed by GC/CIMS, using MeOH as CI ionizing gas. The GC-MS results are shown in and .
Table 1. GC-MS analyses of the essential oils of Turkish H. perforatum samples collected in different phenological stages (1–7)&
Table 2. Typical chemical classes of volatile constituents in the essential oils of Turkish H. perforatum samples collected in different phenological stages (1–7)*.
Statistical analysis
Principal component analysis (PAC) was carried out using the statistical software package SPSS Version 13.0. This analysis is the two-dimensional visualization of the position of investigated exemplars relative to each other. The principal components (PCs) represent the axes which are the orthogonal projection for the values representing the highest possible variances, in this case the first and second PCs.
The obtained data were used to create scatter plot diagrams (CitationBackhaus et al., 1989). Therefore a factor analysis was performed, where each variable was used to calculate relationships between variable and investigated factors. Based on the obtained data also a dendogramme (cluster) was created (CitationBackhaus et al., 1989) showing the relationship of investigated samples regarding their EO composition.
Results and discussion
Significant differences in the EOs yield were observed during the seven phenological stages of the selected plant material. The analyzed samples were collected from different plant organs of the same species H. perforatum var. perforatum during its phonological cycle: leaves at the stages of vegetative, floral budding, flowering and green capsule; buds, full opened flowers and green capsules. The highest and comparable values in terms of the EO yields were obtained for the leaf at vegetative stage (1), leaf at fruiting (4), and full opened flowers (6) (0.44, 0.61, and 0.53% v/w dry plant, respectively). The lowest yield resulted from the floral buds (5) and green capsules (7) (0.04 and 0.07% v/w dry plants, respectively) ().
Few data have been reported in the literature on the EO variations related to the plant development cycle in Hypericum spp. Changes in the content and EO composition during ontogenesis in H. perforatum from wild French populations (CitationSchwob et al., 2004) and field-cultivated plants (CitationAzizi, 2008) were previously reported. However, morphogenetic sampling was not performed in these studies. The present work represents the first report investigating organ-dependence phenologic fluctuations in the EOs content and components in wild H. perforatum plants growing in Turkey. Such a numerous specific phenological stages (1–7) were related to the EOs production in wild H. perforatum for the first time. Comparing with the few previous data (CitationSchwob et al., 2004; CitationAzizi, 2008), the present results confirmed that the EO yield tends to be the highest at full flowering stage ().
The complete GC-MS fingerprint of the volatile constituents detected in each hydrodistilled EO are reported in . The same compounds are grouped in the main chemical classes and submitted in . The PC values calculated regarding the EO constituents of collected samples are shown in . PC1 corresponds to 41.48% of the total variation and PC2 corresponds to 22.28% (totally 63.76%). The obtained scatter plot using these first and second PCs is shown in . Based on the analysis GC-MS results and the obtained scatter plot, it can be concluded that remarkable differences characterized the EOs composition during phenological cycle. In fact, samples collected at different vegetative stages could be clearly differentiated from samples collected at more generative ones.
Table 3. Cumulative values of calculated principal components for H. perforatum plants collected in different stages of plant phenology.
Figure 1. Scatter plot of different parts of H. perforatum plants, harvested at different stages of plant phenology.
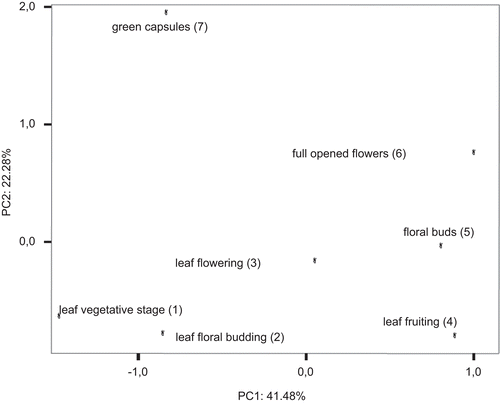
The EOs constistuents of the green capsules allowed separating them clearly from the other samples. Similarly, the full opened flowers collected at flowering stage were different significantly compared with the other samples. The EOs obtained from leaves collected at the fruiting stage were similar to that of the floral buds. Additionally, no significant differences were observed in volatile constituents between the leaves hydrodistilled at floral budding and at the vegetative stages. The cluster analysis supports these results by dendogram in . Results from the second PCA, performed using the relative percentages of the chemical classes revealed significant fluctuations in the content of monoterpens, oxygenated monoterpens and monoterpene hydrocarbons during the all phenological stages. These types of volatiles could be differentiated clearly from the oxygenated hydrocarbons, oxygenated sesquiterpenes and sesquiterpenes (; ) which represented the main volatile constituents of the samples harvested at each phenological stage. The produced first two PCs displayed 97. 44 % of the whole variation.
Figure 2. Results of cluster analysis of different parts of H. perforatum plants, harvested at different stages of plant phenology.
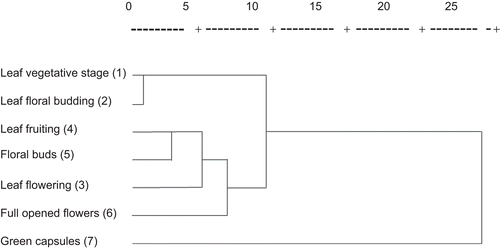
Figure 3. Scatter plot of main chemical classes in essential oil of H. perforatum plants, harvested at different stages of plant phenology.
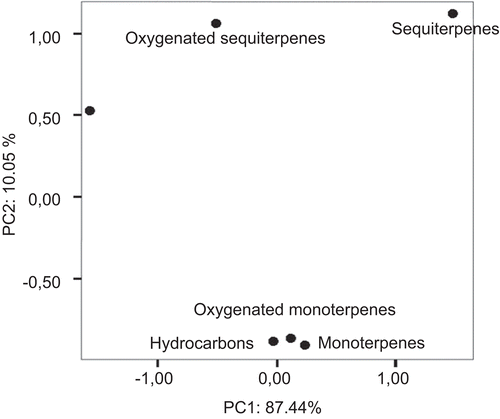
Several peculiar tendencies in specific variations of the typical volatiles were observed during the phenological cycle ():
Oxygenated derivatives of monoterpene hydrocarbons and sesquiterpenes showed high levels at each phenological stage;
Oxygenated hydrocarbons showed a decrease from the first up to the last phenological stage;
Oxygenated sesquiterpenes were the only volatiles which displayed the lowest production at the last phenological stage compared with the other constituents;
Monoterpene hydrocarbons and sesquiterpene hydrocarbons showed higher percentages comparing the first and last phenological stages after increase/decrease fluctuations.
As reported in the literature, huge amounts of sesquiterpenes both as oxygenated and hydrocarbon sesquiterpenes are characteristic for the H. perforatum species native to different regions. In the present study, it was demonstrated that sequiterpenes are regularly produced in significant amounts (oxygenated 26–50% and hydrocarbons 20–40%, ) in all of the seven phenological stages. The same situation was also true for oxygenated hydrocarbons ubiquitarious and abundant in the all analysed samples (13–38%, ). However, even if these two classes of volatiles were the most representative, they showed an opposite trend during phenological cycle. The oxygenated hydrocarbons were predominant especially in the stages 1–2 (38–33%), while their production dropped at the flowering period and the formation of green capsules (3–7, 25–13%). To the contrary, the sesquiterpenes showed the highest levels during the two last stages (40–50%) and the lowest in the first steps (26%). Regarding the other chemical classes of volatile constituents detected in the selected Hypericum stages, they were also useful to relate the EOs composition and plant phenological variations. The monoterpenes (hydrocarbons 0.4–6%; oxygenateds 0.8–6%) and linear hydrocarbons (1–3%) were constantly produced at very low levels at the all growth stages in comparison with the main constituents mentioned above.
These results are in contrast with previous data on Turkish H. perforatum which reported that α-pinene (50%) and carvacrol (22%) were identified as major constituents (CitationErken et al., 2001). Similarly α-pinene (61.7%) was reported again to be the major component as well as 3-carene (7.5%), β-caryophyllene (5.5%), myrcene (3.6%), cadalene (3.2%) and β-pinene (3.0%) for Turkish H. perforatum by CitationCakir et al. (1997).
However, in both studies, the specific ontogenetic stages were not established while sampling performed and only full flowering plants in wild were harvested. More importantly, Hypericum plants were collected in middle part of Anatolia by CitationErken et al. (2001) and in the southeastern part by CitationCakir et al. (1997). In our case, wild plant samples were harvested in the Black Sea region of Anatolia separated from that of CitationErken et al. (2001) by 350 km and that of CitationCakir et al. (1997) by 1200 km distances. Therefore, the different sampling procedures and great differences in habitats among previous studies and ours may be reason for the variations in EOs composition of the same species as already observed in our previous study (CitationCirak et al., 2010).
It is important to point out that a large variability in the EO composition of H. perforatum has generally due to the plant material origin in terms of not only the different countries but also the specific habitat of the collection site (CitationWeyerstahl et al., 1995; CitationCakir et al., 1997; CitationMimica-Dukic et al., 1998; CitationGudzic et al., 2001). In fact, hydrocarbons and sesquiterpenes were dominant in EOs of H. perforatum especially from French and Serbian populations. However, H. perforatum collected from the region Barelic in Serbia was reported to contain important quantity of the monoterpene α-pinene (8.6%), but the same species from the Rujan mountains did not include α-pinene (CitationGudzic et al., 2001). On the other hand α- and β-pinenes have been indicated as the main constituents for EO of H. perforatum, native to Grece (CitationPetrakis et al., 2005). However, it is important to remark that significant differences in the EO composition may be due to a combination of the specific variety and origin of H. perforatum. In fact, the major compounds in EO of Hypericum perforatum var. angustifolium collected in Italy (Sardinia) were 2-methyl octane (21.1%), germacrene D (17.6%) and α-pinene (15.8%) (CitationPintore et al., 2005).
French H. perforatum var. angustifolium samples were characterized by spathulenol (21.1%) and branched tetradecanol (9.1%) (CitationSchwob et al., 2002). Besides, huge amounts of sequiterpenes were detected as the main components such as caryophyllene oxide, β-caryophyllene, spathulenol, β-funebrene, 1-dodecanol, and α-muurolene in EOs of H. perforatum var. perforatum from France population depending on the phenological stages in which sampling performed (CitationSchwob et al., 2004). The results have confirmed those of our study in which H. perforatum var. perforatum was sampled and analyzed.
It is well-known that the EO of H. perforatum is synthesized in specific structures that may be localized in leaves, petals, sepals and pistil (CitationCiccarelli et al., 2001) which are not present at every stages of the developmental cycle. CitationSchwob et al. (2004) considered one population of H. perforatum var. perforatum in one French location to study the presence of different types of secretory structures (translucent glands, black nodules, secretory canals) and the EOs production. In that study, the authors suggested that sesquiterpene variations during the phenological cycle should rather be analysed by taking into account also their oxygenated derivatives, as these molecules share closely metabolic pathways. However, monoterpenoids composition and the levels of aliphatic alcohols seemed to be more related to the phenological cycle than sesquiterpenes (CitationSchwob et al., 2004).
Considering the different chemical classes of volatiles, the monoterpenoids were actually the less represented in the EOs composition among the group of terpenoids during phenological cycle in the wild H. perforatum collected in Turkey (). In fact, they increased especially at the last stage 6–7 where both monoterpenes and their oxygenated derivatives showed the highest production (5–6%, ). Based on the morphological and metabolite modifications observed in French H. perforatum var. perforatum during its phenological cycle by CitationSchwob et al. (2004), the same type of trends was observed for hydrocarbons, monoterpenes and sesquiterpenes during the seven stages of wild Turkish H. perforatum, analyzed in the present study. It is true that important qualitative–quantitative differences were pointed out between the EOs composition of French and Turkish H. perforatum. But, the present study confirms that the morphological modifications occurring during the different phenological stages have followed the same tendency in the variations of the same chemical classes of volatile constituents for the same species native to a different country.
Conclusions
Different plant organs of wild Turkish H. perforatum var. perforatum were collected during seven stages of the phenological cycle: leaves at the stages of vegetative, floral budding, flowering and green capsule; buds, full opened flowers and green capsules of the plant. Based on the EOs composition monitored during these seven morphological stages by GC-MS, PCA and cluster analysis, significant metabolite modifications were observed during the phenological cycle which involved the levels of specific volatile target compounds belonging to the chemical classes of hydrocarbons, monoterpenes, and sesquiterpenes.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adams RP. (1995). Identification of Essential Oil Components by Gas Chromatography-Mass Spectroscopy. Carol Stream, IL, Allured Publishing Corp.
- Azizi M. (2008). Change in content and chemical composition of Hypericum perforatum L. oil at three harvest time. J Herbs Spices Med Plants, 13, 79–85.
- Backhaus K, Erichson B, Plinke W, Weiber R. (1989). Multivariate Analysis Methods. Springer Verlag. Heidelberg 418 S.
- Cakir A, Duru ME, Harmandar M, Ciriminna R, Passannati S, Piozzi F. (1997). Comparison of the volatile oils of Hypericum scabrum L. and Hypericum perforatum in Turkey. Flavour Fragr J, 12, 285–287.
- Cakir A, Kordali S, Kilic H, Kaya E. (2005). Antifungal properties of essential oil and crude extracts of Hypericum linarioides Bosse. Biochem Syst Ecol, 33, 245–256.
- Ciccarelli D, Andreucci AC, Pagni AM. (2001). Translucent glands and secretory canals in Hypericum perforatum, morphological, anatomical and histochemical studies during the course of onthogenesis. Ann Bot-London, 88, 637–644.
- Cirak C, Bertoli A, Pistelli L, Seyis F. (2010). Essential oil composition and variability of Hypericum perforatum from wild populations of northern Turkey. Pharm Biol, 48, 906–914.
- Connolly JD, Hill RA. (1991). Advances in flavours and fragrances In: Dictionary of Terpenoids, Chapman & Hall, London, 677–736.
- Couladis M, Chinou IB, Tzakou O, Petrakis PV. (2003). Composition and antimicrobial activity of the essential oil of Hypericum rumeliacum subsp. apollinis (Boiss. & Heldr.). Phytother Res, 17, 152–154.
- Erken S, Malyer H, Demirci F, Demirci B, Baser KHC. (2001). Chemical investigations on some Hypericum species growing in Turkey. Chem Nat Compd, 37, 434–438.
- Greeson J, Sanford B, Monti DA. (2001). St. John’s wort (Hypericum perforatum L.): A review of the current pharmacological, toxicological and clinical literature. Psychopharmacol, 153, 402–414.
- Gudzic B, Dordevic S, Palic R, Stojanovic G. (2001). Essential oils of Hypericum olympicum L. and Hypericum perforatum L. Flavour Fragr J, 16, 201–203.
- Guedes RC, Eriksson LA. (2005). Theoretical study of hypericin. J Photochem Photobiol A: Chemistry, 172, 293–299.
- Massada Y. (1976). Essential oil, gas chromatography, and mass spectrometry. In Analysis of Essential Oils by Gas Chromatography and Mass Spectrometr., J. Wiley & Sons, New York, 257–336.
- Mathis C, Ourisson G. (1964). Etude chimio-taxonomique du genere Hypericum III. Répartition des carbures saturés et des monoterpènes dans les huiles essentielles d′Hypericum. Phytochemistry, 3, 133–141.
- Mimica-Dukic N, Ivancev-Tumbas I, Zigic R, Popovic M, Gasic O. (1998). The content and composition of essential oil of Hypericum perforatum L. from Serbia. Pharm Pharmacol Lett, 8, 26–28.
- Petrakis PV, Couladis M, Roussis V. (2005). A method for detecting the biosystematic significance of the essential oil composition: The case of five Hellenic Hypericum L. species. Biochem Syst Ecol, 33, 873–898.
- Pintore G, Mario C, GianPiero B, Riccardo C. (2005). Essential oil composition of Hypericum perforatum L. var. angustifolium DC growing wild in Sardinia (Italy). J Essent Oil Res, 17, 120–127.
- Radusiene J, Judzentieneb A, Bernotiene G. (2005): Essential oil composition and variability of Hypericum perforatum L. growing in Lithuania. Biochem Syst Ecol, 33, 113–124.
- Sánchez-Mateo CC, Prado B, Rabanal RM. (2002). Antidepressant effects of the methanol extract of several Hypericum species from the Canary Islands. J Ethnopharmacol, 79, 119–127.
- Saroglou V, Marin PD, Rancic A, Veljic M, Skaltsa H. (2007). Composition and antimicrobial activity of the essential oil of six Hypericum species from Serbia. Biochem Syst Ecol, 35, 146–152.
- Schwob I, Bessière JM, Viano J. (2002). Composition of the essential oils of Hypericum perforatum L. from southeastern France. C R Biol, 325, 781–785.
- Schwob I, Bessiere JM, Masotti V Viano J. (2004). Changes in essential oil composition in Saint John’s Wort (Hypericum perforatum L.) aerial parts during its phenological cycle. Biochem Syst Ecol, 32, 735–745.
- Stenhagen E, Abrahamsson S, McLafferty FW. (1974). Mass spectrometry for chemists and biochemists. In: Registry of Mass Spectral Data, Wiley & Sons, New York, 1974, 294–358.
- Swigar AA, Silverstein RM. (1981). GC and GC/MS integrated analyses of the essential oils. In: Monoterpenes, Aldrich Chem. Comp., Milwaukee, 157–211.
- Toker Z, Kizil G, Ozen HC, Kizil M, Ertekin S. (2006). Compositions and antimicrobial activities of the essential oils of two Hypericum species from Turkey. Fitoterapia, 77, 57–60.
- Vlietinck AJ, De Bruyne T, Apers S, Pieters LA. (1998). Plant-derived leading compounds for chemotherapy of human immunodeficiency virus (HIV) infection. Planta Med, 64, 97–109.
- Weyerstahl P, Splittgerber U, Marschall H, Kaul VK. (1995). Constituents of the leaf essential oil of Hypericum perforatum L. from India. Flavour Fragr J, 12, 285–287.