Abstract
Context: Naringin is a bioflavonoid derivative and is predominantly found in Citrus paradisi Macf., Citrus sinensis (Linn.) Osbeck, Citrus unshiu Marc., Citrus reticulata Blanco cv. Nobilis, Citrus tachibana (Makino) Tanaka, Citrus junos Sieb. ex Tanaka (Rutaceae), and related citrus species. It has anti-inflammatory effects that have been well-documented, but the mechanism is poorly characterized.
Objective: The effect of naringin on production of RANTES (regulated upon activation normal T-cell expressed and secreted) in human HaCaT cells was investigated here for the first time.
Materials and methods: The HaCaT cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) and the proliferation of cell was determined by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT). The cells were divided into three groups including control group, tumor necrosis factor alpha (TNF-α)/interferon gamma (IFN-γ)-stimulated group, and naringin pretreatment group (first incubated in the presence of naringin and then exposed to TNF-α/IFN-γ). The concentration of RANTES in the supernatants was determined by enzyme-linked immunosorbent assay (ELISA). The expression of RANTES mRNA was analyzed by reverse transcription-polymerase chain reaction (RT-PCR). The expression of nuclear factor kappa B (NF-κB) P65 protein was detected with immunocytochemical method and western blot method.
Results: Naringin hardly inhibits HaCaT cells growth at concentrations rising from 0.25 to 1 mmol/L. However, RANTES expression detected in supernatant stimulated with TNF-α/IFN-γ reduced 15 and 16%, respectively, when cultured with 0.25, 0.5 mmol/L naringin. Furthermore, 1 mmol/L naringin significantly decreased RANTES mRNA level. Finally, naringin decreased the expression of NF-κB P65 protein in nuclei.
Discussion and conclusion: Naringin can inhibit the increased production of RANTES, which is partially via NF-κB-dependent signal pathway.
Introduction
The skin epithelium, which is composed mainly of keratinocytes interspersed with dendritic cells, melanocytes, and T-lymphocytes, and monocytes, is highly committed to host defense. Physical, chemical, or immune-specific insults rapidly evoke an epidermal response characterized by activated monocytes and lymphocytes. These activated cells release a large group of cytokines, such as tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ), to stimulate the proliferation and differentiation of keratinocytes (CitationFujisawa et al., 1997; CitationPasparakis et al., 2002). Not only the keratinocytes are the target cells of these cytokines, but also they secrete many kinds of cytokines by themselves, including some chemokines. Thus, the cells cytokines network in the epidermis is formed and involved in the pathogenesis of many kinds of cutaneous diseases (CitationLuger & Schwarz, 1990).
RANTES (regulated upon activation normal T-cell expressed and secreted), also called CC chemokine ligand 5 (CCL5), is predominantly chemotactic for and activates T-cells in chronic inflammatory conditions (CitationSchall, 1991), including cutaneous diseases such as atopic dermatitis and psoriasis (CitationOppenheim et al., 1991; CitationPattison et al., 1995; CitationKrueger & Bowcock, 2005). In the past decade, numerous studies identified RANTES to be associated with atopic dermatitis (CitationHomey et al., 2007). In addition, RANTES has been found overexpressed in the keratinocytes or in the intercellular spaces between epidermal keratinocytes in lesions of psoriasis (CitationFukuoka et al., 1998; CitationRaychaudhuri et al., 1999). Increased amounts of RANTES provide an explanation for migration of the activated T-cells to the epidermis of dermatitis. These results suggest that RANTES may have a significant role in the inflammatory process of cutaneous diseases and substantiate a regulatory role for keratinocytes in the inflammatory process of cutaneous diseases.
Naringin is a bioflavonoid derivative of grapefruit peel and related citrus species and is predominantly found in Citrus paradisi Macf., Citrus sinensis (Linn.) Osbeck, Citrus unshiu Marc., Citrus reticulata Blanco cv. Nobilis, Citrus tachibana (Makino) Tanaka, Citrus junos Sieb. ex Tanaka (Rutaceae), and related citrus species (CitationJourdan et al., 1985; CitationCastillo et al., 1992). It has been described to present antioxidant and anti-inflammatory activity (CitationLimasset et al., 1993; CitationBenavente-García & Castillo, 2008; CitationChen et al., 2009). Although flavonoids have been studied for about 50 years, the cellular mechanisms involved in their biological action are still not completely known. The effects of naringin on RANTES production of keratinocytes and related signal pathways have not been reported in the literature. In this study, the effects of naringin on proliferation and RANTES production of human epidermal keratinocytes cell line (HaCaT cells) were determined as well as the expression of nuclear factor kappa B (NF-κB) in nuclei of HaCaT cells stimulated with TNF-α/IFN-γ.
Materials and methods
Materials
Naringin (from citrus fruit; chemical purity 90%) was purchased from Sigma-Aldrich (St. Louis, MO). HaCaT cell line was purchased from China Center for Type Culture Collection (CCTCC). Mouse monoclonal anti-human NF-κB p65 antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), horseradish peroxidase (HRP)-conjugated goat anti-rabbit/rabbit anti-mouse IgG from Cell Signaling Technology (Beverly, MA), goat polyclonal anti-human β-actin antibody and HRP-conjugated rabbit anti-goat IgG from Beijing Zhongshan Biotechnology Co., Ltd. (Beijing, China). RANTES enzyme-linked immunosorbent assay (ELISA) kit was purchased from Biosource (Wayne, PA), Revert Aid™ First Strand cDNA Synthesis Kit from MBI Co. (produced in Lithuanian, purchased from Xin Hui ze ao Science and Technology Co., Ltd.), and nuclear extract kit from Nanjing KeyGen Biotechnology Co., Ltd (Nanjing, China).
Cell culture and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide assay
HaCaT cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM), with 10% fetal bovine serum (FBS) and antibiotics (100 U/mL penicillin and 100 μg/mL streptomycin). The growth rates of HaCaT cells were measured with the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay. In brief, 1 × 105 cells in 0.2 mL of culture medium were plated in each well of a 96-well culture plate. In total, three concentrations of naringin (0.25, 0.5, and 1 mmol/L) were used for treatment. Cells were analyzed at 44 h by adding 20 μL of MTT to each well of the plate. The cells were incubated at 37°C in a 5% CO2 atmosphere for 4 h, the medium was aspirated, and the cells were then lysed in 100 μL of dimethyl sulfoxide (DMSO). Conversion of MTT to formazan by metabolically viable cells was monitored at 570 nm (A570 nm) in an ELISA reader, and the results were analyzed by regression analysis from triplicate experiments. Cell growth inhibition rate was estimated with the following formula: growth inhibition rate (%) = [1 − A570 nm (treated cells)/A570 nm (control cells)] ×100%.
ELISA for RANTES protein
HaCaT cells (5 × 104 cells per well) were plated in a 96-well culture plate and grown in serum-free medium. Cells were pretreated with two concentrations of naringin (0.25 and 0.5 mmol/L) for 0.5 h. TNF-α 20 ng/mL and IFN-γ 20 ng/mL were added to these cells. The cells were harvested for 48 h and the production of RANTES protein in cell supernatant was measured using an ELISA kit. All procedures were performed in accordance with the manufacturer’s instructions.
Real-time polymerase chain reaction for RANTES gene
HaCaT cells were pretreated with naringin at the indicated doses (0.25 and 0.5 mmol/L) for 48 h before TNF-α/INF-γ treatment. The cells were harvested for 16 h. To quantify relative gene expression, real-time polymerase chain reaction (PCR) was used to amplify and simultaneously quantify the targeted RANTES gene. Total RNA was extracted using Trizol as described by the manufacturer. First strand cDNA was synthesized using RevertAid™ First Strand cDNA Synthesis Kit according to the manufacturer’s instructions: 5 μL of total RNA in 20 μL reaction mixture (final concentrations: 0.01 μg/μL random hexamer primer, 1 mM dNTP mixture, 1 U/μL RNase inhibitor) containing 200 U reverse transcriptase. After the hexanucleotides were annealed for 5 min at 70°C, cDNA synthesis was performed for 60 min at 42°C, followed by an enzyme inactivation step at 70°C for 10 min. The cDNA was stored at −70°C until used.
Total PCR volume was 30 μL, including 5 μL of cDNA products, 1.5 μL of SYBR green I PCR Master (10 μM), 1 μL forward primer (10 μM), and 1 μL reverse primer (10 μM). The tubes were placed in an FTC2000 System for 45 sequential cycles, each comprising denaturing at 94°C for 20 sec followed by annealing at 56°C for 20 sec and extension at 72°C for 30 sec, 80°C for 20 sec, with fluorescence detection after each cycle. The primers were as follows: RANTES (96 bp): sense, 5′-CCAGCAGTCGTCTTTGTCA-3′; antisense, 5′-CTCATCTCCAAAGAGTTGATGT-3′; probe: 5′-FAM-AACCGCCAAGTGTGTGCCAAC-TAMRA-3′. GAPDH (141 bp): sense, 5′-CCTCAAGATCATCAGCAAT-3′; antisense, 5′-CCATCCACAGTCTTCTGGGT-3′; probe, 5′-FAM-ACCACAGTCCATGCCATCAC-TAMRA-3′. The levels of RANTES mRNA were determined by comparing experimental levels to the standard curves and are expressed as fold change in RANTES gene expression normalized to GAPDH gene expression and relative to the untreated control. The data were collected from three independent repeated experiments.
Nuclear protein extraction and western blot analysis
TNF-α 20 ng/mL and IFN-γ 20 ng/mL were added to cell cultures pretreated with or without 0.5 mmol/L naringin for 16 h, and cells were harvested for 0.5 h. Nuclear extracts were prepared using a Nuclear Extraction Kit. In brief, cells from each well were scraped off into 1 mL ice-cold phosphate-buffered saline (PBS), centrifuged at 500 rpm for 3 min, and lysed in 200 μL hypotonic buffer [1 μL dithiothreitol (DTT), 5 μL phenymethylsulfonyl fluoride (PMSF), and 10 μL proteinase inhibitor were added to per milliliter buffer before use]. The lysates were vorticosely oscillated, and then centrifuged for 30 sec at 14,000 rpm in a cold microcentrifuge. The supernatant (cytoplasmic fraction) was removed and the precipitated pellet was the nuclear fraction. The nuclear pellet was resuspended in 100 μL nuclear lysis buffer [1 μL DTT, 5 μL PMSF, and 10 μL proteinase inhibitor were added to per milliliter buffer before use]. The lysates were vorticosely oscillated, and then centrifuged at 14,000 rpm for 10 min at 4°C in a microcentrifuge. The supernatant was the nuclear protein extracts. Protein concentrations in the supernatants were quantified by the Bradford assay using bovine serum albumin (BSA) as a standard and stored at −80°C.
Samples (50 μg) of extracted protein were separated on 12% sodium dodecylsulfate (SDS) polyacrylamide gels, and then electrophoretically transferred to nitrocellulose membranes. The membranes were soaked in 5% nonfat dried milk in Tris–Tween-buffered saline (TTBS) (50 mM Tris, 0.2% Tween 20, 150 mM NaCl, pH 7.5) for 1 h and then incubated with primary antibodies against STAT1 and NF-κB p65 at 4°C overnight. After washing three times with TTBS for 10 min, the membranes were incubated with a HRP-conjugated secondary antibody for 1 h at room temperature. Protein bands were revealed by the ECL kit according to the manufacturer’s protocol, and the antigen–antibody complex was detected using the chemiluminescence detection system. The amount of RANTES protein was corrected by β-actin in the same sample. The integrated optical density (IOD) values of RANTES and β-actin were measured with Image-Pro Plus 5.0 software, and the ratio of IOD values were compared.
Immunocytochemistry
TNF-α 20 ng/mL and IFN-γ 20 ng/mL were added to cell cultures pretreated with or without 0.5 mmol/L naringin for 16 h, and cells were harvested for 0.5 h. To determine the subcellular localization of the NF-κB p65 protein, NF-κB P65 proteins were detected by immunocytochemistry. In brief, HaCaT cells grew on glass coverslips were fixed by 4% paraformaldehyde, rinsed and blocked with 3% hydrogen peroxide for 15 min. The coverslips were re-rinsed, and incubated for 15 min in buffer containing 5% normal goat serum and 2% BSA in order to block unspecific groups. Then the coverslips with cells were incubated with mouse anti-human NF-κB P65 antibody (the first antibody) at 4°C overnight. After incubation, the coverslips were rinsed, and incubated with biotinylated goat anti-mouse antibody (the secondary antibody) at 37°C for 20 min. After incubation with secondary antibody, the coverslips were rinsed. A detection system is then employed to identify the location of the antibodies using marker molecules that can be visually recorded. Streptavidin–biotin immunoperoxidase, currently used as marker molecules, was added on the coverslips and then the coverslips were incubated at 37°C for 20 min. A positive reaction was visualized with diaminobenzidine. Brown staining in cytoplasm or nucleus was determined as positive.
Statistical analysis
Results were expressed as the mean ± standard deviation (SD). One-way analysis of variance (ANOVA) was applied to compare the differences in growth inhibition rates of HaCaT cells and RANTES protein released by HaCaT cells. In the statistical evaluation of RANTES gene expression and NF-κB p65 expression, unpaired Student’s t-test was used to compare the differences between means of two groups. Significance was assumed when P < 0.05.
Results
The inhibitory effects of naringin on HaCaT cells proliferation
Naringin hardly inhibited proliferation of HaCaT cells, and the inhibition rate increased gradually from 4.4, 5.3, to 6.0% with the concentration of naringin rising from 0.25, 0.5, to 1 mmol/L (P ≤ 0.05).
RANTES protein expression in supernatants of HaCaT cells
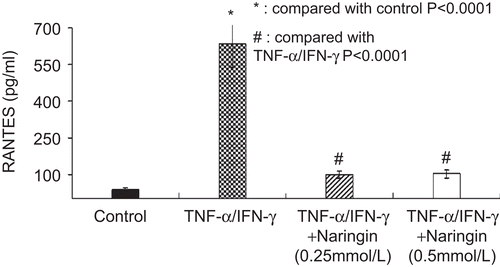
TNF-α/IFN-γ can induce modulation of RANTES release from the HaCaT cell at 24 and 48 h time point. More RANTES is released from HaCaT cell stimulated by TNF-α/IFN-γ at 48 h than that at 24 h (633.900 ± 24.921 pg/mL vs. 37.826 ± 10.394 pg/mL). Naringin inhibited RANTES production of keratinocytes stimulated with TNF-α/IFN-γ (). RANTES protein detected in supernatant of HaCaT cells stimulated with TNF-α/IFN-γ reduced to 15% and 16%, respectively, when cultured with 0.25, 0.5 mM naringin. In order to minimize the cell number-related interference with different concentrations of naringin, cell number-corrected results of RANTES protein were presented.
RANTES mRNA expression detected by real-time PCR
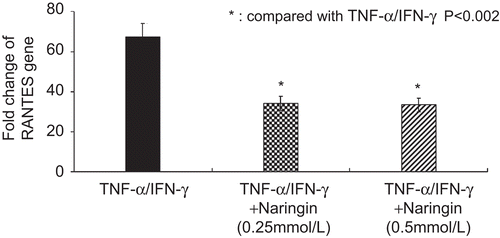
Naringin decreased RANTES mRNA level when HaCaT cells stimulated with TNF-α/IFN-γ (P < 0.002, ). The 0.25 and 0.5 mM naringin decreased the relative copies of RANTES mRNA to 50% of that stimulated cells.
NF-κB p65 expression in western blot and immunocytochemistry
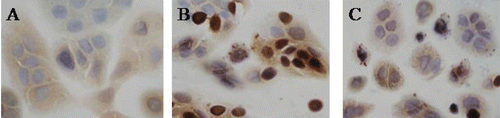
TNF-α/IFN-γ can induce nuclear translocations of NF-κB p65, and 0.5 mmol/L naringin inhibited TNF-α/IFN-γ inducing translocation of NF-κB p65. Western blot showed very weak bands for NF-κB p65 proteins in nuclei of normal HaCaT cells, and the proteins were markedly induced by TNF-α/IFN-γ (n = 3, P < 0.02). Naringin decreased expression of NF-κB p65 in nuclear extracts of HaCaT cells stimulated with TNF-α/IFN-γ (n = 3, P < 0.01) (). In immunocytochemistry, NF-κB p65 expressed in cytoplasm of normal HaCaT cells (), and in nuclei of some HaCaT cells stimulated with TNF-α/IFN-γ for 0.5 h. However, NF-κB p65 proteins were found only in cytoplasm after HaCaT cells were stimulated with TNF-α and IFN-γ for 2 h and pretreated with 0.5 mmol/L naringin ().
Figure 3. Naringin decreased the expression of nuclear factor kappa B (NF-κB) p65 in nuclear extracts of HaCaT cells in western blot. 1, Untreated HaCaT cells, 2, cells treated with 0.5 mmol/L naringin, 3, cells stimulated with tumor necrosis factor alpha (TNF-α)/interferon gamma (IFN-γ). #Compared with untreated cells, P < 0.02. *Compared with cells stimulated with TNF-α/IFN-γ, P < 0.01.
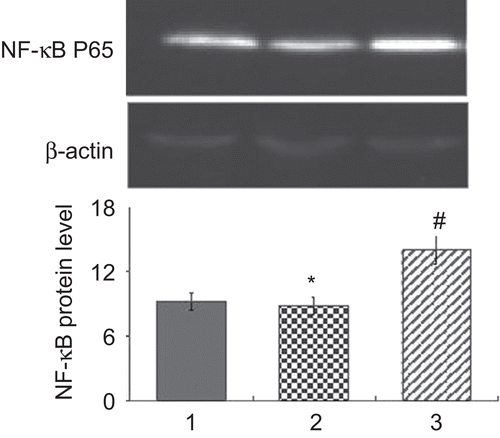
Figure 4. Nuclear factor kappa B (NF-κB) P65-positive expression in HaCaT cells (SP × 400). (A) NF-κB P65-positive expression in cytoplasm of normal cells, (B) NF-κB P65-positive expression in nuclei of some cells stimulated with tumor necrosis factor alpha (TNF-α)/interferon gamma (IFN-γ) for 0.5 h, and (C) NF-κB p65 expression in cytoplasm of cells stimulated with 0.5 mmol/L naringin for 16 h and TNF-α/IFN-γ for 0.5 h.
Discussion
IIn this study, the effects of naringin on RANTES production of human epidermal keratinocytes cell line (HaCaT cells) were determined as well as the expression of NF-κB in nuclei of HaCaT cells was stimulated with TNF-α/IFN-γ. The results of our study indicated that naringin cannot inhibit proliferation of human epidermal keratinocytes; however, RANTES production was inhibited. This means that the inhibition of RANTES production is not due to the destruction of the cells, but may be related to the inhibition of nuclear translocations of NF-κB.
Epidermal keratinocytes, the main constituent of the epidermis, actively participate in innate immune responses by producing cytokines, chemokines (CitationFujisawa et al. 1997; CitationPasparakis et al., 2002). Human RANTES is a proinflammatory chemokine that promotes cell accumulation and activation in chronic inflammatory conditions (CitationOppenheim et al., 1991; CitationPattison et al., 1995; CitationKrueger & Bowcock, 2005). Epidermal keratinocytes can produce RANTES strongly by TNF-α, INF-γ (CitationZhang et al., 2008). In turn, activated monocytes, dendritic cells, and T-cells release potent cytokines that act on cells in the local environment to boost the inflammatory response. In our study, we found that TNF-α/IFN-γ can induce modulation of RANTES release from the HaCaT cell at 24 and 48 h time point. More RANTES is released from HaCaT cell stimulated by TNF-α/INF-γ at 48 h than that at 24 h.
Naringin is the major flavonoid glycoside in grapefruit and gives grapefruit juice its bitter taste. Narinigin exerts a variety of pharmacological effects such as antioxidant activity, anti-inflammatory (CitationLimasset et al., 1993; CitationBenavente-García and Castillo, 2008; CitationChen et al., 2009), and so on. In this study, we chose the TNF-α and IFN-γ to stimulate the HaCaT cell to investigate the effect of naringin on RANTES production of human keratinocytes. We found that naringin at the indicated doses can inhibit the RANTES secretion of HaCaT cell induced by TNF-α/INF-γ. And 0.5 mM, 0.25 mM of naringin show a little bit difference in inhibiting the RANTES secretion of HaCaT cell induced by TNF-α/INF-γ. At the same time, we extracted the mRNA from HaCaT cell induced by TNF-α/INF-γ. We found that the expression of RANTES mRNA was treated by naringin at the indicated doses that significantly decreased relative copies of RANTES mRNA compared with model group.
The transcription factor, NF-κB is known to function as a pleiotropic regulator of many genes that modulate immunological and inflammatory processes. In a study of the mechanism of the anti-inflammatory effect of naringin, naringenin, and resveratrol, these bioflavonoids suppressed the activation of NF-κB in macrophages (CitationTsai et al., 1999). In the tubular cells, up-regulation of RANTES was correlation between the expression of these chemokines and NF-κB activation (CitationMezzano et al., 2004). And NF-κB can regulate the promoter activity of RANTES (CitationMoriuchi et al., 1997). In our study, immunocytochemistry showed that naringin can inhibit the activation and nucleus shifting of the NF-κB. Western blot showed very weak bands for NF-κB p65 proteins in nuclei of normal HaCaT cells, and the proteins were markedly induced by TNF-α/IFN-γ. Naringin can decrease expression of NF-κB p65 in nuclear extracts of HaCaT cells stimulated with TNF-α/IFN-γ.
Conclusion
The present study shows that naringin cannot inhibit proliferation of human epidermal keratinocytes, but can inhibit RANTES production, and the latter may be related to the inhibition of nuclear translocation of NF-κB. The present study provides theoretical basis for clinical use of naringin to cutaneous diseases.
Declaration of interest
The authors report no declarations of interest.
References
- Benavente-García O, Castillo J. (2008). Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J Agric Food Chem, 56, 6185–6205.
- Castillo J, Benavente O, Del Río JA. (1992). Naringin and neohesperidin levels during development of leaves, flower buds, and fruits of Citrus aurantium. Plant Physiol, 99, 67–73.
- Chen JC, Li LJ, Wen SM, He YC, Liu HX, Zheng QS. (2009). [Anti-inflammatory effects and quantitative study of the combinations of active ingredients of Painong powder in mice]. Zhong Xi Yi Jie He Xue Bao, 7, 541–545.
- Fujisawa H, Kondo S, Wang B, Shivji GM, Sauder DN. (1997). The expression and modulation of IFN-alpha and IFN-beta in human keratinocytes. J Interferon Cytokine Res, 17, 721–725.
- Fukuoka M, Ogino Y, Sato H, Ohta T, Komoriya K, Nishioka K, Katayama I. (1998). RANTES expression in psoriatic skin, and regulation of RANTES and IL-8 production in cultured epidermal keratinocytes by active vitamin D3 (tacalcitol). Br J Dermatol, 138, 63–70.
- Homey B, Meller S, Savinko T, Alenius H, Lauerma A. (2007). Modulation of chemokines by staphylococcal superantigen in atopic dermatitis. Chem Immunol Allergy, 93, 181–194.
- Jourdan PS, Weiler EW, Mansell RL. (1985). Naringin levels in citrus tissues: I. Comparison of different antibodies and tracers for the radioimmunossay of naringin. Plant Physiol, 77, 896–902.
- Krueger JG, Bowcock A. (2005). Psoriasis pathophysiology: Current concepts of pathogenesis. Ann Rheum Dis, 64 (Suppl 2), ii30–ii36.
- Limasset B, le Doucen C, Dore JC, Ojasoo T, Damon M, Crastes de Paulet A. (1993). Effects of flavonoids on the release of reactive oxygen species by stimulated human neutrophils. Multivariate analysis of structure–activity relationships (SAR). Biochem Pharmacol, 46, 1257–1271.
- Luger TA, Schwarz T. (1990). Evidence for an epidermal cytokine network. J Invest Dermatol, 95, 100S–104S.
- Mezzano S, Aros C, Droguett A, Burgos ME, Ardiles L, Flores C, Schneider H, Ruiz-Ortega M, Egido J. (2004). NF-kappaB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transplant, 19, 2505–2512.
- Moriuchi H, Moriuchi M, Fauci AS. (1997). Nuclear factor-kappa B potently up-regulates the promoter activity of RANTES, a chemokine that blocks HIV infection. J Immunol, 158, 3483–3491.
- Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K. (1991). Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol, 9, 617–648.
- Pasparakis M, Courtois G, Hafner M, Schmidt-Supprian M, Nenci A, Toksoy A, Krampert M, Goebeler M, Gillitzer R, Israel A, Krieg T, Rajewsky K, Haase I. (2002). TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature, 417, 861–866.
- Pattison JM, Nelson PJ, Krensky AM. (1995). The RANTES chemokine: A new target for immunomodulatory therapy? Clin Immunother, 4, 1–8.
- Raychaudhuri SP, Jiang WY, Farber EM, Schall TJ, Ruff MR, Pert CB. (1999). Upregulation of RANTES in psoriatic keratinocytes: A possible pathogenic mechanism for psoriasis. Acta Derm Venereol, 79, 9–11.
- Schall TJ. (1991). Biology of the RANTES/SIS cytokine family. Cytokine, 3, 165–183.
- Tsai SH, Lin-Shiau SY, Lin JK. (1999). Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol, 126, 673–680.
- Zhang M, Zhu L, Feng Y, Yang Y, Liu L, Ran Y. (2008). Effects of acitretin on proliferative inhibition and RANTES production of HaCaT cells. Arch Dermatol Res, 300, 575–581.