Abstract
Context: Growing adipose tissue is thought to require adipogenesis, angiogenesis, and extracellular matrix (ECM) remodeling. Close examination of developing adipose tissue microvasculature reveals that angiogenesis often precedes adipogenesis. Since our previous study demonstrated that Ob-X, the anti-angiogenic herbal composition composed of Melissa officinalis L. (Labiatae), Morus alba L. (Moraceae), and Artemisia capillaris Thunb. (Compositae), reduced adipose tissue mass in obese mice, we hypothesized that adipogenesis can be inhibited by Ob-X.
Objective: To investigate the effects of the anti-angiogenic herbal extracts Ob-X on adipogenesis in 3T3-L1 adipocytes.
Materials and methods: After differentiated 3T3-L1 adipocytes were treated with Ob-X, we studied the effects of Ob-X on triglyceride accumulation and expression of genes involved in adipogenesis, angiogenesis, and ECM remodeling.
Results: Treatment of cells with Ob-X inhibited lipid accumulation and adipocyte-specific gene expression caused by troglitazone or monocyte differentiation-inducing (MDI) mix. Ob-X reduced mRNA levels of angiogenic factors (vascular endothelial growth factor-A, -B, -C, -D, and fibroblast growth factor-2) and matrix metalloproteinases (MMPs; MMP-2 and MMP-9), whereas it increased mRNA levels of angiogenic inhibitors [(thrombospondin-1, tissue inhibitor of metalloproteinase-1 (TIMP-1), and TIMP-2)] in differentiated cells. MMP-2 and MMP-9 activities were also decreased in Ob-X-treated cells.
Discussion and conclusion: These results suggest that the anti-angiogenic herbal composition Ob-X inhibits differentiation of preadipocytes into adipocytes. These events may be mediated by changes in the expression of genes involved in lipogenesis, angiogenesis, and the MMP system. Thus, by reducing adipogenesis, anti-angiogenic Ob-X provides a possible therapeutic approach for the prevention and treatment of human obesity and its related disorders.
Keywords::
Introduction
Obesity and the related metabolic disorders, such as dyslipidemia, atherosclerosis, and type 2 diabetes, have become global health problems. Obesity is characterized by increased adipose tissue mass that results from both increased fat cell number (hyperplasia) and increased fat cell size (hypertrophy) (CitationCouillard et al., 2000). Fat mass can be regulated by various factors including adipogenesis, angiogenesis, and remodeling of the extracellular matrix (ECM) (CitationCrandall et al., 1997).
During adulthood, most tissues normally do not grow and the supporting vasculature is quiescent. Exceptionally, adipose tissue can grow throughout the life span and is highly vascularized. In growing adipose tissue, each adipocyte is nourished by an extensive capillary network (CitationSilverman et al., 1988; CitationCrandall et al., 1997; CitationBouloumié et al., 2002; CitationCao, 2010). Thus, it can be suggested that the growth and differentiation of adipocytes are angiogenesis-dependent.
Extensive changes in ECM remodeling have also been shown to occur during adipose tissue growth. The matrix metalloproteinases (MMP) play important roles in the development of adipose tissue and microvessel maturation by modulating the ECM (CitationGalardy et al., 1994; CitationBouloumié et al., 2001; CitationLijnen et al., 2002). In most cases, MMPs are expressed at very low levels, but expression is rapidly induced at times of active tissue remodeling associated with adipogenesis. Several lines of evidence suggest that endogenous and exogenous MMPs regulate adipogenesis (CitationKawaguchi et al., 1998; CitationBouloumié et al., 2001; CitationChristiaens & Lijnen, 2006). During obesity, MMP expression is modulated in adipose tissue, and MMPs (e.g., MMP-2 and MMP-9) potentially affect adipocyte differentiation (CitationBouloumié et al., 2001; CitationMaquoi et al., 2002; CitationChavey et al., 2003).
Adipocytes produce angiogenic factors, such as vascular endothelial growth factors (VEGFs) and fibroblast growth factor (FGF)-2, contributing to the formation of new blood vessels inside the fat pad (CitationClaffey et al., 1992; CitationVoros et al., 2005; CitationCao, 2007). VEGFs and FGF-2 stimulate proliferation and migration of endothelial cells (ECs) and enhance adipocyte differentiation (CitationCarmeliet et al., 1996; CitationBikfalvi et al., 1997; CitationKawaguchi et al., 1998; CitationLijnen, 2008). Adipocytes also secrete several MMPs, including MMP-2 and MMP-9 (CitationBouloumié et al., 2001). Indeed, it is well established that degradation of the ECM represents the first step in the angiogenic process, and MMP-2 and MMP-9 have been shown to be necessary for this event (CitationSang, 1998).
We screened anti-angiogenic activity of water extracts from medicinal herbs and plants which have been used for a long period of time, and selected three herbal extracts Melissa officinalis L. (Labiatae), lemon balm, Morus alba L. (Moraceae) white mulberry, and Artemisia capillaris Thunb. (Compositae) injin, to make Ob-X (CitationKim et al., 2006). Moreover, Ob-X reduces adipose tissue mass in both genetically and nutritionally obese mice (CitationKim et al., 2010; Yoon and Kim, accepted). Accordingly, we hypothesized that adipocyte growth and differentiation can also be effectively regulated by Ob-X. Treatment of 3T3-L1 adipocytes with Ob-X reduced lipid accumulation and adipocyte-specific gene expression. The expression of angiogenic factors, MMPs, and their inhibitors in adipose tissue was markedly modulated by Ob-X. These studies suggest that Ob-X can inhibit adipogenesis by targeting adipocytes.
Materials and methods
Preparation of Ob-X
Ob-X was prepared from food grade aqueous extracts of the three herbs M. officinalis (Frutarom, Wadenswil, Switzerland), M. alba (Segae FL, Buyeo, Korea), and A. capillaries (Segae, FL) as previously described (CitationLee et al., 2008). The quality of each herbal extract in Ob-X was controlled by standardization with reference compounds by high-pressure liquid chromatography. The corresponding reference compounds were rosmarinic acid (M. officinalis), 1-deoxynojirimycin (M. alba), and 6,7-dimethylesculetin (A. capillaries).
Induction of preadipocyte differentiation
Mouse 3T3-L1 cells (ATCC) were grown proliferated in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% bovine calf serum (Invitrogen, Carlsbad, CA). After cells were kept confluent for 2 days, they were incubated in monocyte differentiation-inducing (MDI) medium (day 0) containing 0.5 mM 1-methyl-3-isobutyl-xanthin, 1 μM dexamethasone, and 1 μg/mL insulin in DMEM with 10% fetal bovine serum (FBS; Invitrogen). The cultures were continued for 2 more days to induce adipocyte differentiation. Thereafter, cells were cultured in DMEM with 10% FBS for the rest of the differentiation process. All other treatments were administered on day 0 to day 2 only, and the medium was changed every other day. Cells were stained at day 6 with Oil-red O and photographed.
Reverse transcription-polymerase chain reaction
Total cellular RNA from 3T3-L1 cells was prepared using the Trizol reagent (Gibco-Brl, Grand Island, NY). After 2 μg of total RNA was reverse-transcribed using Moloney murine leukemia virus reverse transcriptase (MuLV-RT) and an antisense primer, cDNA was generated. The RNA was denatured for 5 min at 72°C and then immediately placed on ice for 5 min. Denatured RNA was mixed with MuLV-RT, MuLV-RT buffer, and a dNTP mixture, and incubated for 1 h at 42°C. Synthesized cDNA fragments were amplified by polymerase chain reaction (PCR) in an MJ Research Thermocycler (Waltham, MA). The PCR primers used for gene expression analysis are shown in . The cDNA was mixed with PCR primers, Taq DNA polymerase (Solgent, Daejeon, Korea), and a dNTP mixture. The reaction consisted of 30 cycles of denaturation for 1 min at 94°C, annealing for 1 min at 58°C, and elongation for 1 min at 72°C. The PCR products were analyzed by electrophoresis on a 1% agarose gel. Relative expression levels were presented as a ratio of target gene cDNA versus β-actin cDNA. PCR products were quantified from agarose gels using the GeneGenius (Syngene, Cambridge, UK).
Table 1. PCR primers used for cDNA synthesis by RT-PCR.
Zymography
MMP activity was determined by gelatin zymography. 3T3-L1 Cells were extracted with 10 mM sodium phosphate buffer (pH 7.2) containing 150 mM NaCl, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, and 0.2% NaN3 at 4°C. Protein concentrations were quantified using Bicinchonic acid protein assay reagent (Pierce, Rockford, IL). Cell extracts were mixed with zymography sample buffer (63 mM Tris HCl, 10% glycerol, 2% SDS, 0.0025% bromophenol blue, pH 6.8) without heat denaturation. Culture medium of HT1080 cells was used for the molecular weight markers for MMP. Electrophoresis was performed on 10% SDS polyacrylamide gels containing 0.1% gelatin at 125 V. After electrophoresis, the gels were incubated in a renaturing buffer of 0.25% Triton X-100 for 30 min at room temperature, and equilibrated in developing buffer (50 mM Tris base, 40 mM HCl, 200 mM NaCl, 5 mM CaCl2, 0.2% Brij-35) for 30 min at room temperature. The gels were then incubated in developing buffer overnight at 37°C. The gels were stained with 0.1% Coomassie Blue R-250 and destained with 10% acetic acid in 40% methanol.
Statistics
Unless otherwise indicated, all values are expressed as mean ± SD. All data were analyzed by the unpaired Student’s t-test for statistically significant differences between groups.
Results
Effects of Ob-X on 3T3-L1 differentiation
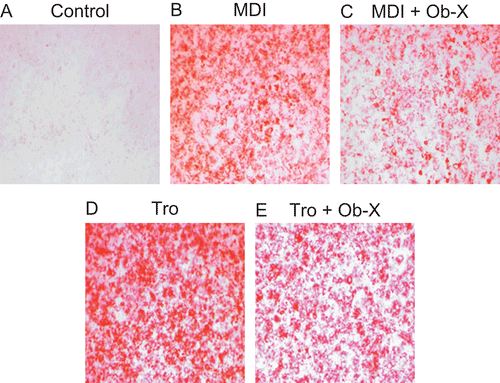
Accumulation of triglyceride droplets in 3T3-L1 cells was evident on the sixth day following 2 days of treatment with MDI differentiation mix (MDI) () or the peroxisome proliferator-activated receptor γ (PPARγ) agonist troglitazone () compared with non-differentiated control (), as shown by positive staining with Oil-red O. Treatment of cells with Ob-X, however, inhibited triglyceride accumulation; the percentage of differentiated cells was ~63% in MDI plus Ob-X-treated cells () and 69% in troglitazone plus Ob-X-treated cells () compared with cells treated with MDI or troglitazone alone, respectively. These results suggest that Ob-X effectively inhibits adipogenesis in 3T3-L1 cells.
Figure 1. Effects of Ob-X on triglyceride accumulation in 3T3-L1 cells. 3T3-L1 preadipocytes were differentiated into mature adipocytes as described in “Materials and Methods”. 3T3-L1 cells were treated with monocyte differentiation-inducing (MDI) differentiation mix (MDI), MDI plus 10 µg/mL Ob-X, 10 µM troglitazone (Tro), or 10 µM Tro plus 10 µg/mL Ob-X. At day 6 post-induction, cells were fixed and stained for neutral lipids with Oil-red O.
Effects of Ob-X on mRNA expression of genes involved in lipogenic pathways
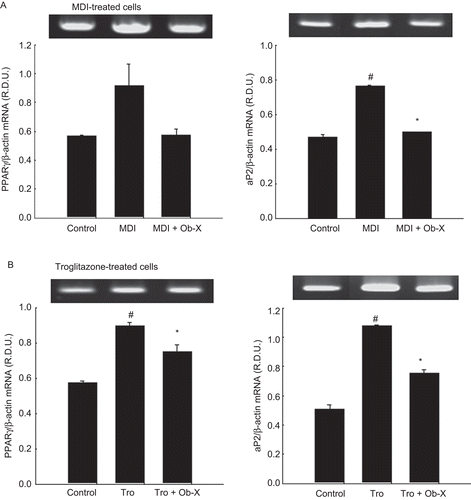
To quantify the changes in differentiation degree by Ob-X, we analyzed mRNA expression of genes involved in adipogenic and lipogenic pathways. PPARγ and adipocyte fatty acid binding protein (aP2) mRNA levels were substantially upregulated by 61 and 62% at day 6 following MDI treatment compared with non-differentiated controls. Importantly, co-administration of Ob-X significantly downregulated MDI-induced PPARγ and aP2 mRNA levels by 34 and 37%, respectively (). Similarly, troglitazone treatment increased PPARγ and aP2 mRNA levels by 112 and 56% compared with non-differentiated controls, whereas Ob-X significantly decreased troglitazone-induced PPARγ and aP2 mRNA levels by 30 and 16%, respectively (). Thus, Ob-X inhibited MDI- or troglitazone-induced expression of genes involved in adipocyte differentiation.
Figure 2. Effects of Ob-X on mRNA expression of adipose-specific genes in 3T3-L1 cells. 3T3-L1 preadipocytes were differentiated into mature adipocytes as described in “Materials and Methods”. 3T3-L1 cells were treated with monocyte differentiation-inducing (MDI) differentiation mix (MDI), MDI plus 10 µg/mL Ob-X, 10 µM troglitazone (Tro), or 10 µM Tro plus 10 µg/mL Ob-X, and the effects of Ob-X on (A) MDI- or (B) troglitazone-induced expression of adipocyte-specific genes were investigated. Total cellular RNA was extracted from differentiated cells on day 6, and mRNA levels of peroxisome proliferator-activated receptor γ, aP2, and β-actin were measured using reverse transcription-polymerase chain reaction (RT-PCR). All values are expressed as the mean ± SD. Insets show representative RT-PCR bands used for quantitation. *Significantly different versus MDI or Tro, respectively, P < 0.05; #significantly different versus control, P < 0.05.
Effects of Ob-X on mRNA expression of angiogenic factors
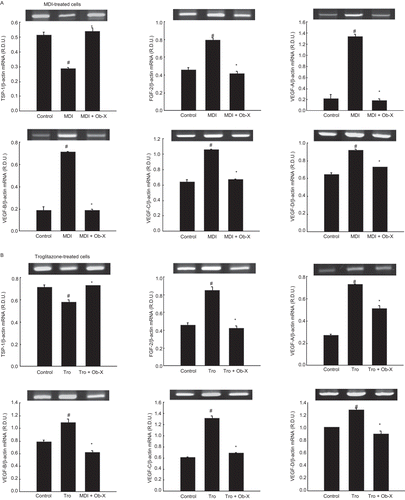
The effects of Ob-X on the expression patterns of genes involved in angiogenesis were investigated in 3T3-L1 cells. The mRNA expression of angiogenic factors was downregulated, and anti-angiogenic factors were upregulated, in Ob-X-treated cells compared with non-differentiated control cells. After MDI treatment, the mRNA levels of angiogenic factors VEGF-A, -B, -C, -D, and FGF-2 were elevated by 516, 275, 65, 42, and 72%, respectively (). However, Ob-X treatment reduced the mRNA levels of VEGF-A, -B, -C, -D, and FGF-2 by 86, 73, 36, 20, and 47%, respectively, in MDI-treated cells. Ob-X also decreased these levels by 29, 43, 47, 29, and 50%, respectively, in troglitazone-treated cells (). In contrast, the mRNA level of the anti-angiogenic molecule thrombospondin-1 (TSP-1) was elevated by 86% in MDI-treated cells, and by 25% in troglitazone-treated cells.
Figure 3. Effects of Ob-X on mRNA expression of genes involved in angiogenesis in 3T3-L1 cells. 3T3-L1 preadipocytes were differentiated into mature adipocytes as described in “Materials and Methods”. 3T3-L1 cells were treated with monocyte differentiation-inducing (MDI) differentiation mix (MDI), MDI plus 10 µg/mL Ob-X, 10 µM troglitazone (Tro), or 10 µM Tro plus 10 µg/mL Ob-X, and the effects of Ob-X on (A) MDI- or (B) troglitazone-induced expression of genes involved in angiogenesis were investigated. Total cellular RNA was extracted from differentiated cells on day 6, and mRNA levels of VEGF-A, -B, -C, and -D, thrombospondin-1 (TSP-1), and β-actin were measured using reverse transcription-polymerase chain reaction (RT-PCR). All values are expressed as the mean ± SD. Insets show representative RT-PCR bands used for quantitation. *Significantly different versus MDI or Tro, respectively, P < 0.05; #significantly different versus control, P < 0.05.
Effects of Ob-X on mRNA expression of MMPs
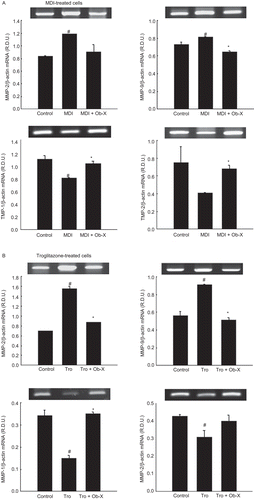
During differentiation of 3T3-L1 cells, MMP-2 and MMP-9 mRNA expression was stimulated by 42 and 10%, respectively, following MDI treatment (), and 125 and 62%, respectively, following troglitazone treatment (). In contrast, Ob-X increased tissue inhibitor of metalloproteinase-1 (TIMP-1) and TIMP-2 mRNA levels by 28 and 65%, respectively, in MDI-treated cells and by 136 and 29%, respectively, in troglitazone-treated cells.
Figure 4. Effects of Ob-X on mRNA expression of matrix metalloproteinases (MMPs) and their inhibitors in 3T3-L1 cells. 3T3-L1 preadipocytes were differentiated into mature adipocytes as described in “Materials and Methods”. 3T3-L1 cells were treated with monocyte differentiation-inducing (MDI) differentiation mix (MDI), MDI plus 10 µg/mL Ob-X, 10 µM troglitazone (Tro), or 10 µM Tro plus 10 µg/mL Ob-X, and the effects of Ob-X on (A) MDI- or (B) troglitazone-induced expression of MMPs and their inhibitors were investigated. Total cellular RNA was extracted from differentiated cells on day 6, and mRNA levels of MMP-2, MMP-9, tissue inhibitor of metalloproteinase-1 (TIMP-1), TIMP-2, and β-actin were measured using reverse transcription-polymerase chain reaction (RT-PCR). All values are expressed as the mean ± SD. Insets show representative RT-PCR bands used for quantitation. *Significantly different versus MDI or Tro, respectively, P < 0.05; #significantly different versus control, P < 0.05.
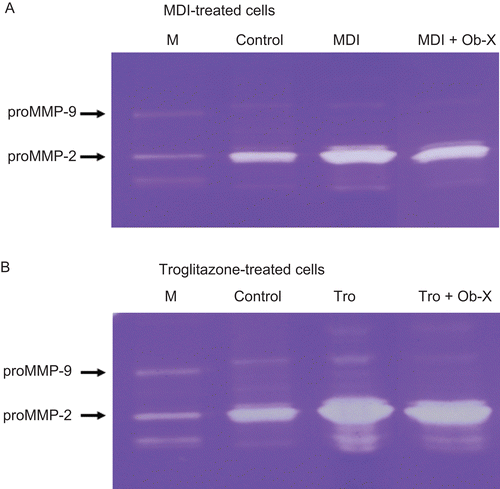
Effects of Ob-X on MMP activity
MMP activity was examined using zymography on gelatin-containing gels. Gelatin zymography revealed that the activity of proMMP-2 and proMMP-9 was significantly reduced in Ob-X-treated cells. Ob-X treatment reduced the proMMP-2 and proMMP-9 activities in MDI-treated cells by 26 and 61%, respectively (). Ob-X also decreased the proMMP-2 and proMMP-9 activities in troglitazone-treated cells by 8 and 50%, respectively ().
Figure 5. Zymographic analysis of 3T3-L1 cells. (A) Extracts from 3T3-L1 cells treated with monocyte differentiation-inducing (MDI) differentiation mix (MDI) or MDI plus 10 μg/mL Ob-X were applied to a gelatin-containing gel. (B) Extracts from 3T3-L1 cells treated with 10 µM troglitazone (Tro) or 10 µM Tro plus 10 µg/mL Ob-X were applied to a gelatin-containing gel. Gelatinolytic activity was measured by zymography. M is the molecular weight marker for matrix metalloproteinases.
Discussion
The development of fat cells from preadipocytes, or adipogenesis, includes morphological changes, expression of many lipogenic enzymes, and extensive lipid accumulation (CitationRosen and Spiegelman, 2000), contributing to the growth and expansion of adipose tissue. Because our previous study demonstrated that Ob-X, a mixture of three herbs with anti-angiogenic and MMP-inhibiting properties, reduced adipose tissue mass (CitationKim et al., 2010; Yoon and Kim, accepted), this study was undertaken to investigate whether Ob-X inhibits adipogenesis and to determine the expression patterns of genes involved in lipogenesis, angiogenesis, and MMPs using an in vitro cell culture system.
We clearly demonstrate that Ob-X is capable of suppressing adipogenesis and adipocyte-specific gene expression. In early stage development of adipose tissue, angiogenesis is tightly associated with angiogenesis, and EC proliferation occurs in expanding adult adipose tissue (CitationCrandall et al., 1997; CitationLijnen, 2008). MMP inhibitors are also known to block adipocyte conversion only when present in the cell culture medium during the initial stages of the differentiation program (CitationCroissandeau et al., 2002), suggesting that this is when angiogenesis and MMP activities play a critical role. Based on these previous results, we treated cells with Ob-X for the first 2 of 6 days. As expected, MDI or troglitazone increased accumulation of triglyceride droplets in 3T3-L1 cells compared with undifferentiated cells. However, Ob-X treatment prevented this MDI- or troglitazone-induced lipid accumulation, indicating that Ob-X has an inhibitory effect on troglitazone- or MDI-induced adipogenesis.
Adipogenesis is initiated by the production of the key transcription factor PPARγ, which is responsible for inducing the expression of adipocyte-specific genes (CitationRosen et al., 1999). Consistent with the effects of Ob-X on lipid accumulation, Ob-X decreased the expression of PPARγ and the PPARγ target gene aP2, which are directly implicated in lipogenic pathways in 3T3-L1 adipocytes. Our results are supported by the finding that blocking differentiation in 3T3-L1 cells is associated with diminished PPARγ expression (CitationCroissandeau et al., 2002). MMP inhibitors are not thought to affect the mitotic clonal expansion, but inhibit adipocyte differentiation through diminished expression of PPARγ (CitationCroissandeau et al., 2002). PPARγ expression is controlled by members of the CCAAT/enhancer-binding protein (C/EBP) (CitationWu et al., 1996) and sterol regulatory element binding protein (CitationFajas et al., 1999) families. In this process, MMP inhibitors decrease the DNA binding capacity of C/EBPβ to its recognition element in the PPARγ gene promoter, resulting in the impairment of PPARγ transcription (CitationCroissandeau et al., 2002). Collectively, angiogenesis and MMP inhibitors are capable of blocking adipose conversion at the early stages of differentiation when C/EBPβ induction is maximal. Thus, Ob-X seems to be an effective angiogenesis and MMP inhibitor to inhibit adipocyte differentiation. Interestingly, inhibition of adipocyte differentiation by overexpression of a dominant-negative PPARg construct leads to impaired development of both adipose tissue and angiogenesis (CitationFukumura et al., 2003).
Growing adipocytes produce multiple angiogenic factors and their inhibitors that regulate adipose angiogenesis. Angiogenic factors such as VEGF-A, -B, -C, and−D, as well as FGF-2, promote the proliferation, differentiation, and migration of ECs within fat and enhance adipocyte differentiation in vivo (CitationCarmeliet et al., 1996; CitationBikfalvi et al., 1997; CitationKawaguchi et al., 1998) whereas TSP-1 inhibits angiogenesis in vivo and impairs migration and proliferation of cultured microvascular ECs (CitationArmstrong and Bornstein, 2003). Blockage of the VEGF receptor 2 (VEGFR2) signaling system by a neutralizing antibody inhibits both angiogenesis and preadipocyte differentiation, suggesting that VEGFs act on ECs to regulate preadipocyte differentiation (CitationFukumura et al., 2003). In addition, angiogenesis inhibitors, such as TNP-470 and VEGFR2-specific inhibitors, have been shown to prevent the development of obesity in genetic mouse models and studies based on high-fat diets (CitationRupnick et al., 2002; CitationFukumura et al., 2003; CitationBråkenhielm et al., 2004; CitationTam et al., 2009). In our present study, Ob-X decreased the mRNA expression of four kinds of VEGFs and FGF-2 that were increased in differentiated 3T3-L1 cells compared with non-differentiated cells. Moreover, Ob-X treatment the increased mRNA level of the anti-angiogenic TSP-1 in 3T3-L1 cells, suggesting that the anti-angiogenic agent Ob-X reduces adipogenesis and can be used for treatment of obesity.
Adipocytes also produce MMPs and MMP inhibitors that are differentially expressed in adipose tissue during obesity in murine obesity models (CitationMaquoi et al., 2002; CitationChavey et al., 2003; CitationVoros et al., 2005). Furthermore, the secretion of MMP-2 and MMP-9 increases during adipocyte differentiation in both human adipocytes and mouse preadipocyte cell lines (CitationBouloumié et al., 2001; CitationMaquoi et al., 2002; CitationChavey et al., 2003), suggesting that MMP-2 and MMP-9 are important for adipocyte conversion. Our reverse transcription-PCR analysis showed that Ob-X decreased MMP-2 and MMP-9 mRNA levels, but increased TIMP-1 and TIMP-2, indicating that Ob-X exerts a specific regulatory effect on genes involved in angiogenesis and the MMP system in 3T3-L1 cells. Several studies demonstrated that MMPs have a novel function in adipogenesis, modulating adipocyte differentiation independent of angiogenesis and therefore, MMP inhibitors can block the adipocyte differentiation process (CitationBouloumié et al., 2001; CitationCroissandeau et al., 2002; CitationMaquoi et al., 2002; CitationChavey et al., 2003). Treatment with MMP inhibitors impairs adipose tissue development in mice fed a high-fat diet (CitationLijnen et al., 2002). Consistent with the inhibitory effects of Ob-X on the mRNA expression of MMP-2 and MMP-9, zymographic analysis revealed that Ob-X suppressed MMP-2 and MMP-9 gelatinolytic activities, since proMMP-2 and proMMP-9 activities were markedly reduced in Ob-X-treated 3T3-L1 cells. It has also been reported that in situ zymography with gelatin-containing gels on cryosections of adipose tissue confirmed lower MMP activity in tissues of the MMP inhibitor galardin-treated animals. Our present results indicate that the reduction of adipogenesis by Ob-X may be due to its anti-angiogenic and MMP-inhibiting actions.
These studies demonstrate that Ob-X, which inhibits angiogenesis and MMP activity, suppresses adipogenesis in 3T3-L1 adipocytes. These events may be mediated by changes in the expression of genes involved in lipogenesis, angiogenesis, and the MMP system. Thus, by reducing adipogenesis, anti-angiogenic Ob-X provides a possible therapeutic approach for the prevention and treatment of human obesity and its related disorders.
Declaration of interest
This work was supported by Biomedical Program (No. 70007823) through CCLIO grant funded by the MKE and Mid-career Researcher Program (No. 2009-0083990) and Female Scientist Program (No. 2010-0017313) through NRF grant funded by the MEST, Korea.
References
- Armstrong LC, Bornstein P. (2003). Thrombospondins 1 and 2 function as inhibitors of angiogenesis. Matrix Biol, 22, 63–71.
- Bikfalvi A, Klein S, Pintucci G, Rifkin DB. (1997). Biological roles of fibroblast growth factor-2. Endocr Rev, 18, 26–45.
- Bouloumié A, Lolmède K, Sengenès C, Galitzky J, Lafontan M. (2002). Angiogenesis in adipose tissue. Ann Endocrinol (Paris), 63, 91–95.
- Bouloumié A, Sengenès C, Portolan G, Galitzky J, Lafontan M. (2001). Adipocyte produces matrix metalloproteinases 2 and 9: involvement in adipose differentiation. Diabetes, 50, 2080–2086.
- Bråkenhielm E, Cao R, Gao B, Angelin B, Cannon B, Parini P, Cao Y. (2004). Angiogenesis inhibitor, TNP-470, prevents diet-induced and genetic obesity in mice. Circ Res, 94, 1579–1588.
- Cao Y. (2007). Angiogenesis modulates adipogenesis and obesity. J Clin Invest, 117, 2362–2368.
- Cao Y. (2010). Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat Rev Drug Discov, 9, 107–115.
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. (1996). Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature, 380, 435–439.
- Chavey C, Mari B, Monthouel MN, Bonnafous S, Anglard P, Van Obberghen E, Tartare-Deckert S. (2003). Matrix metalloproteinases are differentially expressed in adipose tissue during obesity and modulate adipocyte differentiation. J Biol Chem, 278, 11888–11896.
- Christiaens V, Lijnen HR. (2006). Role of the fibrinolytic and matrix metalloproteinase systems in development of adipose tissue. Arch Physiol Biochem, 112, 254–259.
- Claffey KP, Wilkison WO, Spiegelman BM. (1992). Vascular endothelial growth factor. Regulation by cell differentiation and activated second messenger pathways. J Biol Chem, 267, 16317–16322.
- Couillard C, Mauriège P, Imbeault P, Prud’homme D, Nadeau A, Tremblay A, Bouchard C, Després JP. (2000). Hyperleptinemia is more closely associated with adipose cell hypertrophy than with adipose tissue hyperplasia. Int J Obes Relat Metab Disord, 24, 782–788.
- Crandall DL, Hausman GJ, Kral JG. (1997). A review of the microcirculation of adipose tissue: Anatomic, metabolic, and angiogenic perspectives. Microcirculation, 4, 211–232.
- Croissandeau G, Chrétien M, Mbikay M. (2002). Involvement of matrix metalloproteinases in the adipose conversion of 3T3-L1 preadipocytes. Biochem J, 364, 739–746.
- Fajas L, Schoonjans K, Gelman L, Kim JB, Najib J, Martin G, Fruchart JC, Briggs M, Spiegelman BM, Auwerx J. (1999). Regulation of peroxisome proliferator-activated receptor gamma expression by adipocyte differentiation and determination factor 1/sterol regulatory element binding protein 1: implications for adipocyte differentiation and metabolism. Mol Cell Biol, 19, 5495–5503.
- Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, Chatterjee K, Garkavtsev I, Jain RK. (2003). Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res, 93, e88–e97.
- Galardy RE, Grobelny D, Foellmer HG, Fernandez LA. (1994). Inhibition of angiogenesis by the matrix metalloprotease inhibitor N-[2R-2-(hydroxamidocarbonymethyl)-4-methylpentanoyl]-L-tryptophan methylamide. Cancer Res, 54, 4715–4718.
- Kawaguchi N, Toriyama K, Nicodemou-Lena E, Inou K, Torii S, Kitagawa Y. (1998). De novo adipogenesis in mice at the site of injection of basement membrane and basic fibroblast growth factor. Proc Natl Acad Sci USA, 95, 1062–1066.
- Kim MY, Park BY, Lee HS, Park EK, Hahm JC, Lee J, Hong Y, Choi S, Park D, Lee H, Yoon M. (2010). The anti-angiogenic herbal composition Ob-X inhibits adipose tissue growth in obese mice. Int J Obes (Lond), 34, 820–830.
- Kim J, Park BY, Park EK, Lee HS, Halm JC, Bae KH, Kim MY. (2006): Screening of anti-angiogenic activity from plant extracts. Kor J Pharmacogn, 37, 253–257.
- Lee J, Chae K, Ha J, Park BY, Lee HS, Jeong S, Kim MY, Yoon M. (2008). Regulation of obesity and lipid disorders by herbal extracts from Morus alba, Melissa officinalis, and Artemisia capillaris in high-fat diet-induced obese mice. J Ethnopharmacol, 115, 263–270.
- Lijnen HR. (2008). Angiogenesis and obesity. Cardiovasc Res, 78, 286–293.
- Lijnen HR, Maquoi E, Hansen LB, Van Hoef B, Frederix L, Collen D. (2002). Matrix metalloproteinase inhibition impairs adipose tissue development in mice. Arterioscler Thromb Vasc Biol, 22, 374–379.
- Maquoi E, Munaut C, Colige A, Collen D, Lijnen HR. (2002). Modulation of adipose tissue expression of murine matrix metalloproteinases and their tissue inhibitors with obesity. Diabetes, 51, 1093–1101.
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. (1999). PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell, 4, 611–617.
- Rosen ED, Spiegelman BM. (2000). Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol, 16, 145–171.
- Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. (2002). Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci USA, 99, 10730–10735.
- Sang QX. (1998). Complex role of matrix metalloproteinases in angiogenesis. Cell Res, 8, 171–177.
- Silverman KJ, Lund DP, Zetter BR, Lainey LL, Shahood JA, Freiman DG, Folkman J, Barger AC. (1988). Angiogenic activity of adipose tissue. Biochem Biophys Res Commun, 153, 347–352.
- Tam J, Duda DG, Perentes JY, Quadri RS, Fukumura D, Jain RK. (2009). Blockade of VEGFR2 and not VEGFR1 can limit diet-induced fat tissue expansion: role of local versus bone marrow-derived endothelial cells. Plos ONE, 4, e4974.
- Voros G, Maquoi E, Demeulemeester D, Clerx N, Collen D, Lijnen HR. (2005). Modulation of angiogenesis during adipose tissue development in murine models of obesity. Endocrinology, 146, 4545–4554.
- Wu Z, Bucher NL, Farmer SR. (1996). Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol Cell Biol, 16, 4128–4136.
- Yoon M, Kim MY (2010): The anti-angiogenic herbal composition Ob-X from Morus alba, Melissa officinalis, and Artemisia capillaris regulates obesity in genetically obese ob/ob mice. Pharm Biol (accepted)