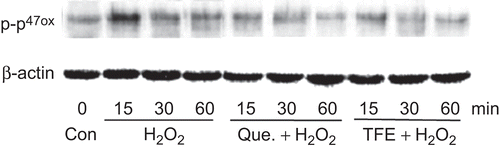Abstract
Context: Inula britanica Linn. (Compositae) is a traditional Chinese medicinal herb that has been used to treat bronchitis and inflammation. The total flavonoid extracts (TFEs) isolated from its flowers can inhibit neointimal formation induced by balloon injury in vivo.
Objective: To investigate the mechanism by which TFE suppresses oxidative stress generation and the subsequent inflammation response in vitro.
Materials and methods: The cultured vascular smooth muscle cells (VSMCs) form rats were exposed to oxidative stress following pretreatment with or without TFE at different concentration. Then, fluorescence staining was used to detect superoxide anion (O2˙−) production, and the lever of maleic dialdehyde (MDA) and superoxide dismutase (SOD) was measured at the same time. Furthermore, tumor necrosis factor-α (TNF-α) was measured by enzyme linked immunosorbent assay (ELISA), reverse transcription-PCR and western blot were performed to detect the expression activity of p47phox gene, and immunoprecipitation was used to test the level of p47phox phosphorylation.
Results: TFE inhibited the production of O2˙− induced by H2O2 in VSMCs, with decrease in secretion of TNF-α; elevated the activity of SOD in the medium, similar to the effect of quercetin; reduced the level of MDA in culture medium of VSMCs. The pretreatment with TFE resulted in decrease the level of p47phox mRNA and protein, and even p47phox phosphorylation in VSMCs, compared with H2O2 control.
Discussion and conclusion: These findings demonstrate that TFE is capable of attenuating the oxidative stress generation and the subsequent inflammation response via preventing the overexpression and activation of p47phox and the increased TNF-α secretion in VSMCs in vitro.
Introduction
The Reactive oxygen species (ROS) are thought to play an important pathophysiological role in endothelial dysfunction and vascular remodeling in atherosclerosis and restenosis (CitationHeckenkamp et al., 2002). ROS derived mainly from vascular smooth muscle cells (VSMCs) contribute to the proliferation and migration of medial VSMCs, leading to neointimal hyperplasia and adverse remodeling (CitationGriendling & FitzGerald, 2003). NADPH oxidase, a multi-subunit enzymatic complex, which are regulated by over-production and phosphorylation of cytoplasmic subunits p47phox (CitationLyle & Griendling, 2006), is the major sources of ROS production in VSMCs (CitationGriendling et al., 2000; CitationJiang et al., 2004). Our recent study has demonstrated that overexpression of p47phox protein occurs in the neointima formation induced by balloon injury, and this increased p47phox protein level is consistent with the increased O2˙− production and neointima thickness found in injured-arteries (CitationZhang et al., 2009).
Flavonoids are a large group of polyphenolic compounds abundantly present in the human diet (CitationHollman & Katan, 1999) and appear to possess a variety of biological activities, including antioxidant, anti-inflammatory, and vasodilator actions (CitationNijveldt et al., 2001). Inula britanica Linn. (Compositae) is a traditional Chinese medicinal herb that has been used to treat bronchitis and inflammation. Recently, the total flavonoid extract (TFE) isolated from Inula britanica has been demonstrated to inhibit neointimal formation induced by balloon injury through reduction of p47phox protein level and oxidative stress generation (CitationZhang et al., 2009). In this study, we investigated the mechanism by which TFE suppresses oxidative stress generation and the subsequent inflammation response via preventing the over expression and activation of p47phox and the increased tumor necrosis factor-α (TNF-α) secretion VSMCs in vitro.
Materials and methods
Extract and reagents
TFE from Inula britannica was prepared by refluxing flowers (3 × 1 h) with water (10:1 water to vegetable material); purified with AB-8 macroporous resin (the concentration of crude extract sample was 0.2 g/mL, introduction rate was 2 mL/min, eluent was 70% alcohol and the eluting rate was 3 mL/min), and then identified by high-performance liquid chromatography as previously described (CitationGeng et al., 2007). According to the extracting conditions, the content of total flavonoids from Inula britannica as 10.9%, TFE used in this experiment contained 45.5% quercetrin, 25.85% luteolin, 12.96% 6-methoxyluteolin, 4.33% spinacetin, and 1.85% isorhamnetin. The purified quercetin, as positive control, was purchased from Sigma Co. Anti-SOD, anti-p47phox antibodies were obtained from Santa Cruz Co.
Cell culture and treatment
VSMCs were isolated from the thoracic aorta of 90–110 g male Sprague–Dawley rats as described previously (CitationHan et al., 2006), and cultured in Dulbecco’s modified Eagle’s medium (Invitrogen) supplemented with 10% fetal bovine serum under 5% CO2 at 37°C. VSMCs grown to 80% confluence were exposed to oxidative stress using hydrogen peroxide (H2O2) at a concentration of 200 μM following pretreatment with or without TFE at different concentration (0–100 mg/L) for the indicated time periods. This study was performed via a protocol approved by the Institutional Animal Care and Use Committee of Hebei Medical University in accordance with the Guide for the Care and Use of Laboratory Animals.
MTT assay
After treatment, the viability of VSMCs cultured in 96-well plates was measured using MTT assay as previously described (CitationZheng et al., 2009). In brief, after cells were collected and incubated in medium containing 2 mg/mL MTT reagent (Sigma-Aldrich Chemical Co.) at 37°C for 4 h, fermazan crystals converted from tetrazolium salts by viable cells were dissolved in DMSO (dimethyl sulfoxide) (200 μL/well) and their absorbance at 570 nm was measured with a microplate reader to reflect cell viability.
Fluorescence staining of superoxide anion (O2˙−) production in VSMCs
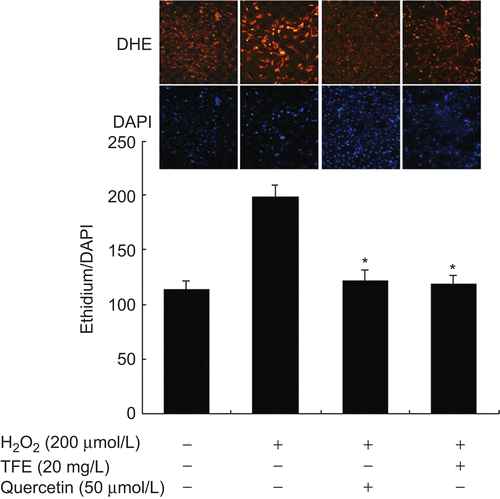
Cells were fixed and immediately incubated with dihydroethidium (DHE, 10 µmol/L). The ethidium from DHE oxidation was examined at 535 nm and photographed on a fluorescence microscope (Olympus IX71), counter-stained with the nuclear stain 4′,6-diamidino,2-phenylindol (DAPI). O2˙− production was estimated from the ratio of ethidium/DAPI fluorescence. Negative controls were obtained in the absence of DHE (CitationMin et al., 2007).
Detection of SOD activity and the level of MDA
VSMCs pretreated with TFE or quercetrin were incubated with H2O2 for 2 h. The media were collected and detected for superoxide dismutase (SOD) activity and maleic dialdehyde (MDA) level as previously described, respectively (CitationSpitz & Oberley, 1989; CitationSrivastava et al., 2002). SOD activity was defined as the degree of inhibition of the pyrogallol autooxidation rate: one unit of SOD activity was defined as the amount of activity inhibiting the autooxidation rate by 50%. Data were expressed as units per milliliter of medium. The MDA content in the medium was measured by thiobarbituric acid reactive substances (TBARS), and MDA generated by acid hydrolysis of tetra-ethoxy- propane was used as a standard.
Measurement of TNF-α by ELISA
The level of TNF-α in culture supernatants was determined by Quantikine ELISA kit (R&D Systems Inc., Minneapolis, MN). The color generated was determined by measuring at 450 nm of spectrophotometric microtiter plate reader. A standard curve was run on using the recombinant protein of TNF-α in serial dilution.
RNA isolation and RT-PCR
Total RNA was isolated from VSMCs using the TRIzol reagent, according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Reverse transcription (RT) was performed with the Superscript First Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). The cDNA was then used as a template for PCR using specific primers for rat p47phox (forward, 5′-TCACCGAGATCTACGAGTTC-3′ and reverse, 5′-TCCCATGAGGCTGTTGAAGT-3′) and GAPDH (forward, 5′-ACCACAGTCCATGCCATCAC-3′and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′. The amplified products were separated on a 2% agarose gel containing ethidium bromide, and the band intensities were quantified using the Image J software provided by the NIH.
Western blot analysis
Whole cell extracts (60–100 μg) were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, and electrotransfered to a polyvinylidine fluoride membrane. Membranes were blocked with 5% bovine serum albumin for 2 h at room temperature, and incubated with specific antibodies overnight, and then with the horseradish peroxidase-conjugated secondary antibody (1:10 000) for 2 h. The blots were evaluated with the enhanced chemiluminescence detection system, and quantified using Quantity One software (version 4.62, Bio-Rad).
Immunoprecipitation assays
Immunoprecipitation assays was performed as described previously (CitationJiang et al., 2006). Briefly, the cell extracts were immunoprecipitated with 2 μg of anti-phosphoserine antibody, followed by incubation with protein A-agarose overnight at 4°C, Protein A-agarose-antigen-antibody complexes were collected by centrifugation. The phosphorylated proteins were analyzed by western blotting with the anti-p47phox antibody.
Statistical analyses
All experiments were performed in triplicate. Results were expressed as mean ± SE and an analysis of variance with Bonferroni’s test was used for the statistical of multiple comparisons of date. A value of P < 0.05 was considered statistically significant.
Results
TFE inhibits the production of O2˙− induced by H2O2 in VSMCs
MTT assay showed that pretreatment with TFE had no significant effect on the cell proliferation, and no cytotoxic evidence of TFE at 100 mg/L was observed in H2O2-treated VSMCs (data not shown). Based on the known antioxidant effect of TFE in vivo (CitationZhang et al., 2009), the effect of TFE on the production of O2˙− caused by H2O2 in VSMCs was detected by DHE staining. The preincubation with of TFE (20 mg/L) resulted in reduction in the intensity of red fluorescence compared with H2O2 alone (P < 0.01) consistant with that of quercetin treatment, suggesting that the production of O2˙− induced by H2O2 is inhibited by TFE in VSMCs ().
TFE enhances SOD activity and decreases the generation of MDA in VSMCs
SOD is an antioxidant enzyme to scavenge oxygen-free radical production. SOD activity in culture medium of VSMCs treated with H2O2 decreased significantly by 42.86%, compared with control cells (P < 0.01). However, TFE pretreatment elevated the activity of SOD in the medium (P < 0.01), similar to the effect of quercetin ().
Figure 2. TFE suppresses H2O2-induced MDA production (A), up-regulates total SOD activity (B) and decreases in secretion of TNF-α (C) in VSMCs. *P < 0.05 compared with H2O2 alone (n = 5).
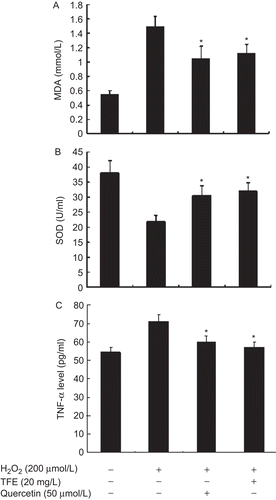
MDA, a production of lipid peroxidation, can be used as a marker of the oxidative stress state and oxidative injury. The level of MDA in culture medium of VSMCs was increased by 1.78-fold following treatment with H2O2 for 24 h, and was significantly reduced by 25.33% in VSMCs pretreated by TFE compared to H2O2 alone (). These results suggest that TFE possess antioxidant capacity and increase the resistance of VSMCs to oxidation in vitro.
TFE suppresses H2O2-induced TNF-α secretion in VSMCs
Because O2˙− production induces inflammation response in cells, to confirm whether TFE inhibits the increased secretion of TNF-α by H2O2, VSMCs were pretreated by TFE for 24 h followed by the addition of H2O2. As shown in Figure 2C, pretreatment with TFE resulted in a significant decrease in secretion of TNF-α by H2O2 in VSMCs, consistent with reduction of O2˙− production.
TFE inhibits the expression of p47phox in VSMCs induced by H2O2
Western blot analysis showed that the expression of p47phox protein increased dramatically in VSMCs treated with H2O2 in dose-and time-dependent manners, respectively (). The pretreatment with TFE or quercetin decreased the level of p47phox protein by 38.2 and 39.4% compared with H2O2 group, respectively ().
Figure 3. Induction of p47phox by H2O2 is inhibited by TFE in VSMCs. A and B, western blotting. β-actin was used as an internal control; C, RT-PCR. GAPDH was used as an internal control. Bar graphs represent the relative level of p47phox protein (A and B) or mRNA (C) of three independent experiments. *P < 0.01 compared with 0 group (A) or H2O2 alone (B and C).
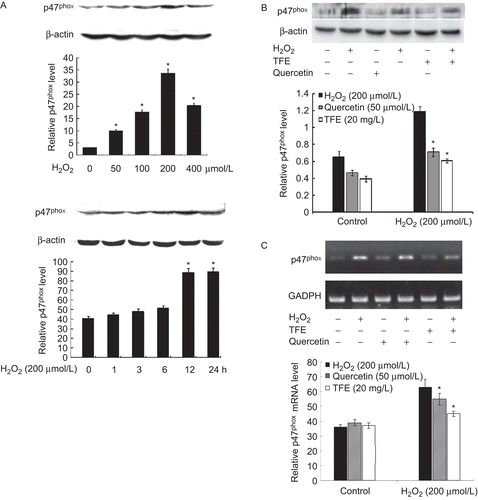
In order to confirm whether TFE inhibits the expression of p47phox mRNA, total RNA was extracted from VSMCs and then the mRNA of p47phox were detected by RT-PCR. The result showed that pretreatment with TFE resulted in a reduction of the transcriptional activities of p47phox gene, and the level of p47phox mRNA decreased by 17.0%, compared with H2O2 control (). However, TFE treatment did not change the expression of p47phox in quiescent VSMCs. These data suggest that the reduction of p47phox protein level may be associated with the inhibition of the transcription of p47phox gene by TFE in VSMCs.
TFE inhibits the H2O2-induced phosphorylation of p47phox in VSMCs
Immunoprecipitation analysis showed that H2O2 triggered p47phox phosphorylation, which reached to peak at 15 min and kept high level up to 30 min and then decreased. The pretreatment with TFE resulted in 47.2% decrease in the level of p47phox phosphorylation (P < 0.01), and the inhibitory effect of TFE on p47phox phosphorylation was similar to quercetin (). These results suggest that the inhibition of p47phox activation by TFE may be attributable at least in part to reduction of ROS generation in VSMCs.
Discussion
The vascular wall is the first target for oxidative stress caused by ROS, resulting in diverse damage of VSMCs. Cardiovascular antioxidant defense is equipped with enzymatic scavengers such as SOD, but also non-enzymatic ones which include flavonoids. Our previous study has indicated that TFE inhibits neointimal hyperplasia via reduction of O2˙− generation through inhibiting the expression of p47phox protein. In this study, we show that H2O2-induced oxidative stress of VSMCs in vitro can be prevented by TFE. Moreover, the TFE also prevented H2O2-induced overexpression and phosphorylation of p47phox in VSMCs and the increase in O2˙− production. The antioxidant effect of TFE was similar to quercetin that was used as a positive control in this experiment. Previous reports have shown that oxidative stresses play an important role in neointimal formation via inducing vascular inflammation and VSMC proliferation (CitationGriendling & Harrison, 1999; CitationTaniyama and Griendling, 2003; CitationChan et al., 2007). Antioxidants are considered as potent anti-inflammation stratagem. Here, we showed that treatment with TFE reduced the secretion of TNF-α induced by H2O2 in VSMCs. However, TFE had no significant effect on the cell proliferation, implying that the inhibition of neointimal hyperplasia by TFE in vivo may be resulted from antioxidant and antiinflammation.
Excess of O2˙− generation is critically involved in endothelial dysfunction in the vessel. To confirm that TFE inhibits generation of O2˙− in VSMCs in vitro, we used DHE, a widely used indicator of O2˙− production. H2O2 increased the red ethidium fluorescence from DHE oxidation in VSMCs and this increase was markedly reduced by TFE, consistent with the involvement of changes in SOD activity and MDA level that is a marker of oxidative damage to lipids. The results suggest that TFE facilitates the eliminating of oxygen-free radicals in VSMCs. An increased production of superoxide anions and metabolites and/or a reduced bioavailability of antioxidants cause oxidative damage to cells and tissues. An imbalance between pro-oxidant and antioxidant levels in the body gives rise to cellular oxidative stress that plays an important role in the genesis of cardiovascular diseases (CitationDhalla et al., 2000). Large studies have shown potential protective effects of flavonoids in cardiovascular diseases via improving endothelial dysfunction (CitationMiddleton et al., 2000). We first time demonstrated that the TFE is an effective antioxidant component of Inula britannica.
The major source of intracellular ROS in vascular cells is NADPH oxidase, which are regulated by cytoplasmic subunits such as p47phox. The translocation of cytosolic p47phox to the membrane is essential in the assembly process of this complex and plays a major role in NADPH oxidase activity in VSMCs (CitationTouyz et al., 2003). The previous study has reported that quercetin prevent AngII-induced endothelial dysfunction by inhibiting the overexpression of p47phox and the subsequent increased O2˙− production in the medial layer of the vessel (CitationSanchez et al., 2007). Quercetin that is the main component of TFE, about 45%, as a member of the flavonoid family is widely discussed as an antioxidant. Most in vitro studies with quercetin used higher concentrations to prove antiproliferative effects in cancer lines. Quercetin also exerts vascular effects and reduces blood pressure, cardiac hypertrophy, endothelial dysfunction, vascular remodeling and oxidative status in several rat models of hypertension (CitationDuarte et al., 2001; CitationSánchez et al., 2006). However, the TFE had no significant effect on the proliferation of VSMCs in vitro. In addition to quercetin, the TFE also contains luteolin, 6-methoxyluteolin, spinacetin and isorhamnetin. The multiple components of the TFE generate complex effects. We speculated that the inhibition of neointimal hyperplasia may be indirect effect of TFE, resulting from antioxidant.
Conclusion
This is the first report to show that TFE isolated from Inula britanica is capable of attenuating the oxidative stress generation and subsequent inflammation response, and we believe, this occurs via inhibiting the overexpression and activation of p47phox and the increased TNF-α secretion in VSMCs in vitro. The inflammatory response to O2˙− production shown here to be inhibited by TFE are crucial events underlying the remodeling of VSMC phenotypes. Besides providing novel insights into the protective action of TFE in the oxidative damage response, these results offer a therapeutic potential of TFE in the prevention and treatment of vascular diseases and restenosis after angioplasty.
Declaration of interest
This work was supported by the National Natural Science Foundation of China (No. 30971457 and 30770787), the “973” Program of China (No. 2008CB517402) and the Hebei Province Natural Science Foundation (No. 08966129D and C2007000831).
References
- Chan EC, Datla SR, Dilley R, Hickey H, Drummond GR, Dusting GJ. (2007). Adventitial application of the NADPH oxidase inhibitor apocynin in vivo reduces neointima formation and endothelial dysfunction in rabbits. Cardiovasc Res, 75, 710–718.
- Dhalla NS, Temsah RM, Netticadan T. (2000). Role of oxidative stress in cardiovascular diseases. J Hypertens, 18, 655–673.
- Duarte J, Pérez-Palencia R, Vargas F, Ocete MA, Pérez-Vizcaino F, Zarzuelo A, Tamargo J. (2001). Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br J Pharmacol, 133, 117–124.
- Geng HM, Zhang DQ, Zha JP, Qi JL. (2007). Simultaneous HPLC determination of five flavonoids in Flos inulae. Chromatographia, 66, 271–275.
- Griendling KK, FitzGerald GA. (2003). Oxidative stress and cardiovascular injury: Part II: animal and human studies. Circulation, 108, 2034–2040.
- Griendling KK, Harrison DG. (1999). Dual role of reactive oxygen species in vascular growth. Circ Res, 85, 562–563.
- Griendling KK, Sorescu D, Ushio-Fukai M. (2000). NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ Res, 86, 494–501.
- Han M, Wen JK, Zheng B, Cheng Y, Zhang C. (2006). Serum deprivation results in redifferentiation of human umbilical vascular smooth muscle cells. Am J Physiol, Cell Physiol, 291, C50–C58.
- Heckenkamp J, Gawenda M, Brunkwall J. (2002). Vascular restenosis. Basic science and clinical implications. J Cardiovasc Surg (Torino), 43, 349–357.
- Hollman PC, Katan MB. (1999). Dietary flavonoids: Intake, health effects and bioavailability. Food Chem Toxicol, 37, 937–942.
- Jiang F, Drummond GR, Dusting GJ. (2004). Suppression of oxidative stress in the endothelium and vascular wall. Endothelium, 11, 79–88.
- Jiang GJ, Han M, Zheng B, Wen JK. (2006). Hyperplasia suppressor gene associates with smooth muscle alpha-actin and is involved in the redifferentiation of vascular smooth muscle cells. Heart Vessels, 21, 315–320.
- Lyle AN, Griendling KK. (2006). Modulation of vascular smooth muscle signaling by reactive oxygen species. Physiology (Bethesda), 21, 269–280.
- Middleton E Jr, Kandaswami C, Theoharides TC. (2000). The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol Rev, 52, 673–751.
- Min LJ, Mogi M, Iwanami J, Li JM, Sakata A, Fujita T, Tsukuda K, Iwai M, Horiuchi M. (2007). Cross-talk between aldosterone and angiotensin II in vascular smooth muscle cell senescence. Cardiovasc Res, 76, 506–516.
- Nijveldt RJ, van Nood E, van Hoorn DE, Boelens PG, van Norren K, van Leeuwen PA. (2001). Flavonoids: A review of probable mechanisms of action and potential applications. Am J Clin Nutr, 74, 418–425.
- Sánchez M, Galisteo M, Vera R, Villar IC, Zarzuelo A, Tamargo J, Pérez-Vizcaíno F, Duarte J. (2006). Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J Hypertens, 24, 75–84.
- Sanchez M, Lodi F, Vera R, Villar IC, Cogolludo A, Jimenez R, Moreno L, Romero M, Tamargo J, Perez-Vizcaino F, Duarte J. (2007). Quercetin and isorhamnetin prevent endothelial dysfunction, superoxide production, and overexpression of p47phox induced by angiotensin II in rat aorta. J Nutr, 137, 910–915.
- Spitz DR, Oberley LW. (1989). An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem, 179, 8–18.
- Srivastava S, Chandrasekar B, Bhatnagar A, Prabhu SD. (2002). Lipid peroxidation-derived aldehydes and oxidative stress in the failing heart: Role of aldose reductase. Am J Physiol Heart Circ Physiol, 283, H2612–H2619.
- Taniyama Y, Griendling KK. (2003). Reactive oxygen species in the vasculature: Molecular and cellular mechanisms. Hypertension, 42, 1075–1081.
- Touyz RM, Yao G, Schiffrin EL. (2003). c-Src induces phosphorylation and translocation of p47phox: Role in superoxide generation by angiotensin II in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol, 23, 981–987.
- Zhang HB, Wen JK, Wang YY, Zheng B, Han M. (2009). Flavonoids from Inula britannica L. inhibit injury-induced neointimal formation by suppressing oxidative-stress generation. J Ethnopharmacol, 126, 176–183.
- Zheng B, Han M, Bernier M, Zhang XH, Meng F, Miao SB, He M, Zhao XM, Wen JK. (2009). Kruppel-like factor 4 inhibits proliferation by platelet-derived growth factor receptor beta-mediated, not by retinoic acid receptor alpha-mediated, phosphatidylinositol 3-kinase and ERK signaling in vascular smooth muscle cells. J Biol Chem, 284, 22773–22785.