Abstract
Context: The variety of pathways for the introduction of a species into the medical collection of traditional communities has led many researchers to question the processes of selection and the use of these resources. A better comprehension of these processes will allow us to understand the cultural dynamics that are related to traditional medical practices, as well as to provide us with new ways in which to facilitate the exploration of natural products.
Objective: This study aims to test the predictive power of the plant apparency hypothesis as it relates to medicinal plant selection by the rural communities of the Caatinga and the Atlantic Forest in northeast Brazil.
Material and methods: Initially, a survey of the medicinal plants used by these communities was conducted using semistructured interviews. Subsequently, data on the life strategies and the habits of each species were collected. More detailed data on the specific plant parts indicated in interviews were also collected. A phytochemical screening for seven classes of chemical compounds was carried out to test the predictions of the plant apparency hypothesis.
Results: The medicinal plants from the Caatinga (especially those that are considered to be trees in habit) have a strong ability to accumulate quantitative compounds, and these species are most likely to be plants with significant biological activity related to these compounds; the medicinal plants from the Atlantic Forest, on the other hand, tend to have a high occurrence of qualitative compounds, especially in herbaceous life forms.
Discussion and conclusion: It was concluded that the plant apparency hypothesis does not adequately explain the selection of medicinal plants in the two environments studied. Our findings highlight some important implications for bioprospecting that need to be further tested experimentally, and through systematic studies, in different regions.
Introduction
The various methods of the introduction of a species into the pharmacopoeia of traditional communities (CitationBennett & Prance, 2000; CitationJanni & Bastien, 2004; CitationPalmer, 2004) has led many researchers to question the processes of selection & the use of medicinal plants (CitationVoeks, 1996; CitationStepp and Moerman, 2001; CitationAlbuquerque, 2010). The complete comprehension of these processes can lead us to a better understanding of the cultural dynamics related to traditional medical practices, as well as to the development of new ways in which to facilitate the exploration of natural products.
Accordingly, some researchers have questioned whether the habits of plants and their ecological biochemistry could somehow be predictors of which plants might possess medicinal properties (CitationJohns, 1996; CitationGottlieb et al., 1998; CitationStepp & Moerman, 2001). For example, CitationStepp & Moerman (2001) observed a high frequency of medicinal herbs in various traditional pharmacopoeias, suggesting that this may be related to the chemical and ecological aspects of these species. The authors suggest that the plant apparency hypothesis presents a possible scenario for understanding the patterns of selection of certain medicinal plants by different cultures.
The apparency hypothesis of plant defensive strategies was proposed by researchers studying insect–plant relationships (see CitationAlbuquerque & Lucena, 2005). CitationFeeny (1976), for example, believes that plants have developed two types of chemical defense against herbivory and they can therefore be classified as “apparent” and “non-apparent” plants. The “apparent” species (plants that are more susceptible to attack by herbivores due to the characteristics of their habits and life cycle) developed quantitative chemical defenses that work by reducing their digestibility and not through the development of high toxicity. These plants are also most often trees and plants with long life cycles. The “non-apparent” species accumulate qualitative defenses that are highly toxic in small quantities because they are highly active compounds. These species are most commonly herbs and plants with short life cycles. For example, the defensive ecology of the family Cruciferaceae seems to be typical of herbaceous plants, where chemical resistance presents itself in the form of small quantities of toxic compounds (CitationFeeny, 1977). Based on these generalizations, and on the selection strategies of medicinal plants used by traditional communities, the high occurrence of herbs in different pharmacopoeias is clearly present and consistent with these principles (see CitationStepp & Moerman, 2001).
If human populations guide the selection of medical resources, even unconsciously, then a test of the chemical–ecological strategies of these particular plants is necessary to determine if the plant apparency hypothesis can be used as a guide for future strategies in bioprospecting. CitationAlbuquerque and Lucena (2005) admit that people can also act as fodder for this theory, and thus, it is necessary to test the predictions of the plant apparency hypothesis within socioenvironmental systems.
This hypothesis is very difficult to evaluate, because there are not clear quantitative variables to test it. Only two studies have tested the relationship of this hypothesis to the ethnobotanical data for semi-arid environments (CitationAlmeida et al., 2005; CitationAlencar et al., 2009, Citation2010).
Thus, this study aims to test the predictions of the plant apparency hypothesis in two different environments: an area of dry forest (the Caatinga from northeastern Brazil) and an area of humid forest (the Atlantic Forest), using the local ethnobotanical data. The following issues are raised: Does the habit and life strategy of a plant predict the occurrence of certain phytocompound classes? Can the habit, life strategy, and chemical composition of a plant explain its relative local importance, as measured by the mean of the versatility of the plant?
Material and methods
Study area
Caatinga
In the Caatinga, the study was performed in the Soledade municipality, located in the Soledade microregion and in the mesoregion of Agreste, Paraíba state, at the coordinates 07°03′26′′ S and 36º21′46′′ W. Soledade is in the Paraíba River Basin and Taperoá River sub-basin (CitationLacerda et al., 2005), crossed by perennial rivers, but with small flow and low potential for groundwater. The seat of the Soledade municipality is 165.5 km from the capital of Joao Pessoa, 634.9 km2 in area and 521 m above sea level (CitationMascarenhas et al., 2005). The climate is semi-arid with warm summer rains (BSwh′), 7 to 8 months of drought and precipitation of only 400 to 600 mm per year (CitationSEBRAE, 1998). Due to the warm and dry climate, the vegetation consists of deciduous and thorny Caatinga forests (CitationBeltrão et al., 2005), with a dominant shrub layer and few individual trees. This study was conducted in three communities in the Caatinga where the main economic activity is subsistence agriculture: Cachoeira, Bom Sucesso, and Barrocas. Each community is located near Soledad city in the state of Paraiba; Cachoeira is 14 km away, Barrocas is about 18 km away, and Bom Sucesso is about 21 km. The distance between each city is about 7 km.
Atlantic Forest
In the Atlantic Forest, the municipality studied was Igarassu, located in the Itamaracá microregion and the mesoregion of Recife, Pernambuco state, at the coordinates 7°50′00′′ S and 34°54′30′′ W, 30 km from the state capital (CitationCONDEPE/FIDEM, 2007). The climate is tropical, hot, and humid, with rainfall in autumn and winter (As′). The average annual temperature is 25°C, and the average annual rainfall is around 2000 mm (CitationCONDEPE/FIDEM, 2007). The predominant vegetation includes the remnants of Atlantic Forest, scrubs, mangroves, palm trees, and areas of commercial and subsistence agriculture. There are ecological reserves in the municipality, such as the Usina São José Mata (State Law No. 9989 from January 13, 1987), with dense and widespread vegetation of 323.30 hectares located on the Transcanavieira Highway (PE-41) (CitationIgarassu, 2007). The study was conducted in the district of Três Ladeiras, 30 km north of the municipality headquarters (CitationIgarassu, 2007), which is inhabited by 1472 people, of which 876 are adults (436 women and 440 men) (CitationCONDEPE/FIDEM, 2007). Residents of the Três Ladeiras village interact with the native flora in a fragments of the Atlantic Forest, which has been declared as an ecological reserve (January 13, 1987, No. 9989). The surrounding area is a monoculture cane sugar plantation from Usina de São José.
Ethnobotanical data collection
Initially, the Free Informed Consent Term (FICT) was arranged with the residents who agreed to participate in the study. The legal and ethical aspects from resolution 196/96 of the Research Ethics Committee were followed closely to obtain permission to work with and interview the local residents about the plants that are known as medicinal in their culture.
The free list technique was used in the interviews to obtain the largest number of known ethnospecies and their uses in the region (CitationAlbuquerque et al., 2008). The following question was used to acquire general information on the botanical species: “What medicinal plants do you know?” Subsequently, a semistructured interview was conducted (CitationAlbuquerque et al., 2008) to obtain information on the plant parts used, the method of preparation, and identification. The interviews were conducted in the presence of the household head (over 18 years of age) on each occasion.
In the Caatinga, ethnobotanical data were collected in the municipality of Soledade, with sampling of all households in the communities of Cachoeira, Bom Sucesso, and Barrocas, totaling 55 interviews (40 women and 15 men, between 20 and 88 years), from January to July, 2006. In the Atlantic Forest, the work was performed in the municipality of Igarassu in the community of Três Ladeiras. As a community with a high number of residents, sampling was carried out in 53% of the households, totaling 203 interviews (148 women and 55 men, between 18 and 93 years) from June 2007 to January 2008.
In the Caatinga, the study resulted in the documentation of 100 species, but only 65 were sampled. In the Atlantic Forest, 177 species were recorded and 75 were sampled (for more information, CitationAlmeida et al., 2010). The collections were carried out after each interview. In the case of species that were not located near the interviewees’ homes, subsequent collection trips were made to the forest areas with the aid of a forester. The difference in the number of species cited during the interviews and the number of collected species is due to the exclusion of traded species and species that were mentioned by only one interviewee.
Species studied were identified and deposited in the Geraldo Mariz Herbarium (UFP), Federal University of Pernambuco and Vasconcelos Sobrinho Herbarium (PEUFR), Federal Rural University of Pernambuco.
Analysis and data processing
To test the predictions of the plant apparency hypothesis, the collected plants were identified and classified according to their habits and life strategies. In the field, data on the life strategies and habits were obtained for each species, as well as for all of the parts of the plant that were indicated as important for medical use (the whole plant, stem, bark, leaves, flowers, fruits, seeds, root, or latex). For species that had more than one part mentioned, the material was collected from the specific plant organ that was mentioned in the majority of the interviews.
The life strategies of the plants were defined according to CitationBegon et al. (1988), where the letters “r” and “K” are the parameters of the logistic equation. Individuals selected as “r” have high rates of reproduction and growths, being the more likely colonizers of an area, whereas the “K” individuals are species that have a lower potential for reproduction, but have better competitive survivability related to the density of the previous life stage and are present in constant habitats.
To categorize the type of plant habit each species possessed, the concepts proposed by CitationBegon et al. (1988) were adopted. Trees are considered to be perennial woody plants, usually with a single axis stem. Shrubs are also perennial woody plants but are of low stature and typically have many branches. Herbs are not woody plants and have relatively small shoots. The Cactaceae, Agavaceae, and Arecaceae, which occurred only in restricted environmental contexts, were classified using the categories of universal relevance: “tree,” “shrub,” and “herb” (CitationBrown, 1977).
Phytochemical tests were performed for the following compound classes: tannins, saponins, quinones (naphthoquinones and anthraquinones), coumarins, flavonoids, terpenoids (mono-, di-, tri-, and sesquiterpenes), and alkaloids. These compounds were selected due to their pronounced biological activity (CitationFalkenberg, 2004; CitationHenriques et al., 2004; CitationKuster & Rocha, 2004; CitationSantos & Mello, 2004; CitationSchenkel et al., 2004; CitationSpitzer, 2004; CitationZuanazzi & Mountain, 2004) and the need to work on compounds of both low and high molecular weight, as are predicted in the plant apparency hypothesis (CitationFeeny, 1976).
The selected compounds were divided into two categories according to CitationFeeny (1976): quantitative compounds (those of high molecular weight and low biological activity, such as tannins, saponins, quinones) and qualitative compounds (those of low molecular weight and high biological activity, such as coumarins, flavonoids, terpenoids, and alkaloids).
Tests were performed with the plant parts described by the respondents as medicinally useful, which were dried in the dark at room temperature after collection. Afterward, 20 g of plant material was crushed and sieved through 2.5-mm mesh. The resulting powder was subjected to cold extraction in 70% ethanol for 5 days. After this period, the extract was filtered with qualitative filter paper. The crude extracts were evaporated until the solvent was completely removed and was kept in a desiccator for 1 week. Subsequently, 20 mg of the dried extract was dissolved in 1 mL ethanol.
The phytochemical testing was performed using thin-layer chromatography (TLC) using Merck silica gel with 60 chromatoplates and with an F254 as a fluorescent indicator measuring 0.2 mm in thickness. The elution systems were specifically selected for each of the compound classes following the methods described by CitationWagner and Bladt (1996) and CitationHarbone (1982). This chromatographic method was chosen because it provides reasonably rapid results and repeatability, and because it is able to qualitatively detect the compounds selected for evaluation. Only the presence and absence of each chemical class was analyzed for each plant species that was indicated as medicinal based on traditional knowledge.
Data analysis
The relative importance (RI) of each plant species was calculated according to the method proposed by CitationBennett and Prance (2000), with “2” being the maximum value that a species can obtain. Species that obtained higher results were considered more versatile, and present a higher number of properties and bodily systems that can subsequently be treated. This analysis was performed only in order to test if the habit of a species could be a predictor of the versatility of a plant that already possesses medicinal uses. We applied the Kruskal–Wallis test (CitationSokal & Rholf, 1995) to examine if the RI of local species is associated with the life strategy, habit, chemical composition, and the plant parts used (P < 0.05).
To test the plant apparency hypothesis, the G-test was used (CitationSokal & Rholf, 1995) to verify the percentage of species with positive results for each class of chemical compounds in relation to the life strategy and habit (P < 0.05). Statistical tests were performed using the statistical package BioEstat 5.0 (CitationAyres & Ayres-Júnior, 2007).
Results and discussion
From the 65 species identified in the Caatinga region, 41.5% are trees, 17% are shrubs, and 41.5% are herbs. The 75 species of the Atlantic Forest are composed of 39% trees, 30.5% shrubs, and 30.5% herbs. An analysis of the life strategies showed that both species from the Caatinga and those from the Atlantic Forest were predominantly K-strategy species [68 and 77%, respectively ()], which are considered perennial species.
Table 1. Medicinal plants collected to study the criteria to be used for the selection and use in the Caatinga and the Atlantic Forest, northeastern Brazil.
RI of species
Of the five Caatinga species that obtained better results on their RI, four are native and are classified as trees: Maytenus rigida Mart. (Celastraceae) (1.78), Cnidoscolus quercifolius Pohl. (Euphorbiaceae) (1.66), Bauhinia cheilantha (Bong.) Steud. (Caesalpiniaceae) (1.64), and Commiphora leptophloeos (Mart) J.B. Gillet (Burseraceae) (1.61). The other species, Mentha piperita L. (Lamiaceae), is exotic and herbaceous and has a RI of 1.76 (). In a study conducted in the Caatinga by CitationAlencar et al. (2010), plants with a great versatility, as measured by RI, were also native trees, including: Caesalpinia ferrea Mart. (Caesalpiniaceae) (1.81), Myracrodruon urundeuva Allemão (Anacardiaceae) (1.75), and Mimosa tenuiflora (Willd.) Poir. (Mimosaceae) (1.50). CitationAlencar et al. (2009, Citation2010) also emphasize that from the 10 species with the highest RI values, nine were trees, which again shows the versatility of species with this habit.
In the Atlantic Forest, from the five species that stood out, just one was native and a tree, Schinus terebinthifolius Raddi (Anacardiaceae) (1.60). The others were exotic and herbaceous in habit, such as Mentha piperita (2.00), Lippia alba (Mill.) N.E..Br. (Verbenaceae) (1.86), Cymbopogon citratus (DC.) Stapf. (Poaceae) (1.47), and Plectranthus amboinicus (Lour.) Spreng. (Lamiaceae) (1.22). One exotic species is highlighted in both study sites, Mentha piperita ().
The trees in the Caatinga tended, on average, to receive higher scores in RI in relation to the shrubs (H = 6.7313, P < 0.05) and the herbs (H = 4.66, P < 0.05) (). These results differ from those obtained by CitationAlmeida et al. (2005), in which the highest RI scores obtained from the species studied in the other regions of the Caatinga where independent of habit, life strategy, and compounds classes, despite woody plants tending to have higher scores.
Table 2. Summary of the Kruskal–Wallis test based on the relative importance (RI), life strategies, habit, and phytocompounds, to study the criteria for the selection and use of medicinal plants in the Caatinga and the Atlantic Forest, northeastern Brazil (mean + standard deviation).
Thus, in the variety of species studied in this work, the RI was independent of the variables analyzed, except in the Caatinga region where the habit of the species was related to the RI. In the Atlantic Forest, there was no significant difference for the variables analyzed (), although herbs tended to have the highest RI scores.
Apparency hypothesis: compounds classes versus habit
All of the species studied possessed at least one of the seven classes of compounds. The Caatinga species presented the following configuration: saponins (76.9%), terpenes (72.3%), and tannins (60%). In the Atlantic Forest species, the configuration was different and was constituted by: flavonoids (86.7%), quinones (76%), and saponins (54.7%), which stood out (). We believe that the next step in future studies is to include quantitative tests for total phenols.
Figure 1. Positive correlations among the species studied related to phytocompounds of the medicinal plants used in the Caatinga and Atlantic Forest, northeastern Brazil.
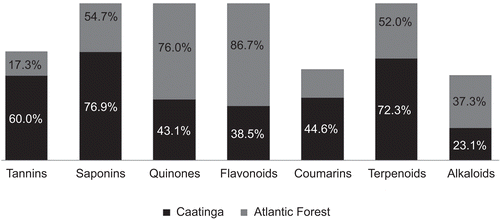
In the Caatinga, tannins were predominantly found in all of the habits studied, including trees (G = 10.13, P < 0.01), shrubs (G = 22.74, P < 0.0001) and herbs (G = 8.40, P < 0.01), whereas in the Atlantic Forest, tannins stood out only in shrubs (G = 16.70, P < 0.0001) and herbs (G = 9.52, P < 0.01) (). The plant apparency hypothesis predicts that qualitative compounds, such as alkaloids, flavonoids, and terpenoids, will have a higher occurrence in herbs. Our results were contradictory to this prediction. We found qualitative phytocompounds, flavonoids (G = 50.54, P < 0.0001) and alkaloids (G = 16.40, P < 0.01), to be more common in trees from the Caatinga, whereas in the Atlantic Forest, there was no distinction in the qualitative compounds between the different habits (see ). There is a clear trend in the quantity and quality of compounds and that occur in the Caatinga and in the Atlantic Forest. We expected that the “apparent” species would tend to accumulate quantitative chemical defenses, whereas the “non-apparent” species would present qualitative chemical defenses (CitationFeeny, 1976). The findings of our study allow us to reject the plant apparency hypothesis.
Table 3. Summary of the G-test related to the presence of all compound classes from the medicinal plants collected to study the criteria to be used for the selection and use of medicinal plants in the Caatinga and the Atlantic Forest, northeastern Brazil.
According to the results presented in our work, and based on all of the study species collected from the Caatinga and the Atlantic Forest, the habit and the life strategy of the plant are not predictors of the occurrence of certain phytocompound classes.
The herbs from Caatinga demonstrated a high occurrence of quantitative compound, such as tannins (G = 8.40, P < 0.01) and quinones (G = 47.99, P < 0.0001). In the Atlantic Forest, it was demonstrated that herbs possessed more qualitative compounds, such as flavonoids (G = 18.16, P < 0.0001), terpenoids (G = 10.35, P < 0.001), and alkaloids (G = 79.19, P < 0.0001) ().
Even with the low occurrence of alkaloids in the Caatinga species (see ), trees (G = 16.40, P < 0.01) and shrubs (G = 25.80, P < 0.0001) showed significant results for the presence of these compounds. However, CitationPelletier (1983) maintains that alkaloids are fairly common in plants. Contrary to our results, CitationLevin (1976) reports that there is no difference in the percentages of alkaloids between herbaceous and woody plants, although annual plants can contain more alkaloids than perennial species. This assertion can only be checked for the medicinal plants from Atlantic Forest.
Overall, our findings for the Caatinga plants are corroborated by previous tests of the plant apparency hypothesis by using the same methodological procedures (CitationAlmeida et al., 2005; CitationAlencar et al., 2009). Although these results lead to a rejection of the plant apparency hypothesis, a curious pattern emerges; quantitative compounds (i.e., tannins) have a broad occurrence in plants from the Caatinga, independent of habit, whereas in the Atlantic Forest there is a significant occurrence of qualitative compounds (i.e., alkaloids). In other phytochemical and herbivory studies, the findings followed the expected distributions of compounds, in contrast to the present study (see, e.g., CitationMcKey, 1984; CitationHeil et al., 2002). This apparent discrepancy needs to be further discussed.
The production of bioactive compounds in plants is due to different environmental influences. In general, in addition to adaptations to UV radiation (CitationGottlieb, 1987), there are other phenomena in the environment which require special adaptations including defense mechanisms of these plants to environmental conditions, microorganisms, insects, and animals (CitationMontanari & Bolzani, 2001), as well as adaptation to low density, humidity, and other environmental challenges.
Accordingly, bioprospecting studies for certain compounds should take into consideration the environment in which plants are developed. CitationGottlieb (1987), for example, argues that plants in arid and semi-arid regions, such as those in the Caatinga, tend to have favored the metabolic pathways that lead to the production of high-molecular-weight compounds (such as tannins). There is evidence, for example, that plants that are commonly used as anti-inflammatory agents in the Caatinga tend to have higher concentrations of tannic compounds, rather than other compounds that could also justify this activity, such as flavonoids (de Araújo et al., 2008). Flavonoids were very frequent in medicinal plants from both regions. This may be related to the deposition of flavonoids in the leaf surface for protection against UV radiation, heat reduction, herbivory and as an antimicrobial agent (CitationGottlieb, 1987).
Our findings indicate some important patterns that can be applied to bioprospecting that need to be tested with both experimental and systematic studies in different regions. The patterns indicate that medicinal plants (especially trees) from semi-arid regions (i.e. the Caatinga) are strongly orientated to accumulate quantitative compounds (such as tannins and saponins), and it is therefore more likely to find plants with significant biological activity related to these types of compounds in these habitats. The medicinal plants of wet forests (i.e. the Atlantic Forest), for example, tend to have a high occurrence of qualitative compounds (such as coumarins and alkaloids), and these compounds are especially prevalent in herbs.
Acknowledgements
The authors thank CNPq (“edital universal”) for financial support to the PhD student Cecilia de Fatima C.B.R. de Almeida and grants to U.P. Albuquerque. The Iana Moura and Reinaldo Lucena for help in ethnobotanical data collection in Soledade (PB) and the study participants from Cachoeira, Barrocas, Bom Sucesso, and Três Ladeiras for participating in this study and for valuable exchange of knowledge. The authors also thank Camila C.B.R. de Almeida, and Ana Carolina O. da Silva for technical assistance, and to the anonymous reviewers for suggestions and critical ideas.
Declaration of interest
We also thank the contribution of the project “Sustainability of remnants of the Atlantic rainforest in Pernambuco and its implications for conservation and local development”, a Brazilian-German scientific cooperation within the program “Science and Technology for the Atlantic Rainforest” funded by CNPq (590039/2006-7) and BMBF (01 LB 0203 A1).
References
- Albuquerque UP. (2010). Implications of ethnobotanical studies on bioprospecting strategies of new drugs in semi-arid regions. Open Complementary Med J, 2, 21–23.
- Albuquerque UP, Lucena RFP. (2005). Can apparency affect the use of plants by local people in tropical forests? Interciencia, 30, 506–511.
- Albuquerque UP, Lucena RFP, Alencar NL. (2008). Métodos e técnicas para a coleta de dados etnobotânicos. In: Albuquerque UP, Lucena RFP, Cunha LVFC, orgs. Métodos e técnicas na pesquisa etnobotânica. Recife: Editora Comunigraf/NUPEEA, pp. 41–72.
- Alencar NL, Araújo TAS, Amorim ELC, Albuquerque UP. (2009). Can the apparency hypothesis explain the selection of medicinal plants in an area of Caatinga vegetation? A chemical perspective. Acta Bot Bras, 23, 908–909.
- Alencar NL, Araújo TAS, Amorim ELC, Albuquerque UP. (2010). The inclusion and selection of medicinal plants in traditional pharmacopoeias—evidence in support of the diversification hypothesis. Econ Bot, 64, 68–79.
- Almeida CFCBR, Silva TCL, Amorim ELC, Maia MBS, Albuquerque UP. (2005). Life strategy and chemical composition as predictors of the selection of medicinal plants from the Caatinga (Northeast Brazil). J Arid Environ, 62, 127–142.
- Almeida Cde F, Ramos MA, Amorim EL, Albuquerque UP. (2010). A comparison of knowledge about medicinal plants for three rural communities in the semi-arid region of northeast of Brazil. J Ethnopharmacol, 127, 674–684.
- Ayres M, Ayres-Júnior M. (2007). BioEstat 5.0— Aplicações estatísticas nas áreas das ciências bio-médicas. Pará: Universidade Federal do Pará.
- Begon M, Harper JL, Townsend CR. (1988). Ecologia: Individuos, Poblaciones y Comunidades. Barcelona: Ediciones Omega.
- Beltrão BA, Morais F, Mascarenhas JC, Miranda JLF. (2005). Diagnóstico do município de Soledade. Projeto Cadastro de Fontes de Abastecimento por Água Subterrânea, estado da Paraíba. Recife: CPRM/PRODEEM.
- Bennett BC, Prance GT. (2000). Introduced plants in the indigenous pharmacopoeia of Northern South America. Econ Bot, 54, 90–102.
- Brown CH. (1977). Folk botanical life-forms: Their universality and growth. Am Anthropol, New Series, 79, 317–342.
- CONDEPE/FIDEM—Agência Estadual de Planejamento e Pesquisa de Pernambuco. (2007). Igarassu. Recife: FIDEM.
- Falkenberg MB. (2004). Quinonas. In: Simôes CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz LA, Petrovick PR, orgs. Farmacognosia—da Planta ao Medicamento, 5a. edição. Porto Alegre/Florianópolis: Editora Universitária/UFRGS/UFSC, pp. 657–684.
- Feeny P. (1977). Defensive ecology of the Cruciferae. Ann Missouri Bot Garden, 64, 221–234.
- Feeny PP. (1976). Plant apparency and chemical defense. In: Wallace JW, Mansell RL. eds. Recent Advances in Phytochemistry. New York: Plenum Press, pp. 1–40.
- Gottlieb OR. (1987). Evolução química vegetal. Cienc Cult, 39, 357–360.
- Gottlieb OR, Borin MRMB, Pagotto CLAC, Zocher DHT. (1998). Biodiversidade: o enfoque interdisciplinar brasileiro. Cien & Saúde Colet, 3, 97–102.
- Harbone JB. (1982). Phytochemical Methods, 2nd ed. London: Chapman & Hall.
- Heil M, Delsinne T, Hilpert A, Schürkens S, Andary C, Linsenmair KE, Sousa SM, McKey D. (2002). Reduced chemical defence in ant-plants? A critical re-evaluation of a widely accepted hypothesis. Oikos, 99, 457–468.
- Henriques AT, Limberger RP, Kerber VA, Moreno PRH. (2004). Alcalóides: Generalidades e aspectos básicos. In: Simôes CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz LA, Petrovick PR, orgs. Farmacognosia—da Planta ao Medicamento, 5a. edição. Porto Alegre/Florianópolis: Editora Universitária/UFRGS/UFSC, pp. 765–792.
- Igarassu—Secretaria de turismo, cultura e esportes do município de Igarassu. (2007). Inventário do Potencial Turístico de Igarassu. Igarassu: Prefeitura Municipal.
- Janni KD, Bastien JW. (2004). Exotic botanicals in the Kallawaya pharmacopoeia. Econ Bot, 58, S274–S279.
- Johns T. (1996). The Origins of Human Diet & Medicine. Arizona: The University of Arizona Press.
- Kuster RM, Rocha LM. (2004). Cumarinas, cromonas e xantonas. In: Simôes CMO, Schenkel EP, Gosmann G, Mello, JCP, Mentz LA, Petrovick PR, orgs. Farmacognosia—da Planta ao Medicamento, 5a. edição. Porto Alegre/Florianópolis: Editora Universitária/UFRGS/UFSC, pp. 573–556.
- Lacerda AV, Nordi N, Barbosa FM, Watanabe T. (2005). Levantamento florístico do componente arbustivo-arbóreo da vegetação ciliar na bacia do rio Taperoá, PB, Brasil. Acta Bot Brasíl, 19, 647–656.
- Levin DA. (1976). Alkaloid bearing plants: An ecogeographic perspective. Amer Nat, 110, 261–284.
- Mascarenhas JC, Beltrão BA, Souza Júnior LC, Morais F, Mendes VA, Miranda JLF. (2005). Projeto Cadastro de Fontes de Abastecimento por Água Subterrânea. Diagnóstico do município de Soledade, estado da Paraíba.
- McKey D. (1984). Interaction of the ant-plant Leonardoxa africana (Caesalpiniaceae) with its obligate inhabitants in a rain forest in Cameroon. Biotropica, 16, 81–99.
- Montanari CA, Bolzani VS. (2001). Planejamento racional de fármacos baseado em produtos naturais. Quím Nova, 24, 105–111.
- Palmer C. (2004). The inclusion of recently introduced plants in the Hawaiian ethnopharmacopoeia. Econ Bot, 58, S280–S293
- Pelletier SW. (1983). The nature and definition of an alkaloid. In: Pelletier SW, ed. Alkaloids: Chemical and Biological Perspectives. New York: Wiley, pp. 1–31.
- Santos SC, Mello JCP. (2004). Taninos. In: Simôes CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz LA, Petrovick PR, orgs. Farmacognosia—da Planta ao Medicamento, 5a. edição. Porto Alegre/Florianópolis: Editora Universitária/UFRGS/UFSC, pp. 615–656.
- Sousa Araújo TA, Alencar NL, Amorim EL, Albuquerque UP. (2008). A new approach to study medicinal plants with tannins and flavonoids contents from the local knowledge. J Ethnopharmacol, 120, 72–80.
- Schenkel EP, Gosmann G, Athayde ML. (2004). Saponinas. In: Simões CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz LA, Petrovick PR, orgs. Farmacognosia—da Planta ao Medicamento, 5a. edição. Porto Alegre/Florianópolis: Editora Universitária/UFRGS/UFSC, pp. 711–740.
- SEBRAE—Serviço Brasileiro de Apoio às Micro e Pequenas Empresas. (1998). Série Diagnóstico Sócio-Econômico. Soledade. João Pessoa: Proder.
- Sokal RR, Rholf FG. (1995). Biometry. New York: Freeman and Company.
- Spitzer CMOSV. (2004). Óleos voláteis. In: Simôes CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz LA, Petrovick PR, orgs. Farmacognosia—da Planta ao Medicamento, 5a. edição. Porto Alegre/Florianópolis: Editora Universitária/UFRGS/UFSC, pp. 467–496.
- Stepp JR, Moerman DE. (2001). The importance of weeds in ethnopharmacology. J Ethnopharmacol, 75, 19–23.
- Voeks RA. (1996). Tropical forest healers and habitat preference. Econ Bot, 50, 381–400.
- Wagner H, Bladt S. (1996). Plant Drug Analysis—A Thin Layer Chromatography Atlas, 2ª. edição. Berlin: Springer-Verlag Berlin.
- Zuanazzi JAS, Montanha JA. (2004). Flavonóides. In: Simôes CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz LA, Petrovick PR, orgs. Farmacognosia—da Planta ao Medicamento, 5a. edição. Porto Alegre/Florianópolis: Editora Universitária/UFRGS/UFSC, pp. 577–614.