Abstract
Context: Light microscopy is in most cases a quick method for the identification and discrimination of medicinally used plant drugs; moreover, this technique is very inexpensive. Reliable descriptions of the anatomy of plants and their adulterations are prerequisites for necessary purity controls.
Objective: The anatomy of the subterranean organs of 18 pharmaceutically useful as well as related but inconsiderable Asteraceae species from nine genera (Taraxacum F. H. Wigg., Leontodon L., Scorzoneroides Moench, Hypochaeris L., Crepis L., Aposeris Neck., Cichorium L., Scorzonera L., and Tragopogon L.; tribe Cichorieae, Asteraceae) is described in detail and graphically illustrated. Features characterizing and discriminating the studied taxa are presented and discussed.
Materials and methods: The roots/rhizomes of various species were examined by means of light microscopy.
Results: Useful anatomical characters were found for the discrimination between the species, and some of them were examined for the first time.
Discussion and conclusion: Discrimination of most genera and species investigated was possibly based on the anatomy of their underground parts. The identified characters may be effectively used for quality control of commercial drugs and the identification of adulterations.
Keywords::
Introduction
The Asteraceae, the sunflower family, comprise about 23,000 species and 1600 genera (CitationJeffrey, 2007), many of which have long been used for medicinal purposes. Taraxacum F. H. Wigg. sp., Scorzonera L. sp., and Cichorium intybus L. (Asteraceae) are few examples out of the existing diversity with a long tradition in conventional and traditional medicine all over the world.
The genus Taraxacum is a widespread taxon, first mentioned against ailments of the eyes in the 13th century (CitationMadaus, 1938), and its species have been used for diverse cures since then without any special differentiation. Until now, Taraxacum is popular for its effects as an amarum and cholereticum against absence of appetite and disorders of the gastrointestinal tract (CitationFrohne, 2006). Taraxacum is also famous for its use in slimming diets and spring makeovers due to its diuretic effects and against diabetes (CitationGerlach et al., 2006; CitationŠarić-Kundalić et al., 2010). The herb and the roots are established not only in traditional but also in conventional medicine, for example, compound of species cholagogae (CitationOEAB, 2009) and various preparations against diseases of liver and gall (CitationLänger & Kubelka, 2001).
C. intybus is an ancient medical plant. Dioskurides (according to CitationBerendes, 1902) mentioned it to be an astringent and good for the stomach. CitationMattioli (1563) applied a kind of syrup of the roots against liver problems and packs of leafs and roots in combination with rose water against skin diseases. The roots also served as a substitute for coffee. Nowadays, the roots are mainly used for dyspeptically disorders and are ingredients of various teas (CitationFrohne, 2006).
Scorzonera hispanica L. is mainly known as a vegetable used for salads or as a side dish. Like C. intybus, its roots are also served as a kind of coffee. Until the 16th century, S. hispanica was considered efficacious against snake bites (CitationDiemair, 1945; CitationGenaust, 1996). A study about 10 years ago (CitationJantzen, 1999) confirmed the potency of this plant against hemorrhages caused by snake venoms. Investigations among people these days showed that S. hispanica is still being used in traditional medicine against disorders of stomach, gall bladder, and liver, and against adenophyma and is compiled in the so-called VOLKSMED database (CitationGerlach et al., 2006).
Both modern and traditional medicine use plant organs for the preparation of drugs, usually as a cut formulation or as a powder. In praxis, the identification of drugs and required purity tests are mainly done by microscopic means based on discriminative anatomical features. Hence, proper knowledge on the anatomy of the diverse parts of the plants used as drugs is required for drug analysis.
However, respective comparative studies on subterranean organs of pharmaceutically exploited Asteraceae species and their close relatives (occurring as adulterations) are rare. Some works have been done on resin ducts and cavities as well as on laticiferous vessels of diverse Asteraceae (CitationVan Tieghem, 1883, Citation1884; CitationVan Vuillemin, 1884; CitationCol, 1903, Citation1904) in the second half of the 19th century. Most available studies though dealt with the aerial vegetative organs. A few recent results concern the anatomy, particularly the secretory structures, of roots and rhizomes (CitationRagonese, 1988; CitationMelo-de-Pinna & Menezes, 2003; CitationAppezzato-da-Gloria et al., 2008), although they do not cover species interesting in the course of medicine and growing in Western Europe.
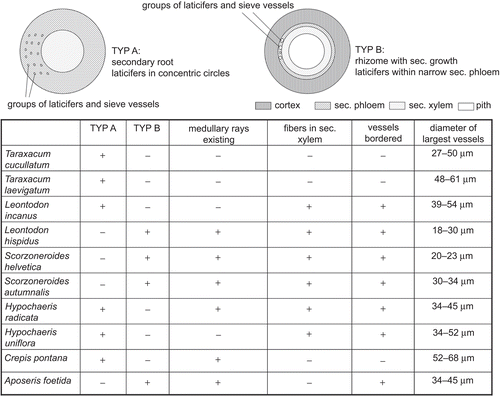
Comparative studies on the root anatomy of the Cichorieae are available so far for Taraxacum species and the genera Hypochaeris L., Leontodon L., and Aposeris Neck. (CitationLänger, 1990). Taraxacum, for instance, could be discriminated from the other genera by the lack of libriform fibers and of bordered vessels, the presence of laticifers arranged in concentric circles, and the diameter of the vessels. However, these characters may discriminate among the chosen restricted set of taxa only, but might fail if further species are added. In addition, intraspecific and even intraindividual variation has to be carefully considered in order to identify reliable taxonomically informative characters. Depending on the position along the root axis, the dimensions of vessels, for instance, may differ largely within one plant (CitationFritz & Saukel, 2011). Hence, standardization of the part of an analyzed root, as well as selection of a representative taxonomic sample, is an urgent requirement in comparative anatomical studies.
In this study, we will analyze the anatomical structure of the subterranean plant parts of nine Asteraceae genera (Taraxacum, Leontodon, Scorzoneroides Moench, Hypochaeris, Crepis L., Aposeris, Cichorium, Scorzonera, and Tragopogon L.) from the tribe Cichorieae (Asteraceae). The anatomy of roots and rhizomes will be analyzed in detail by means of light microscopy and a database of identified anatomical features created. Based on these results, we will try to extract characters useful for the discrimination of the analyzed species. This will involve pharmaceutically useful plants as well as inconsiderable relatives.
Materials and methods
Plant material
The plant material used for examination included 19 species from the Cichorieae occurring naturally in Austria as listed in (plants arranged according to classification in CitationFischer et al., 2008). Taxa were chosen, respectively, to their use in medicine (e.g., Taraxacum sp., C. intybus) and their role as possible adulteration. Fully development roots were collected during or following antheses.
Table 1. List of species examined in this study (plants arranged according to the classification applied by CitationFischer et al., 2008).
Vouchers are deposited in the herbarium of the Department of Pharmacognosy, University of Vienna (WUP). The plant material was identified by the authors using floristic manuals covering the sampled geographic areas (CitationPawłowskiego & Jasiewicza 1972; CitationSzafer et al., 1976; CitationFischer et al., 2008). As the anatomy may vary with environmental conditions (e.g., soil parameters) and geography, species were collected from three to six localities if possible.
Anatomical analysis
The samples were examined by means of light microscopy. For preparation, based on a traditional method of the University of Vienna, the desiccated roots were boiled in water for 10 min to soften the tissue and subsequently inundated in 96% ethanol for dehydration. After evaporating the alcohol for a few minutes, transverse and longitudinal sections were obtained by free hand sectioning about 1.5 cm below the hypocotyle as this position proofed to provide the most information about the root anatomy. Sections obtained by hand cutting were embedded in few drops of chloral hydrate (60% in water) and examined using a Nikon Optiphot-2 light microscope equipped with a Samsung Digimax V50 Digital Camera.
Results
Taraxacum sp.
In line with the results of CitationLänger (1990), the studied Taraxacum species, Taraxacum cucullatum Dahlst. and Taraxacum laevigatum DC., were indistinguishable from each other based on root anatomy. The following description applies to both taxa: the plants possess an allorhizous root system with a taproot ().
Figure 1. Taproot of (A) T. cucullatum and (B) T. laevigatum. (C) Schematic view of Taraxacum sp. secondary root in transverse section: small ellipses mark the position of the laticifers within the dominating secondary phloem (gray), secondary xylem with vessels dispersed; medullary rays are not found.
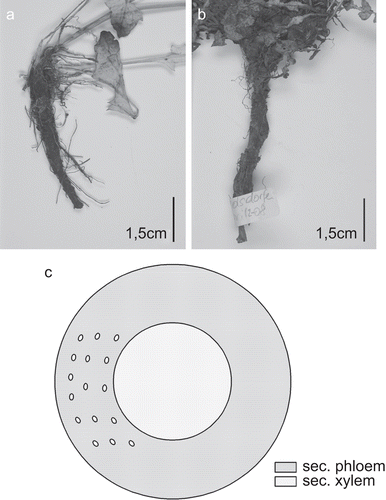
Microscopical description of the secondary root ( and ): The root develops a thin-walled phellem, the cortex gets lost due to the secondary growth and the formation of rhytidome. Within the secondary phloem, which is dominating in extension, laticiferous vessels are arranged in concentric circles. Phloem fibers are absent. The secondary xylem comprises tracheary elements and xylem parenchyma only, with reticulate vessels (in diameter up to 50 µm in T. cucullatum and 61 µm in T. laevigatum) irregularly arranged. Medullary rays are not found. No secretory structures besides the laticifers are differentiated, crystalloids and sclereids are absent.
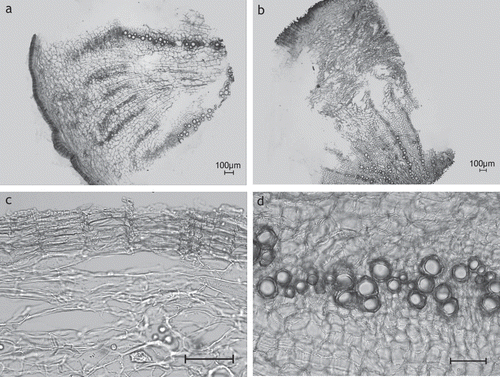
Figure 2. Taraxacum roots. (A) Overview showing the extension and arrangement of the diverse tissues: cortex lost due to formation of rhytidome, secondary phloem dominating, laticiferous vessels within secondary phloem circularly arranged, medullary rays in secondary xylem missing; (B) secondary phloem with laticiferous vessels arranged in concentric circles; (C) secondary xylem with vessels irregularly dispersed over transverse section, fibers missing; (D) reticulate vessels of secondary xylem [(A, C) T. laevigatum (TL01-08), (B, D) T. cucullatum (TC03-08)]; (A–C) transverse sections; (D) longitudinal section; scale bars = 50 µm.
![Figure 2. Taraxacum roots. (A) Overview showing the extension and arrangement of the diverse tissues: cortex lost due to formation of rhytidome, secondary phloem dominating, laticiferous vessels within secondary phloem circularly arranged, medullary rays in secondary xylem missing; (B) secondary phloem with laticiferous vessels arranged in concentric circles; (C) secondary xylem with vessels irregularly dispersed over transverse section, fibers missing; (D) reticulate vessels of secondary xylem [(A, C) T. laevigatum (TL01-08), (B, D) T. cucullatum (TC03-08)]; (A–C) transverse sections; (D) longitudinal section; scale bars = 50 µm.](/cms/asset/32547c91-0c15-4915-9b0c-cacb54cc95af/iphb_a_548390_f0002_b.gif)
The analysis of various roots and rhizomes of Taraxacum sp. and related taxa (Leontodon sp., Scorzoneroides sp., Hypochaeris sp., Crepis pontana Druce, Aposeris foetida Cass.) showed the following anatomical features to be the most important to be used in their identification and differentiation: the overall structure of the vascular cylinder, with the distribution of vessels, and the presence or absence of medullary rays, the occurrence of fibers in the secondary xylem, and the types of perforation plates in the vessels ().
The diameter of the largest vessels cannot serve as a useable feature, as the ranges of the diverse species mainly overlap. Besides, our studies confirm the importance to define a point of the root where the cross section for the measurements is to be taken as the diameter of the vessels may vary greatly even within a single plant (CitationFritz & Saukel, 2011), for example, samples of Hypochaeris radicata showed a great variability with 34 µm 1.5 cm below the hypocotyle, 72 µm at the tip of the root. The numbers given in were all derived from sections beneath the hypocotyle as mentioned in “Material and Methods.”
Scorzonera hispanica
S. hispanica develops a long rhizome, presumably with taproot ().
Figure 4. (A) Rhizome of S. hispanica. (B) Schematic section of rhizome: small ellipses mark the position of the laticifers; vascular cylinder with vessels (dots) arranged in single or multiple radial rows.
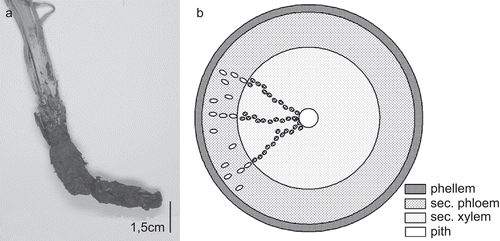
Microscopical description of the secondary root ( and ): The cortex gets lost in course of the formation of rhytidome; the emerging phellem is thin-walled and thoroughly parallel-laminated, sometimes with crystalloids. The layer of secondary phloem is dominant, of similar extension as the vascular cylinder, with laticiferous vessels arranged in rows in fascicular position in continuation of the vessels of the secondary xylem as well as in interfascicular position, also irregularly dispersed. Outer parts of the secondary phloem often appear obliterated and collapsed. No phloem fibers were found. The secondary xylem is dominated by parenchymatous cells, and the vessels (reticulate, up to 60 µm in diameter) are aggregated into single or double radial rows, separated by wide parenchymatous rays. The pith is often collapsed. Xylem fibers and sclereids are absent.
Figure 5. S. hispanica rhizome. (A, B) Overview showing the extension and arrangement of tissues: secondary phloem with laticiferous vessels arranged in rows, fibers are missing (A), secondary xylem dominated by parenchymatous cells with fibers missing, vessels arranged in single to multiple rows (B) (SHI03); (C) phellem thin-walled, thoroughly parallel-laminated (SHI02); (D) vessels of secondary xylem more or less in single or double rows (SHI02); (A–D) transverse sections; scale bars = 50 µm.
The root anatomy of the other examined species of the genus Scorzonera (Scorzonera humilis L., Scorzonera purpurea L., Scorzonera aristata Ramond ex DC., Scorzonera rosea Waldst. & Kit.) with the exception of Scorzonera austriaca Willd. showed to be similar to S. hispanica apart from the structure of the phellem (either thin-walled as within S. purpurea, S. humilis (additional sclereids) or thick-walled as within S. aristata, S. rosea).
S. austriaca represented considerably different anatomical structure from all other taxa investigated due to its irregular secondary growth. Bundles of phloem and xylem separated by a cambium are irregularly dispersed over the transverse section, each undergoing secondary growth (illustrated in ).
Tragopogon orientalis L. and Tragopogon dubius Scop.
The following description applies to both examined species of the genus: The root system is allorhizous, and the species possess taproots.
Microscopical description of the secondary root: A periderm with a thin-walled phellem, sometimes containing crystalloids, replaces the rhizodermis. A distinct endodermis separates the small layer of the enduring cortex from the broad secondary cortex, whose extension is comparable with the vascular cylinder. Within the secondary phloem, laticiferous vessels are aggregated into rows in fascicular position “continuing” the formation of the vessels of the secondary xylem, and they may also be irregularly dispersed within the outer part of the phloem. The vessels of the secondary xylem are arranged in single and double rows, cumulative at the outer part, separated by broad, and often obliterated medullary rays. Mainly strongly bordered pits are present. The diameter of the tracheary elements may reach up to 68 µm. Fibers occur particularly within the outer part of the xylem, and sclereids are not found.
Cichorium intybus
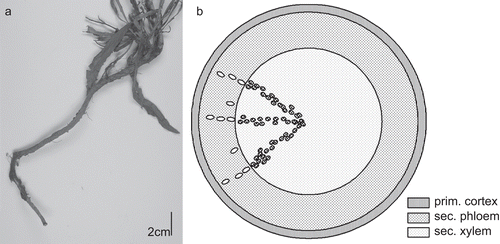
Typical for C. intybus is its long taproot ().
Figure 6. (A) Taproot of C. intybus. (B) Schematic view of C. intybus root in transverse section: secondary root: small ellipses mark the laticifers, dots within xylem are vessels.
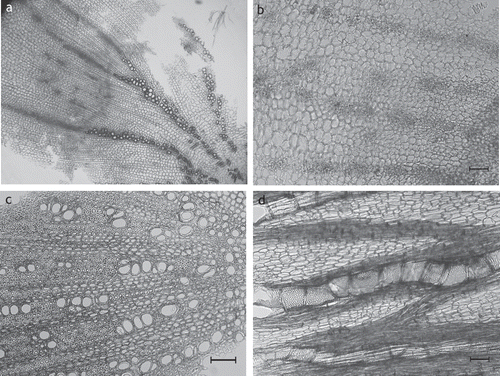
Microscopical description of the secondary root ( and ): On the surface of the root, a thin-walled phellem, rarely comprising crystalloids, develops. A small layer of the cortex remains despite secondary growth. The secondary phloem is dominant, comparable in extension to the vascular cylinder, with laticiferous vessels arranged in rows. Phloem fibers are missing. The secondary xylem contains fibers, and the vessels are arranged in rows or dispersed groups, short in longitudinal view, reticulate, mainly strongly bordered, up to 109 µm in diameter. The root lacks sclereids.
Figure 7. C. intybus root. (A) Overview showing the extension and arrangement of tissues: secondary phloem and secondary xylem are of similar extension (S5); (B) secondary phloem with lactiferous vessels arranged in rows (CI1-08); (C) secondary xylem with vessels in groups and short rows (CI1-08); (D) secondary xylem: vessels and rays (CI2-07); (A–C) transverse sections; (D) longitudinal section; scale bars = 50 µm.
Discussion
The studied Taraxacum species can be characterized as well as distinguished from related genera based on various anatomical root features—amongst others, the absence of medullary rays, the lack of fibers in the secondary xylem, and the types of perforation plates of the vessels (). Taraxacum is one of the few (along with A. foetida and C. pontana) plants with underground organs completely missing fibers. Furthermore, root vessels are of the reticulate type in Taraxacum, but bordered in all other studied taxa except for C. pontana.
The diameter of vessels cannot be used as reliable taxonomic characters as the size ranges of the taxa overlap. Furthermore, our studies confirmed a possibly great variability of the dimensions of the vessels even within one plant depending on the position along the root axis from which the sample is taken (CitationFritz & Saukel, 2011). Hence, measurements of vessels have to be carefully considered with regard to their pharmaceutical use for the identification of cut roots/rhizomes in which case a localization of a certain position within a root cannot be provided.
The root anatomy of S. hispanica was similar to that of the other species of the genus as well as to Tragopogon. However, the two genera can be distinguished based on the vessels. In Scorzonera, the vessels were reticulate, whereas Tragopogon mainly possessed strongly bordered vessels. Nevertheless, no distinction could be made between T. orientalis and T. dubius by means of light microscopy. In contrast, diverse species of the genus Scorzonera could be distinguished based on phellem characteristics ().
Figure 8. Differentiation of Scorzonera sp. and Tragopogon sp.; S. austriaca: transverse section treated with phloroglucine-HCL, lignified tracheids of the xylem parts red-colored.
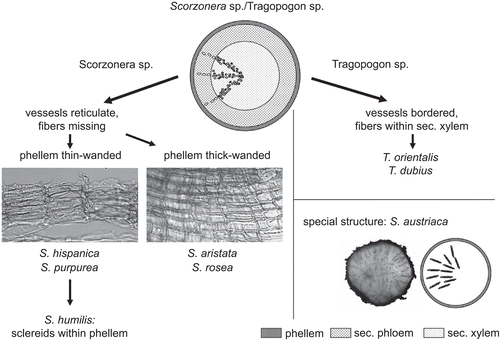
An exceptional case represents S. austriaca, whose anatomical structure differs clearly from all other species investigated due to its irregular secondary growth.
The root of C. intybus is anatomically indistinguishable from Tragopogon. As these species are morphologically largely distinct, adulterations can easily be identified based on above ground organs.
Conclusions
The identification and discrimination of used drugs by microscopical means is a well-known and often used method in pharmacognosy. According to the diverse anatomical characters identified in this study, the nine investigated genera could be distinguished based on root and rhizome anatomy. Taraxacum, as a pharmaceutically important plant, can thus be clearly discriminated from all other genera examined. A mix-up with other medicinally used species may easily be identified, as the root anatomy of C. intybus (differing in the arrangement of laticifers, the possession of libriform fibers) and of Scorzonera (differing in the formation of vascular cylinder and the phellem) is largely different.
A further discrimination of species within a single genus, however, may be restricted using root anatomy alone. Respective results differed, with Taraxacum species being indistinguishable, but members of the genera Scorzonera and Tragopogon could be differentiated using phellem and vessel characteristics, respectively.
Acknowledgement
We would like to thank our colleagues Christoph Dobeš and Valerie Klatte-Asselmeyer for their help with the collection of the plant material.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Appezzato-da-Gloria B, Hayashi AH, Cury G, Soares MKM, Rocha R. (2008). Occurrence of secretory structures in underground systems of seven Asteraceae species. Bot J Linn Soc 157:789–796.
- Berendes J. (1902). Des Pedanius Dioskurides aus Anazarbos Arzneimittellehre in fünf Büchern. Stuttgart: Ferdinand Enke Verlag.
- Col MA. (1903). Recherches sur l’appareil sécréteur interne des composées. J Bot (Desvaux) 17:252–318.
- Col MA. (1904). Recherches sur l’appareil sécréteur interne des composées (Suite). J Bot (Desvaux) 18:110–174.
- Diemair W. (1945). Die Haltbarmachung von Lebensmitteln. Stuttgart: Ferdinand Enke Verlag, p. 52.
- Fischer MA, Oswald K, Adler W. (2008). Exkursionsflora für Österreich, Liechtenstein und Südtirol, 3rd ed. Austria: OÖ Landesmuseen, Land Oberösterreich.
- Fritz E, Saukel J. (2011). Anatomy of subterranean organs of medicinally used Cardueae and related species and its value for discrimination. SciPharm. 79:157–174.
- Frohne D. (2006). Heilpflanzenlexikon. Stuttgart: Wissenschaftliche Verlagsgesellschaft mbH.
- Genaust H. (1996). Etymologisches Wörterbuch der botanischen Pflanzennamen, 3rd ed. Basel Boston Berlin: Birkhäuser.
- Gerlach S, Saukel J, Kubelka W. (2006). Pflanzen in der österreichischen Volksmedizin—die “Volksmed-Datenbank.” SciPharm 74 (Suppl. 1):36.
- Jantzen K. (1999). Chemische und biochemische Untersuchungen von Pflanzeninhaltsstoffen aus Calendula officinalis und Scorzonera hispanica und ihre Wirksamkeit gegen Schlangengifte. Ph.D. Thesis, University of Hannover.
- Jeffrey C. (2007). Compositae. In: Kubitzki K, ed. The Families and Genera of Vascular Plants. VIII Flowering Plants-Eudicots. Berlin, Heidelberg: Springer Verlag, pp. 61–77.
- Länger R. (1990). Orientierende Untersuchungen zur Wurzelanatomie einiger Cichorieaceen. SciPharm 58:417–422.
- Länger R, Kubelka W. (2001). Phytokodex. Pflanzliche Arzneispezialitäten in Österreich 2001/2002. Gablitz: Krause&Pachernegg GmbH.
- Madaus G. (1938). Lehrbuch der Biologischen Heilmittel. Leipzig: Olms Verlag.
- Mattioli PA. (1563). New Kreüterbuch. Prag: Georgen Melantrich von Auentin.
- Melo-de-Pinna G, Menezes N. (2003). Meristematic endodermis and secretory structures in adventitious roots of Richterago Kuntze (Mutisieae–Asteraceae). Rev Bras Bot 26:1–10.
- Oesterreichisches Arzneibuch (OEAB). (2009). Pharmacopoeia Austriaca. Wien: Verlag Österreich GmbH.
- Pawłowskiego B, Jasiewicza A. (1972). Flora Polska TOM XIII, Warszawa: Polska Akademia Nauk.
- Ragonese AM. (1988). Canales secretores en los organos vegetativos de Eupatorium inulaefolium H.B.K. (Compositae). Acta Farm Bonaerense 7:161–168.
- Šarić-Kundalić B, Dobeš Ch, Klatte-Asselmeyer V, Saukel J. (2010). Ethnobotanical study on medicinal use of wild and cultivated plants in middle, south and west Bosnia and Herzegovina. J Ethnopharmacol 131:33–55.
- Szafer W, Kulczyński S, Pawłowski B. (1976). Rośliny Polskie, 4th ed. Warszawa: Panstwowe Wydawnictwo Naukowe.
- Van Tieghem M. (1883). Sur la situation de l’appareil sécréteur dans les composées. B Soc Bot Fr 30:310–313.
- Van Tieghem M. (1884). Sur la situation de l’appareil sécréteur dans la racine des composées. B Soc Bot Fr 31:112–116.
- Van Vuillemin M. (1884). Remarques sur la situation de l’appareil sécréteur des composées. B Soc Bot Fr 31:108–110.