Abstract
Context: Crocus sativus Linn. (Iridaceae), commonly known as saffron, becomes more and more popular due to its versatile biological and medicinal properties. At present, studies mainly focus on the traditional medicinal part, the saffron stigma, with less attention to the other parts of saffron, such as the perianth, the stamen, and the corm, which are high yield compared to the stigma and also possess various pharmacological effects.
Objective: To determine the chemical compositions, antifungal, cytotoxic, and antioxidant activities of the ether fractions from the stamen, perianth, and stigma of saffron.
Materials and methods: The chemical constituents of the ether fractions from different parts of saffron were investigated by gas chromatography/mass spectrometry. Several pathogenic fungi isolates and tumor cell lines were employed to evaluate the antifungal and cytotoxic activities of these three ether fractions. 1,1-Diphenyl-2-picrylhydrazyl assay was used to determine the free radical-scavenging activity.
Results: The ether fractions composition of the three C. sativus parts are different from each other, but lauric acid, hexadecanoic acid, 4-hydroxydihydro-2(3H)-furanone, and stigmasterol were the common constituents shared by all the three fractions. The stamen ether fraction displayed the strongest antifungal and cytotoxic activities, whereas both of the saffron stamen and perianth ether fractions exhibited significant antioxidant activities.
Discussion and conclusion: These findings demonstrate that the saffron stamen and perianth possess significant antifungal, cytotoxic, and antioxidant activities as well as the stigma, though not to the same extent, prompting us to expand the medicinal resource and make best use of this valuable plant.
Introduction
C. sativus Linn. (Iridaceae), commonly known as saffron, is a perennial stemless plant. It is originally distributed in southern Europe and Asia Minor and is cultivated not only in some areas of the Mediterranean basin (North Africa, Spain, Greece, etc.) and the Near and Middle East (Asia Minor, Iran, etc.), but also in India and China (CitationAbdullaev, 1993; CitationRios et al., 1996; CitationGiaccio, 2004).
The saffron stigma, which is basically formed commercial saffron, is a very expensive spice appreciated as a colorant for foodstuffs as well as for its aromatic and flavoring property (CitationRios et al., 1996). Moreover, saffron stigma has been used as a drug in folk medicine since ancient times for various purposes such as an aphrodisiac, antispasmodic, andexpectorant, antidepressant, and stomachic agent (CitationAbdullaev, 1993; CitationRichelson, 1994; CitationWang et al., 2010). In some folk remedies, it was also used against scarlet fever, smallpox, colds, asthma, eye, and heart diseases (CitationRios et al., 1996; CitationAbdullaev and Espinosa-Aguirre, 2004; CitationCarmona et al., 2007; CitationNemati et al., 2008).
Recently, C. sativus has become more and more popular due to its versatile biological and medicinal properties. Especially, saffron stigma extracts and the associated carotenoid ingredients are extensively studied for their chemopreventive potential against cancer (Abdullaev and Frenkel, Citation1992a,b; CitationEscribano et al., 1996; CitationAbdullaev and Espinosa-Aguirre 2004; CitationPremkumar et al., 2006; CitationChryssanthi et al., 2007; CitationTavakkol-Afshari et al., 2008). Furthermore, antioxidant activity of saffron stigma and its active constituents were frequently reported (CitationNair et al., 1991; CitationAssimopoulou et al., 2005; CitationNaghizadeh et al., 2008). The presence of carbohydrates, minerals, mucilage, vitamins, and pigments (including crocin, anthocianin, carotene, lycopene, zigzantin, and flavonoids) has been reported in saffron stigmas, among which, crocin, picrocron, crocitin, and safranal are regarded as the main active ingredients (CitationAbdullaev & Espinosa-Aguirre, 2004).
At present, studies mainly focus on the traditional medicinal part, the saffron stigma, with less attention to the other parts of saffron, such as the perianth, the stamen, and the corm, which are high yield compared to the stigma and also possess various pharmacological effects. Previous studies have already demonstrated that the ethanol extracts of saffron petal has antidepressant effect in a pre-clinical study (CitationMoshiri et al., 2006), and petroleum ether and dichloromethane fractions of saffron corms produce antidepressant-like effects in behavioral models predictive of antidepressant properties (CitationWang et al., 2010). It is also reported that a kind of glucoconjugate, isolated from saffron corms and calluses has cytotoxic activity property against tumor cells (CitationEscribano et al., 2000).
Our preliminary investigations on the three parts (stamen, perianth, and stigma) of C. sativus indicated that their liposoluble fractions possess significant anti-Pyricularia oryzae activities which predict potential antitumor and antifungal properties. Therefore, the present study is conducted to determine the chemical compositions of the ether fractions of the stamen, perianth, and stigma of C. sativus and to investigate their antifungal, cytotoxicity properties, and antioxidant activity, so as to expand the medicinal resource and make best use of this valuable plant.
Materials and methods
Chemicals
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical (Sigma, Chemical Co., St. Louis, MI) was used for testing the radical-scavenging activity of extracts. Ascorbic acid (Sigma) and butylhydroxyanisole (BHA) (Sigma) were used as references. All other regents and solvents were analytically pure and purchased from Sinopharm Chemical Reagent Co. Ltd., Shanghai, China.
Plant materials
Samples were collected from the growing fields of saffron in the Changxing Island, Shanghai (China) in November 2008. Taxonomic identification was performed by LPQ. A voucher specimen of the plant was deposited at the herbarium of the Department of Pharmacognosy, School of Pharmacy, Second Military Medical University, Shanghai, China. The three parts of C. sativus, namely stamen (#2008-225), perianth (#2008-226), and stigma (#2008-227), were separated by hand.
Samples preparation
Following desiccation and comminution, three powdered samples were soaked in 70% ethanol aqueous solution for 24 h, then ultrasonic-assisted extraction was performed three times (30 min/time). The materials were filtered, and the clear supernatant was then concentrated under reduced pressure at 60°C with a vacuum rotary evaporator. Samples of the concentrated ethanol extracts were partitioned between water and ethyl ether. After removing the ethyl ether fraction, the aqueous layers were further partitioned by n-butanol. The fractions of ethyl ether, n-butanol, and aqueous layer remains were evaporated and the residues were used in the following experiments. Then the extracts kept at 4°C.
Gas chromatography/mass spectrometry analysis
Gas chromatography (GC)/mass spectrometry analysis of ethyl ether fractions of the three samples were carried out on a Thermo Focus DSQ gas chromatograph-mass spectrometry system fitted with a VF-5 ms capillary column (30 m × 0.25 mm; 0.25 μm film thickness). Helium was used as the carrier gas with a flow rate of 1.0 mL/min, 1:30 split ratio, and 1 μL of the sample was injected. The injection and ion source temperatures were both 250°C. The GC oven temperature was programed from 60°C, held for 2 min, and then raised from 60°C to 300°C at 10°C/min, and finally held at 300°C for 10 min. The electron impact technique of 70 eV was used and the mass range scanned was 41–450 amu in full-scan acquisition mode. The identification of the compounds was based on comparison of their retention indexes (RI), obtained using n-alkanes (C7–C30), and retention time. Compounds were also confirmed by comparison of their mass spectra with the NIST/NBS-Wiley library spectra. The relative amounts of individual components of the ethyl ether fractions of the three samples were expressed as percentages of the peak area relative to the total peak area. Relative percentage amounts were calculated from the total ion current by the computer.
Anti-P. oryzae assay
P. oryzea P-2b, a phytopathogenic fungus, has been used as a test microorganism for the primary screening of antineoplastic and antifungal agents (CitationKobayashi et al., 1996). This bioassay method, detecting deformations of mycelia germinated from conidia of P. oryzae P-2b, has been used to give quantitative estimations. Antimitotic and antifungal agents revealed characteristic curling effect of morphological deformations including curling, swelling, hyper-divergency, beads shape, and so on. The bioassay method was proved to be a quick, easy, and applicable throughput screening estimation for antimitotic and antifungal substances from natural sources.
Anti-P. oryzae activity was evaluated by the method as follows. P. oryzae was grown on a slant potato-dextrose-agar culture medium at 27°C. The conidia were collected on 10 days after inoculation by suspending in sterilized water, and were filtered to separate from the mycelia. The conidia were then adjusted to 4 × 104 for further experiment. Sample 50 μL in a solvent of 10% methanol and 50 μL mycelia suspension were poured into 96-well plate. The assay plates were incubated at 27°C for 15 h. The shape of mycelia germinated from conidia was observed and compared with ketoconazole to determine the minimum morphological deformation concentration.
Antifungal activity assay
The antifungal activities of the samples were individually tested against pathogenic fungi including Candida albicans (ATCC 76615), Cryptococcus neoformans (32609), Trichophyton rubrum, and Aspergillus fumigatus. Antifungal activities were determined using the modified liquid dilution method. Sabouraud-dextrose-agar slant medium was employed for pathogenic fungal growth. Dilutions of the ether fractions were prepared in dimethyl sulfoxide (DMSO). The fraction solutions were serially diluted (4:1) in 96-well plates. Organisms at a concentration of ~1–5 × 103 colony forming units/mL were then added to each well. Plates were made in triplicate and incubated at 35°C for about 24 h for C. albicans, about 72 h for C. neoformans and about 168 h for T. rubrum and A. fumigatus, then their turbidity obtained by measuring optical density at 630 nm.
The standard antifungal agent IC amphotericin B was used as a positive control and experiments were repeated at least three times. Test substance concentrations at which fungi proliferation was reduced by 80% are given as MIC80 values and the values presented are an average of triplicate.
Cytotoxic activity assay
The human lung tumor cell line A549, human promyelocytic leukemia cell line HL-60, human gastric tumor cell line MKN-45 and human hepatocellular liver carcinoma cell line HepG2 were employed in the test. The cells viability were determined using a modified 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay (CitationMosmman, 1983; CitationSharifi et al., 2005). Briefly, A549, HL-60, MKN-45, and HepG2 cells were grown in RPMI 1640 including 100 units/mL penicillin and streptomycin supplemented with 15% new-born bovine serum at 37°C in a 5% CO2 atmosphere. For experimentation, the exponentially growing cells (4–6 × 104) were used. Then, cells were incubated in the presence of 1000 μg/mL samples in DMSO for 72 h at 37°C. After removing the sample solution and washing with phosphate-buffered saline (pH 7.4), 10 μL/well of 0.5% MTT bromide cells phosphate-buffered saline solution was added. After a further 4 h of incubation, 0.04 M HCl was added. Viable cells were determined by measuring the absorbance at 570 nm. Measurements were performed three times, and the concentration required for a 50% inhibition of viability (IC50) was determined. The values presented in are an average of triplicate.
DPPH free radical-scavenging assay
The DPPH free radical-scavenging activities of ether, n-butanol fractions and aqueous layer remains of three parts of saffron flower were determined according to the method previously described with modification (CitationChen et al., 2008). Briefly, a 100 μL solution of sample at different concentration (0.0125–0.500 mg/mL) was added to 200 μL of methanol DPPH solution (40 mg in 100 mL). The absorbance was measured at 517 nm after 30 min incubation at room temperature in dark place. Lower absorbance of the reaction mixture indicates higher free radical-scavenging activity. And the capability to scavenge the DPPH radical was calculated using the following formula:
DPPH scavenging effect (%) = [A0 − (A1 − AS)]/A0 × 100,
where A0 is the absorbance of the control solution containing DPPH; A1 is the absorbance of the DPPH solution containing samples, and AS is the absorbance of the sample solution without DPPH. The experiment was carried out in triplicate and the results are mean values.
Results and discussion
Chemical components of the stamen, perianth, and stigma of C. sativus
The ether fractions yields of the stamen, perianth, and stigma were 5.17, 3.42, and 12.23%, respectively. The components identified from the ether fractions of the stamen, perianth, and stigma of C. sativus, along with the RI and their percentage, are presented in .
Table 1. Chemical composition of the ether fractions from different parts of C. sativus.
A total of 46 components were detected in the ether fraction of stamen, of which 29 compounds were identified, representing 81.19% (area percent) of the total fractions. According to our results, 4-hydroxydihydro-2(3H)-furanone (22.01%), hexadecanoic acid (12.09%), tyrosol (7.52%), benzeneacetic acid (5.23%), linolenic acid (4.96%), linoleic acid (3.86%), 1-docosene (3.85%), and vitamin E (3.63%) were the major components. These components accounted for 63.15% of the total fractions while the other minor components made up the balance.
Out of 50 peaks detected in the ether fractions of perianth, 23 compounds were identified (81.70% of the total fractions), with 4-hydroxydihydro-2(3H)-furanone (22.12%), hexadecanoic acid (18.14%), linolenic acid (7.73%) and stigmasterol (4.20%) being the major components. The four predominant components occupied 52.19% of the total fraction of the perianth.
A total of 34 components were detected in the ether fractions of stigma with 19 compounds identified, which represented 81.25% (area percent) of the total fractions, with 1,3,3-trimethyl-2-vinyl-1-cyclohexene (22.36%), diisooctyl phthalate (14.77%), hexadecanoic acid (9.48%), cis-9,cis-12-octadecadienoic acid (7.49%), 4-hydroxy-3,5,5-trimethyl-2-cyclohexen-1-one (4.74%) and stigmasterol (3.31%) being the most abundant. Safranal, the main aroma factor in saffron, was detected in the ether fraction of the stigma at 2.05%.
The chemical composition of the stamen and the perianth is very similar. Seventeen compounds were shared, which accounted for 66.07 and 62.71% of the total fractions of the stamen and the perianth, respectively, with 4-hydroxydihydro-2(3H)-furanone and linolenic acid as the main constituent in both of them.
The ether fraction composition of the stigma showed much difference from the other two parts. Only five compounds were shared with the fraction of the stamen as well as six compounds were shared with that of the perianth. The other compounds varied from one fraction to another.
Anti-P. oryzae assay
The tested samples displayed a variable degree of anti-P. oryzae activity. As shown in , n-butanol fractions and aqueous layer remains of the three parts of the saffron flower had weak effect on the P. oryzae bead-shape deformation, demonstrating the fractions were void of antifungal activities. However, all ethyl ether fractions showed significant antifungal activities in vitro against P. oryzae. Among the three ether fractions, the biological activity of stigma ether fractions proved to be better than the other two, with a minimum inhibited concentration value of 7.8 μg/mL. Then, we continued to test the bioactivities of the ether fractions of the three samples of saffron flower against pathogenic fungi and human tumor cell lines.
Table 2. Anti-Pyricularia oryzae activity of different fractions from the stamen, perianth, and stigma of C. sativus.
Antifungal activity
All three ether fractions displayed promising effects against C. albicans, with MIC80 values of 32.0, 128.0, and 64.0 μg/mL, respectively. Both of the stamen and stigma fractions were found to be active against C. neoformans and T. rubrum. However, none of these fractions showed inhibition on A. fumigatus.
Cytotoxic activity
The cytotoxic activity of the ether fractions from the stamen, perianth and stigma of C. sativus was quantitatively assessed, and the results were represented in . The tested ether fractions displayed a variable degree of cytotoxic activity against the different cell lines tested.
Table 3. Antifungal activity of the ether fractions from different parts of C. sativus [MIC80 (μg/mL)].
Table 4. The in vitro cytotoxic activity of the ether fractions from the stamen, perianth and stigma of C. sativus.
It is interesting that the fractions of the stamen and stigma exhibited noticeable inhabitation on all the tested tumor cells when compared with the perianth ether fractions. The IC50 values of the ether fraction of stamen against the four human tumor cell lines of A549, MKN-45, HL-60, and HepG2 are 48.53 ± 2.68, 21.18 ± 1.36, 14.11 ± 0.98, and 63.39 ± 3.26 μg/mL, respectively, whereas, the values of the ether fractions of stigma are 37.12 ± 2.21, 80.24 ± 4.55, 28.87 ± 0.74, and 77.71 ± 2.90 μg/mL. The stamen fraction possessed better cytotoxic activity than the stigma with most of IC50 values below 50 μg/mL. The fraction of the perianth also showed moderately inhibitory to all test tumor cells.
To our knowledge, this is the first report that investigated the cytotoxic effects of ether fractions of the stamen, perianth and stigma against A549, MKN-45, HL-60, and HepG2 cell lines. Our results showed that the ether fractions of the stamen and stigma of C. sativus possessed notable cytotoxic activity against these four cell lines. This suggested that the bioactive compounds in the ether fractions are responsible for a share of the total activity. Therefore, the ether fraction of the stamen may be a potential source of antitumor agent.
Cancer is a growing health problem around the world. Natural products have been used to prevent and treat many diseases for a long time, including cancer and thus they are good candidates for the development of anticancer drugs (CitationTavakkol-Afshari et al., 2008). At present, the saffron stigma is paid more attention for its potential use in cancer therapy and chemoprevention trials. However, the low production and the high price limit the utilization of the saffron stigma on a large scale. Moreover, the saffron corm and other parts of the flower take the majority part of the harvest weight, and this prompt us to find ways to screen cytotoxicity materials in them and then expand the resource for saffron plants.
Based on our above cytotoxic assay, future research should focus on other active parts of saffron (except for the stigma) and active fractions/components from the whole plant. Additional work is necessary to unravel the specificity and usefulness of active components as cancer chemotherapeutic agents.
DPPH scavenging assay
DPPH is a stable free radical and accepts electron or hydrogen radical to become a stable diamagnetic molecule. A freshly prepared DPPH solution exhibits a deep purple color with an absorption maximum at 517 nm. This purple color generally fades when an antioxidant is present in the medium. Thus, antioxidant molecules can quench DPPH and convert it to a colorless product, resulting in a decrease in absorbance at 517 nm. In short, the reduction capacity of DPPH was determined by the decrease in its absorbance at 517 nm, which is reduced by antioxidants (CitationLoo et al., 2007). The scavenging effects of fractions under investigation on DPPH radicals were significant. As shown in , all fractions and positive drugs (ascorbic acid and BHA) exhibited appreciable scavenging properties against DPPH radicals in a concentration dependent manner. The ether fractions of saffron stigma (ES) exhibited the weakest antioxidant activity, while ether fractions of saffron stamen (EST) and perianth (EP) and n-butanol fractions of saffron stigma (BS) showed better radical-scavenging activity among these tested samples.
Figure 1. DPPH (1,1-ddiphenyl-2-picrylhydrazyl) free radical-scavenging activity of fractions from the stamen, perianth and stigma of saffron. Ascorbic acid/butylhydroxyanisole (BHA), positive control; BP: n-butanol fraction of perianth; BS: n-butanol fraction of stigma; BST: n-butanol fraction of stamen; EP, ether fraction of perianth; ES, ether fraction of stigma; EST, ether fraction of stamen.
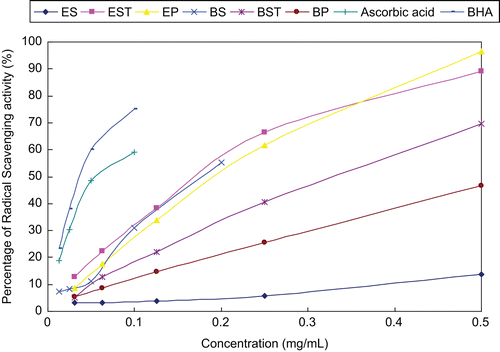
Despite some investigations aimed to evaluate the antioxidant potential of saffron stigma and its active constituents in vitro and in animals (CitationAssimopoulou et al., 2005; CitationChen et al., 2008; Asdaq et al., 2009), to our knowledge, the main antioxidant constituent of the saffron stigma remains unknown. CitationAssimopoulou et al. (2005) reported that methanol extract of C. sativus (stigma) grown in Greece exhibited high antioxidant activity. CitationChen et al. (2008) evaluated the antioxidant capacity of crocins (crocin 1–3), ethanol extracts of saffron and gardenia, and macroporous resin fractions of gardenia, and they suggested that crocins probably did not play a main role in antioxidant capacities in assays of anti-hemolysis, DPPH radical-scavenging and lipid peroxidation. CitationAsdaq and Inamdar (2010) reported saffron extract is superior to crocin in hypolipidemic and antioxidant potential assay in hyperlipidemic rats and they thought there were other constituents apart from crocin responsible for synergistic antihyperlipidemic and antioxidant potential of saffron stigma. Moreover, there are no experimental reports on the screening of antioxidative fractions from saffron stigma and other parts of saffron.
In our study, n-butanol fractions of saffron stigma, ether fractions of saffron stamen and perianth displayed DPPH free radical-scavenging ability and the stamen and perianth may be promising sources with antioxidant activity as well as the stigma as food supplements in functional foods, beverages, in pharmaceutical preparations and cosmetic formulations. Further investigation concerning isolation, identification and quantification of components responsible for the antioxidant activity of the saffron stamen and perianth is currently being conducted in our lab.
In conclusion, our study revealed that the ether fractions compositions of the three parts of C. sativus are different from each other, while lauric acid, hexadecanoic acid, 4-hydroxydihydro-2(3H)-furanone, and stigmasterol were the common constituents shared by all three fractions. This is the first report to show the antifungal activity, cytoxicity and DPPH radical-scavenging activity of the ether fractions from different parts of C. sativus. The stamen fractions display a wide range of antifungal and cytotoxic activities, while both ether fractions of stamen and perianth possess antioxidant capacity in DPPH assay, which prompts us to expand the medicinal resource and make best use of this valuable plant and thus merit further investigation regarding the bioactive constituents and mechanism of action.
Acknowledgment
The authors are grateful for the assistance of Lei Guo, Lili Xu, and Hongsheng Yu of the Department of Pharmacognosy, School of Pharmacy, Second Military Medical University.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abdullaev FI, Espinosa-Aguirre JJ. (2004). Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev, 28, 426–432.
- Abdullaev FI, Frenkel GD. (1992a). The effect of saffron on intracellular DNA, RNA and protein synthesis in malignant and non-malignant human cells. Biofactors, 4, 43–45.
- Abdullaev FI, Frenkel GD. (1992b). Effect of saffron on cell colony formation and cellular nucleic acid and protein synthesis. Biofactors, 3, 201–204.
- Abdullaev FI. (1993). Biological effects of saffron. Biofactors, 4, 83–86.
- Asdaq SM, Inamdar MN. (2010). Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl Biochem Biotechnol, 162, 358–372.
- Assimopoulou AN, Sinakos Z, Papageorgiou VP. (2005). Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res, 19, 997–1000.
- Carmona M, Sάnchez AM, Ferreres F, Zalacain A, Tomάs-Barberάn F, Alonso GL. (2007). Identification of the flavonoid fraction in saffron spice by LC/DAD/MS/MS: Comparative study of samples from different geographical origins. Food Chem, 100, 445–450.
- Chen Y, Zhang H, Tian X, Zhao C, Cai L, Liu Y, Jia L, Yin H X, Chen C. (2008). Antioxidant potential of crocins and ethanol extracts of Gardenia jasminoides Ellis and Crocus sativus L.: A relationship investigation between antioxidant activity and crocin contents. Food Chem, 109, 484–492.
- Chryssanthi DG, Lamari FN, Iatrou G, Pylara A, Karamanos NK, Cordopatis P. (2007). Inhibition of breast cancer cell proliferation by style constituents of different Crocus species. Anticancer Res, 27, 357–362.
- Escribano J, Alonso GL, Coca-Prados M, Fernandez JA. (1996). Crocin, safranal and picrocrocin from saffron (Crocus sativus L.) inhibit the growth of human cancer cells in vitro. Cancer Lett, 100, 23–30.
- Escribano J, Díaz-Guerra MJ, Riese HH, Alvarez A, Proenza R, Fernández JA. (2000). The cytolytic effect of a glycoconjugate extracted from corms of saffron plant (Crocus sativus) on human cell lines in culture. Planta Med, 66, 157–162.
- Giaccio M. (2004). Crocetin from saffron: An active component of an ancient spice. Crit Rev Food Sci Nutr, 44, 155–172.
- Kobayashi H, Namikoshi M, Yoshimoto T, Yokochi T. (1996). A screening method for antimitotic and antifungal substances using conidia of Pyricularia oryzae, modification and application to tropical marine fungi. J Antibiot, 49, 873–879.
- Loo AY, Jain K, Darah I. (2007). Antioxidant and radical scavenging activities of the pyroligneous acid from a mangrove plant, Rhizophora apiculata. Food Chem, 104, 300–307.
- Moshiri E, Basti AA, Noorbala AA, Jamshidi AH, Hesameddin Abbasi S, Akhondzadeh S. (2006). Crocus sativus L. (petal) in the treatment of mild-to-moderate depression: a double-blind, randomized and placebo-controlled trial. Phytomedicine, 13, 607–611.
- Mosmman T. (1983). Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods, 65, 55–63.
- Naghizadeh B, Boroushaki MT, Vahdati Mashhadian N, Mansouri MT. (2008). Protective effects of crocin against cisplatin-induced acute renal failure and oxidative stress in rats. Iran Biomed J, 12, 93–100.
- Nair SC, Pannikar B, Panikkar KR. (1991). Antitumour activity of saffron (Crocus sativus). Cancer Lett, 57, 109–114.
- Nemati H, Boskabady MH, Ahmadzadef Vostakolaei H. (2008). Stimulatory effect of Crocus sativus (saffron) on beta2-adrenoceptors of guinea pig tracheal chains. Phytomedicine, 15, 1038–1045.
- Premkumar K, Thirunavukkarasu C, Abraham SK, Santhiya ST, Ramesh A. (2006). Protective effect of saffron (Crocus sativus L.) aqueous extract against genetic damage induced by anti-tumor agents in mice. Hum Exp Toxicol, 25, 79–84.
- Richelson E. (1994). Pharmacology of antidepressants–characteristics of the ideal drug. Mayo Clin Proc, 69, 1069–1081.
- Rios JL, Recio MC, Giner RM, Manez S. (1996). An update review of saffron and its active constituents. Phytother Res, 10, 189–193.
- Sharifi AM, Mousavi SH, Bakhshayesh M, Tehrani FK, Mahmoudian M, Oryan S. (2005). Study of correlation between lead-induced cytotoxicity and nitric oxide production in PC12 cells. Toxicol Lett, 160, 43–48.
- Tavakkol-Afshari J, Brook A, Mousavi SH. (2008). Study of cytotoxic and apoptogenic properties of saffron extract in human cancer cell lines. Food Chem Toxicol, 46, 3443–3447.
- Wang Y, Han T, Zhu Y, Zheng CJ, Ming QL, Rahman K, Qin LP. (2010). Antidepressant properties of bioactive fractions from the extract of Crocus sativus L. J Nat Med, 64, 24–30.