Abstract
Context: Vitex negundo Linn. (Verbenaceae) is an indigenous tree species in India. This tree species has been of interest to researchers because traditionally its roots are reported in the treatment of ulcer and colic pain.
Objective: The present work was undertaken to validate its folk use in the treatment of ulcerative colitis (UC) by using the method of acetic acid-induced colitis in mice.
Materials and methods: Ethanol and aqueous extracts of roots of V. negundo (100 mg/kg) were screened for use in the treatment of UC by the method of acetic acid-induced UC in mice. Macroscopical study of the colon, level of myeloperoxidase (MPO), malondialdehyde (MDA) in colon tissue and blood and histopathology of the colon tissue were studied for the assessment.
Results: Ethanol extract (100 mg/kg) reduced the level of MPO in blood from 355 ± 0.39 to 240 ± 0.36 U/mL and from 385 ± 0.35 to 257 ± 0.36 U/mg in tissue. Similarly, it reduced the level of MDA in blood from 9.40 ± 0.42 to 6.10 ± 0.36 nmol/mL and from 9.38 ± 0.56 to 5.89 ± 0.56 U/mg in tissue. Both the results are comparable with the standard drug, prednisolone (5 mg/kg). This preventive effect was observed by morphological and histopathological study.
Discussion and conclusion: Results showed that ethanol extract of V. negundo root is effective in the treatment of UC and results are comparable with the standard drug, prednisolone, and thus possessing a great potential in the treatment of UC.
Introduction
Ulcerative colitis (UC) is a subcategory of inflammatory bowel disease (IBD). Colitis affects the inner most lining or mucosa of the colon and rectum. A continuous area of inflammation and ulceration with no segments of normal tissue is observed (CitationMohan, 2005). The two primary types of IBD are Crohn’s disease and UC. In IBDs, the intestine (bowel) becomes inflamed, often causing recurring abdominal cramps and diarrhea. Although the exact cause of UC remains undetermined, the condition appears to be related to a combination of genetic and environmental factors. Among the pathological findings associated with IBD are increase in certain inflammatory mediators, signs of oxidative stress, a deranged colonic milieu, abnormal glycosaminoglycan content of the mucosa, decreased oxidation of short-chain fatty acids, increased intestinal permeability, increased sulfide production, and decreased methylation. No single factor has been identified as the initial trigger for IBD (CitationRutgeerts & Geboes, 2001). Hypochlorous acid, produced by the action of myeloperoxidase (MPO) on hydrogen peroxide in the presence of chloride ions is importantly involved in the inflammatory reaction in colitis (CitationCetinkaya et al., 2005). One of the most frequently used biomarkers providing an indication of the overall lipid peroxidation level is the plasma concentration of malondialdehyde (MDA) and it is used to measure the level of oxidative stress in organism (CitationChurch & Pryor, 1985). Advances in our knowledge of the pathophysiology of UC have highlighted the importance of cytokines such as tumor necrosis factor-α (TNF-α) in the inflammatory process. TNF-α is a proinflammatory mediator that plays an integral role in the pathogenesis of IBD. In addition, mounting evidence indicates a genetic association between TNF-α and UC. Furthermore, increased TNF-α levels have been demonstrated in studies of patients with UC. TNF-α is likely an important component in the pathophysiology of UC, and thus, agents targeting TNF-α in UC have been studied. Recent randomized controlled trials have confirmed that biologic anti-TNF-α therapy is effective in UC. Soluble TNF-α receptors or biologic agents that suppress or inhibit TNF-α production may also show therapeutic promise (CitationSands and Kaplan, 2007). The role of prostaglandins in the course of IBDs and their possible usefulness as predictive indicators of inflammation, remains largely speculative. Recently, it was found that plasma and mucosal prostaglandin E2 (PGE2) rise simultaneously with the degree of colonic injury. Because of a good correlation with mucosal injury and PGE2 content, measurement of plasma PGE2 could be considered as a possible surrogate marker of bowel inflammation (CitationWiercinska-Drapalo et al., 1999).
Vitex negundo L. (Verbenaceae) is a branched shrub that occurs throughout India, commonly known as Nirgundi (CitationKirtikar & Basu, 1956). Nirgundi is an important drug of indigenous systems of medicine and its root part has been attributed a number of medicinal properties in ayurveda (CitationNadkarni, 1991). Traditionally, roots of V. negundo are reported for the treatment of UC (CitationNadkarni, 1991); the present work verified its use in the treatment of UC using the method of acetic acid-induced colitis in mice.
Material and methods
Plant material
The roots of V. negundo were collected from Ahmednagar district (M.S.) in May 2010 and authenticated by Mr. P. G. Diwakar, Deputy Director, Botanical Survey of India, Pune (Voucher specimen—VBBZN1).
Extraction
The roots were dried under the shade and then powdered. The dried powdered material (500 g) was subjected to extraction with ethanol AR in Soxhlet apparatus and then the marc left was extracted with water in a reflux condenser. Both the extracts were vacuum dried to yield 8.16% of ethanol extract and 4.48% of aqueous extract.
Animals
Male Swiss mice (20–25 g) were used. The animals were housed under standard laboratory conditions and fed with standard rodent diet and water ad libitum. Rodent diet is composed of crude proteins 16%, crude fats 3.8%, crude fibers 2%, amino acids, vitamins, and minerals. The animals were kept in constant temperature (22 ± 2°C), humidity (55%), and light-dark condition (12/12 h light/dark). The experimental protocol was approved by institutional animal ethical committee (Approval No. CPCSEA/C/448/08-0-/17).
Pharmacological screening
Induction of experimental colitis
Animals were divided into four groups. Control group mice received vehicle (0.2 mL of 5% Tween 80 in distilled water). Standard group received prednisolone at the dose of 5 mg/kg, i.p. Animals from group III and IV were received ethanol and aqueous extracts of roots of V. negundo at the dose of 100 mg/kg suspended in vehicle for 7 days. On the eighth day, colitis was induced by intrarectal administration of 150 µL of 5% acetic acid (pH 2.5), 3 cm from the anal margin. Extract treatment was continued up to the tenth day (CitationNakhai et al., 2007).
Determination of ulcer index
The entire colon was isolated, opened longitudinally, and rinsed with phosphate buffer saline (PBS; freshly prepared). The ulceration of the opened colon was measured with help of a magnifying glass and the ulcer index was calculated by following formula (CitationGoel & Sairam, 2002).
Assessment of colitis severity
After 48 h of colitis induction mice were sacrificed by cervical dislocation and dissected upon to remove the colon. The entire colon was isolated, opened longitudinally, and rinsed with PBS. Histological scoring of the colon damage was performed. For each mouse, the ulcer area was determined by summing the sizes of lesions measured macroscopically. The total area of damage was expressed as the relative percentage of the total surface area of the colon (CitationMahgoub et al., 2003).
Determination of colonic MPO activity
After the macroscopic measurements, the excised colon (100–150 mg) were homogenized with PBS (pH 7.4) and centrifuged at 1000 rpm for 20 min at 4°C. MPO activity of supernatants was then assayed by mixing the supernatant with citric phosphate buffer (pH 5.0) containing 0.4 mg/mL O-phenylene diamine LR and 0.015% hydrogen peroxide LR. The change in absorbance at 492 nm was measured spectrophotometrically and compared with the standard dilution with horseradish peroxidase LR (CitationEvans et al., 2000).
Determination of MDA level
The reaction mixture containing 0.1 mL tissue sample, 0.2 mL 8.1% sodium dodecyl sulfate LR, 1.5 mL 2% acetic acid, and 1.5 mL 0.8% aqueous solution of thiobarbituric acid LR. The mixture pH was adjusted to 3.5 and volume was finally made up to 4 mL with distilled water and 5 mL of mixture of n-butanol and pyridine (15%) was added. The mixture was shaken vigorously. After centrifugation at 4000 rpm for 10 min, the absorbance of organic layer was measured at 532 nm. MDA was expressed as nmol/mg of protein (CitationBuege and Aust, 1978).
Histopathological study
Tissue was fixed with 10% formalin for 24–36 h and then trimmed at suitable site and washed under running tap water for 2 h then the tissue is dehydrated with help of increasing grades of alcohol LR (50% alcohol overnight, 70% alcohol for 2 h, 80% alcohol for 2 h, 90% alcohol for 2 h, and absolute alcohol for 2 h). Then, the tissue was cleaned with xylene LR for 1 h and embedded with paraffin wax at 60°C. Blocks were prepared and stored in the freezer for 45 days. Slices of tissue were cut at 5-mm thickness. Slices were taken on to clean grease-free glass slides smeared with egg albumin in water bath at 60°C. Tissue was deparafinnated partially with heat and followed by immersing in the xylene LR for 3 min in each (three changes of 3 min each). Sections were rehydrated with decreasing grades of the alcohol LR [100, 90, 80, and 50% (3 min in each)]. Slides were kept in distilled water (5 min) and in hematoxylin LR (10 min). One dip in 1% ammonia water was done and immediately washed under running tap water (5 min). Two to three drops of alcoholic eosin were given and shades again dehydrated with increasing grades of alcohol [70, 80, 90, and 100% (3 min in each)]. Slices were cleaned with xylene (3 min and three changes), mounted with DPX mountant, and observed under suitable magnification.
Statistical analysis
All results are expressed as mean ± SEM (n = 6). The statistical significance was calculated by the software “Prismprimer”. The statistical significance (p < 0.05) of differences between mean was assessed by analysis of variance followed by Tukey–Kramar multiple comparison test.
Results and discussion
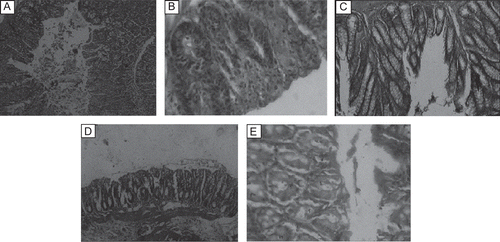
Histopathological observation showed hemorrhage, ulceration, hyperemia, destruction of mucosal epithelium, and edema in the colon of mice treated with acetic acid. Treatment with ethanol extract (100 mg/kg) showed absence of hemorrhage (, ) and least ulceration, hyperemia, necrosis, edema, cellular infiltration, and globlet-cell hyperplasia in the colon (, ). Results from ulcer index showed better protective effect of ethanol extract than aqueous extract (). This showed protective effect of ethanol extract in the treatment of colitis.
Table 1. Histopathological observations after the treatment with extracts of Vitex negundo roots.
Table 2. Histopathological observations after the treatment with extracts of Vitex negundo roots.
Table 3. Effect of extracts of Vitex negundo roots on ulcer protection.
Figure 1. Histopathological observations of colon tissue after the treatment with ethanol and aqueous extracts (100 mg/kg) of Vitex negundo roots. (A) Normal; (B) control (5% acetic acid); (C) standard; (D) ethanol extract; (E) aqueous extract.
Acetic acid causes increase in MPO level in blood from 75 U/mL to 355 U/mL. After treatment with ethanol extract (100 mg/kg), the MPO level in blood was decreased significantly to 240 U/mL. Similar results was observed in tissue. MPO level in tissue was reduced significantly to 257 U/mg from 385 U/mg increased due to acetic acid treatment (). Ethanol extract (100 mg/kg) significantly reduced MDA level in blood from 9.4 nmol/mL to 6.1 nmol/mL and from 9.38 U/mg to 5.89 U/mg in tissue (). Ethanol extract was found better than aqueous extract to reduce increased MPO and MDA levels in blood and tissue and the activity was compared with standard drug prednisolone. As ethanol extract reduced both MPO and MDA level significantly, it may have potential anti-inflammatory role in the treatment of colitis because MPO is involved in the inflammatory reaction in colitis (CitationCetinkaya et al., 2005) and increased level of MDA is indicating oxidative stress in organ and thus causes inflammation (CitationChurch & Pryor, 1985).
Table 4. Effect of extracts of Vitex negundo roots on myeloperoxidase activity.
Table 5. Effect of extracts of Vitex negundo roots on malondialdehyde activity.
Cytokines are responsible for modulating intestinal inflammation and injury (CitationArdizzone & Bianchi Porro, 2005; CitationNakamura et al., 2006). Increased levels of TNF-α and PGE2 may cause epithelial cell necrosis, edema, and neutrophil infiltration, as proved by the histopathological study. Recently CitationStucchi et al. (2006) found that LITAF (lipopolysaccharide-induced TNF-α), which mediates TNF-α expression in human macrophages, is significantly elevated above controls in macrophages of ileal and colonic tissues from patients with either Crohn’s disease or UC. Elevated levels of PGE2, goes in harmony with CitationOtani et al. (2006) who proved that the increased level of PGE2 is attributed to its enhanced synthesis rather than reduced catabolism, both of which are mediated by TNF-α. On the other hand ethanol extract of V. negundo roots decreased significantly the gross lesion scores, and may be production of TNF-α and PGE2. Inhibition of PGE2, on the other hand, may follow that of TNF-α (CitationOtani et al., 2006), or may result from its ability to inhibit cycloxygenase enzymes (CitationGrzanna et al., 2005). Since, the intestine is in a constant state of controlled inflammation, the amplification of the inflammatory response activates infiltration of inflammatory cells that triggers pathological responses and symptoms of IBD (CitationSartor, 1997). Our study showed that acetic acid raised the levels of colonic MPO, indicating infiltration of neutrophils and perturbation of the inflammatory system (CitationKrawisz et al., 1984). This fact is documented in both animal models (CitationAkgun et al., 2005; CitationCetinkaya et al., 2005), and patients with IBD (CitationKruidenier et al., 2003). Ethanol extract of V. negundo ameliorated neutrophil infiltration as evidenced by suppression of colon MPO and improvement of histological features. This action lends pharmacological support to folkloric, ethno-medical uses of the plant in the management of inflammatory gastrointestinal tract disorders.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Akgun E, Caliskan C, Celik HA, Ozutemiz AO, Tuncyurek M, Aydin HH. (2005). Effects of N-acetylcysteine treatment on oxidative stress in acetic acid-induced experimental colitis in rats. J Int Med Res, 33, 196–206.
- Ardizzone S, Bianchi Porro G. (2005). Biologic therapy for inflammatory bowel disease. Drugs, 65, 2253–2286.
- Buege JA, Aust SD. (1978). Microsomal lipid peroxidation. Meth Enzymol, 52, 302–310.
- Cetinkaya A, Bulbuloglu E, Kurutas EB, Ciralik H, Kantarceken B, Buyukbese MA. (2005). Beneficial effects of N-acetylcysteine on acetic acid-induced colitis in rats. Tohoku J Exp Med, 206, 131–139.
- Church DF, Pryor WA. (1985). Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect, 64, 111–126.
- Evans M, Laszlo R, Brendan J, Whitlle R. (2000). Site specific lesion formation inflammation and inducible nitric oxide synthase expression by indomethacin in the rat intestine. Eur J Pharm, 388, 281–285.
- Goel RK, Sairam K. (2002). Antiulcer drugs from indigenous sources with emphasis on Musasapientum tamrabhasma, Asparagus racemosus and Zingiber officinale. Indian J Pharmacol, 34, 100–110.
- Grzanna R, Lindmark L, Frondoza CG. (2005). Ginger-an herbal medicinal product with broad anti-inflammatory actions. J Med Food, 8, 125–132.
- Kirtikar KR, Basu BD. (1956). Indian Medicinal Plants, second edition. Deharadun, Uttarakhand, India: International Book Distributors, 723.
- Krawisz JE, Sharon P, Stenson WF. (1984). Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology, 87, 1344–1350.
- Kruidenier L, Kuiper I, Lamers CB, Verspaget HW. (2003). Intestinal oxidative damage in inflammatory bowel disease: Semi-quantification, localization, and association with mucosal antioxidants. J Pathol, 201, 28–36.
- Mahgoub AA, El-Medany AA, Hager HH, Mustafa AA, El-Sabah DM. (2003). Evaluating the prophylactic potential of zafirlukast against the toxic effects of acetic acid on the rat colon. Toxicol Lett, 145, 79–87.
- Mohan H. (2005). Textbook of Pathology, fifth edition. New Delhi, India: Jaypee Brothers Medical Publishers, 580.
- Nadkarni AK. (1991). Indian Materia Medica, third edition. Bombay, Maharashtra, India: Bombay Popular Prakashan, 1197–1198.
- Nakamura K, Honda K, Mizutani T, Akiho H, Harada N. (2006). Novel strategies for the treatment of inflammatory bowel disease: Selective inhibition of cytokines and adhesion molecules. World J Gastroenterol, 12, 4628–4635.
- Nakhai LA, Nakhai LA, Yasa N, Boushe VS. (2007). Benefits of Zataria multiflora Boiss in experimental model of mouse inflammatory bowel disease. Evid Based Complement Alternat Med, 4, 43–50.
- Otani T, Yamaguchi K, Scherl E, Du B, Tai H, Greifer M, Petrovic L, Daikoku T, Dey SK, Subbaramaiah K, Dannenberg AJ. (2006). Levels of NAD+-dependent 15 hydroxyprostaglandin dehydrogenase are reduced in inflammatory bowel disease: Evidence for involvement of TNF-α. Am J Physiol Gastrointest Liver Physiol, 290, G361–G368.
- Rutgeerts P, Geboes K. (2001). Understanding inflammatory bowel disease–the clinician’s perspective. Eur J Surg Suppl, 586, 66–72.
- Sands BE, Kaplan GG. (2007). The role of TNFalpha in ulcerative colitis. J Clin Pharmacol, 47, 930–941.
- Sartor RB. (1997). Pathogenesis and immune mechanisms of chronic inflammatory bowel disease. Am J Gastroenterol, 92, 5S–11S.
- Stucchi A, Reed K, O’Brien M, Cerda S, Andrews C, Gower A, Bushell K, Amar S, Leeman S, Becker J. (2006). A new transcription factor that regulates TNFalpha gene expression, LITAF, is increased in intestinal tissues from patients with CD and UC. Inflammatory Bowel Disease, 12, 581–587.
- Wiercinska-Drapalo A, Flisiak R, Prokopowicz D. (1999). Mucosal and plasma prostaglandin E2 in ulcerative colitis. Hepatogastroenterology, 46, 2338–2342.