Abstract
Context: Joloo is a Nigerian herbal decoction used for managing breast tumor, ulcer, pain, fever and general malaise in southwestern Nigeria.
Objective: The evaluation of the sub-chronic toxicity of Joloo, a Nigerian herbal decoction, is done by investigating its effects on biochemical, antioxidant, histopathologic and hematologic indices in normal albino rats.
Materials and methods: Albino rats of either sex weighing between 128 and 160 g were divided into 4 groups of 10 rats each. Three test groups were orally administered 400, 800 and 1600 mg kg−1 body weight (b. wt.) doses of Joloo while control animals received distilled water over 28 days. Animal were weighed weekly and sacrificed after day 28. Organs were harvested, weighed and subjected to histopathologic assessment. Liver and blood samples were used for biochemical, antioxidant and hematological studies.
Results: Mortality and signs of toxicity were absent in animals treated with 400 and 800 mg kg−1 doses of Joloo. At 1600 mg kg−1 dose, 20% mortality occurred. Decreased body weight and red blood cells (P < 0.05) observed at 1600 mg kg−1 differed significantly from control animals. No significant changes in body and organ weights presented. Significant increases in biochemical analytes and histopathologic parameters were unobserved. Rather, Joloo increased leukopoiesis and exhibited antioxidant activities at all doses.
Discussion: Joloo proved safe at lower doses. The mortality at 1600 mg kg−1 could be due to disturbances in the physiology of the animals. The significant reduction in erythropoiesis could indicate early signs of toxicity. However, the unremarkable increases in hepatic and antioxidant enzymes may suggest that Joloo modulated oxidative status in the animals.
Conclusion: Joloo seems safe at lower doses, but caution is advised at higher doses.
Introduction
Medicinal plants play significant roles in health management in Africa. They are also reliable sources of raw materials for both traditional and modern medicines.
Joloo is a Nigerian formulation extracted from seven medicinal plants used in folk medicine practice for the management and treatment of breast cancer in southwestern Nigeria. CitationOloyede et al. (2008) reported the analgesic and anti-inflammatory activities of Joloo. A recent investigation reported the antimitotic activities of Joloo suggesting the likely presence of some anti-cancer properties (CitationOloyede et al., 2009).
Some of the medicinal uses of the individual plants used in formulating Joloo have been documented in literature. In traditional medicine, the whole plant of Allium ascalonicum Linn. (Liliaceae: Alliaceae) has been used both as a hypoglycemic and bacteriostatic agent (CitationBurkill, 1995). The plant has been reported also to possess some antitumor properties (CitationOdugbemi, 2006). Butyrospermum paradoxum Gaertn (Sepotaceae) has been used as a stimulant, carminative, antihelminthic and antihypertensive in Nigeria (CitationBurkill, 2000; CitationOdugbemi, 2006). CitationBurkill (1995) reported that Hoslundia opposita Vahl (Labitae) has antipyretic, diuretic, cholagogic, antimalarial and anticonvulsant properties while Olax subscorpioidea Olive (Olacaceae) is used traditionally as an anti-arthritic, antirheumatic (CitationBurkill, 1997) and antimicrobial remedy (CitationAyandele and Adebiyi, 2007). Xylopia aethiopica Dunal A. Richard (Annonaceae) has been associated with the treatment of cancer and ulcer in Nigeria (CitationBurkill, 1985), while Securidaca longepedunculata Fresen (Polygalaceae) is reported to possess antirheumatic, antipyretic and anti-inflammatory properties (CitationAsres et al., 2001). The pod extract of Tetrapleura tetraptera Schum. and Thonn. (Leguminosae: Mimosidae) is used to treat chest pain, female sterility, ulcer, convulsion and arthritis (CitationBurkill, 1995; CitationOdugbemi, 2006). Although these plants have been utilized individually in various ways to treat different ailments, to the best of our knowledge, there is no known report on the sub-chronic toxicity and antioxidant activities of these plants when used in combination.
The objective of this study, therefore, was to evaluate the sub-chronic toxicity of Joloo using its effect on hematologic, biochemical, antioxidant and histopathologic indices as a measure of toxicity in normal albino rats. This study will assist in ascertaining the safety of this widely used local decoction.
Materials and methods
Plant materials
The plants A. ascalonicum PCGH 440, B. paradoxum PCGH 473, H. opposita PCGH 322, O. subscorpioidea PCGH 438, X. aethiopica Dunal PCGH 441, S. longepedunculata PCGH 439, and T. tetraptera PCGH 382, were collected in March, 2009 from a traditional medicine practitioner in Totoro village, Abeokuta, Ogun State, Nigeria. They were identified and authenticated by Mrs. Olorunyomi Moji of the Department of Pharmacognosy, Faculty of Pharmacy, University of Lagos, Lagos, Nigeria, where the voucher specimens were also deposited.
Preparation of the decoction
The decoction was prepared as described by CitationOloyede et al. (2008). The seeds of B. paradoxum, whole plant of H. opposita, roots of O. subscorpioidea, fruits of X. aethiopica, roots of S. longepedunculata, whole plant of A. ascalonicum and pods of T. tetraptera were air-dried and mixed in the ratio of 5:2:1:4:1:3:3, respectively, to give the desired preparation. The plant materials (1 kg) were subsequently powdered and soaked in 4000 mL of 95% cold ethanol (based on the traditional mode of use of the extract) for 72 h and then decanted and sieved using muslin cloth. Thereafter, the ethanol extract obtained was filtered with muslin cloth and then evaporated to dryness in an oven set at 40°C to yield 89.6 g (8.96%) extract for each week. This extraction was carried out weekly with same materials from the same collection for 4 weeks to derive fresh and adequate amount of extract each week. The powdered extract (89.6 g) was reconstituted in distilled water according to doses selected through LD50 (CitationOloyede et al., 2009). The required concentration served as the decoction used for the experiments.
Animals
Normal albino rats of either sex, weighing between 128 and 160 g, were purchased from the Nigerian Institute of Medical Research, Yaba, Lagos, Nigeria. The experimental protocol was approved by the University of Lagos Ethical Committee on Animal Use. Animals were housed in aerated metal cages in the animal house of the University of Lagos, acclimatized for 1 week before experiments and fed with standard rat chow manufactured by Ladokun Feeds Limited, Ibadan, Nigeria, and water ad libitum. Rats were maintained under standard environmental conditions (CitationMbagwu et al., 2007) throughout the experimental period.
Sub-chronic oral toxicity study of Joloo in rats for 28 days
Study design
Animals divided into 4 groups of 10 rats each were treated with Joloo for 28 days by oral gavage using a curved blunt tipped stainless steel feeding needle. Group one (control) was treated with distilled water 10 mL kg−1 while groups two, three and four received Joloo at doses of 400, 800 and 1600 mg kg−1 b. wt., respectively. The weights of the animals were monitored and recorded weekly for 4 weeks after which animals were sacrificed. The organs and tissues were then collected, weighed and processed on day 28 for relevant assays and histopathologic analysis.
Mortality and clinical signs
During the administration period of 28 days, animals were observed for general appearance, mortality and clinical signs.
Hematology, relative organ weight and necropsy
The rats were fasted for 16–19 h on the autopsy day and anesthetized with ethyl ether. All the animals were then euthanized by exsanguination and blood samples collected from the abdominal aorta into EDTA vials for routine hematological investigation.
The spleen, heart, liver, kidneys, and lungs were harvested and weighed to determine the absolute organ weight. The relative organ weight of each animal was then calculated based on body weight measured on the day of sacrifice as follows:
Absolute organ weight (g)
Relative organ weight (g) = × 100
Body weight of rat on sacrifice day (g)
The organs were thereafter preserved in 10% buffered formalin for histopathologic examinations. The tissue biopsies were dehydrated and embedded in paraffin, cut into 4–5 μm sections with rotary microtome (LEICA RM 2235 Rotary Microtome), and stained with hematoxylin-eosin for photomicroscopic examination.
Biochemical analysis
The livers were homogenized in 0.1 mol/L sodium phosphate buffer (pH 7.4) and centrifuged (8000 rpm for 20 min at 4°C). The homogenate derived from each sample was used for all assays which included aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total cholesterol, and creatinine kinase. Spectrophotometer optimal sp 3000 model was used for this study.
Antioxidant assay
Antioxidant assay was not the primary focus of this study. It was only included to see the effect of Joloo on superoxide dismutase (SOD) and catalase (CAT). Therefore, detailed studies on antioxidant assay were not undertaken. Direct connection with traditional uses has been discussed in this paper.
The liver homogenate was used for the estimation of SOD according to the method described by CitationEl-Beshbishy (2008). SOD activity was measured at 37°C by the xanthine oxidase method using purified bovine erythrocyte SOD (5000 units mg−1 solid) as standard. The activity is expressed as the amount of the erythrocyte SOD showing activity equivalent to the determined activity. CAT activity was determined as per the method modified by CitationEl-Beshbishy (2008). The activity was measured at 37°C by recording the rate of H2O2 decomposition at 240 nm. One unit (U) of this activity is defined as the amount of enzyme decomposing one μmol H2O2 as a substrate per minute.
Results
Clinical signs and mortality
During the 28 days treatment period, 20% mortality was recorded among the animals treated with 1600 mg kg−1 dose of Joloo. These animals showed signs of emaciation (). On the other hand, neither mortality nor any other adverse clinical manifestations were observed in the 400 and 800 mg kg−1 dose test groups.
Table 1. Effect of Joloo on some blood parameters and body weight of albino rats in 28 days.
The rats in the control group gained weight throughout the duration of treatment. Variable changes like frequent urination and restlessness were observed in rats treated with Joloo at all doses used (400, 800 and 1600 mg kg−1). However, these changes were not significantly different compared to rats in the control group.
Hematologic indices
Variable changes with no defined patterns were observed in the level of neutrophils while the WBC, on the other hand, increased with increasing doses (). There was a significant graded dose-dependent reduction in RBC.
Biochemical indices
The biochemical indices clearly showed that the effect of Joloo was dose-dependent and that no statistically significant adverse effects were observed in all the parameters (). Generally, except in C/Knac, there was no significant change in all the enzyme activities and biochemical analytes at all doses. ALP activity in the rats treated with 1600 mg kg−1 of Joloo was slightly higher than the value in the control group. However, the increases were not significant in all the cases.
Table 2. Effect of Joloo on rat liver enzymes activities and serum biochemical analytes post 28 days treatment.
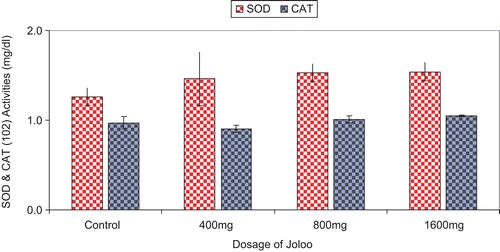
SOD and CAT activities
The activities of SOD and CAT were not significantly different from control, but SOD showed a gradual dose-dependent increase at all doses of Joloo used ().
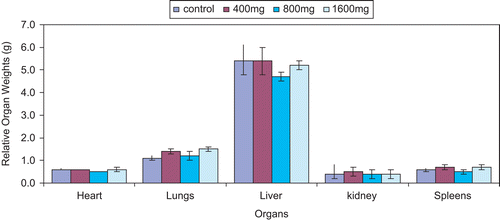
Histopathologic studies
Mean relative organ weights in the Joloo treated groups were comparable to control (P > 0.05) (). There were no significant changes in the relative weight of the heart, lungs, and kidney. However, variations were observed in the weight of the liver, but it was not dose-dependent.
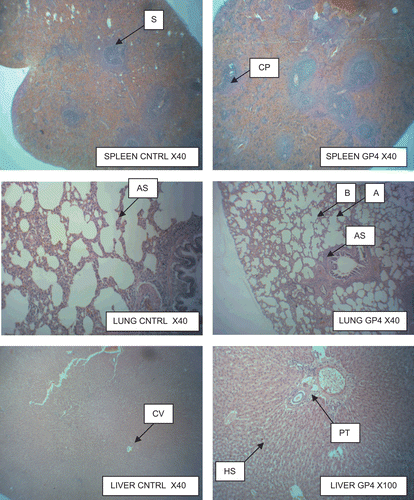
The photomicrographs of the spleen, lungs, liver, kidney and heart from rats treated with 800 and 1600 mg kg−1 doses of Joloo and control in a 28-day sub-chronic oral toxicity study are shown in . A cross section of the spleen shows the sinusoid (S) and congested pulp (CP) clearly conserved. The lung cross section shows alveolar space (AS), blood vessel (BV), and bronchiole (B) all conserved. Cross section of the liver shows central vein (CV), portal tract (PT), and hepatic artery (HA) all conserved. Cross section of the kidney shows proximal convoluted tubule (PCV), artery (A), and glomerulus (G) all conserved. The heart cross section illustrates connective tissue (CT) and muscle fiber (MF) conserved.
All the structures were conserved totally. GP refers to group as indicated in the micrographs.
Discussion
This study was designed to evaluate the sub-chronic toxicity of Joloo in normal albino rats. The absence of mortalities, adverse clinical signs, decrease in body weight as well as the lack of decrease in mean relative organ weight at doses of 400 and 800 mg kg−1 suggests that the decoction (Joloo) may be relatively safe at low doses.
However, the 20% mortality recorded in the 1600 mg kg−1 dose group and the observed signs of emaciation and significant increase in weight may be attributed to increase in bone mass. This is because the surviving animals in this group were healthy as observed in the histopathologic studies. The Society of Toxicologic Pathology (STP) has established the evaluation of organ weights in toxicologic studies as an integral component in the assessment of pharmaceuticals, chemicals and medical devices (CitationSellers et al., 2007). The histopathologic evaluation of vital organs in this study did not reflect any injury or damage. Due to the absence of any significant changes in organ weights between the animals treated with Joloo and the control group, it is suggested that Joloo does not exhibit toxicity at the tissue level under the studied period. The STP had reported that organ weight alone should not be interpreted but alongside histopathologic studies (CitationSellers et al. 2007).
Biological markers like endogenous enzymes have been shown and established to be organ-specific and can leak from a damaged or an injured organ (CitationKubavat and Asdaq, 2009). Hepatic function has been monitored by the evaluation of the levels of ALP, ALT and AST in conjunction with biochemical analytes such as cholesterol, creatine kinase and creatinine in the liver. The insignificant differences in the levels of the hepatic enzymes and biochemical analytes between the treated and control rats at lower doses may suggest that Joloo has no notable toxicity at the sub-chronic level. Although the rats treated with 1600 mg kg−1 dose of Joloo appeared to show slight increase in enzyme activities, these increases were not significantly different from the control. Further studies are, however, ongoing to assess the effects of long-term treatment with Joloo.
SOD is an enzyme with the capacity to catalyze the transformation of hypoxanthine to xanthine and subsequently into urate. During the reoxidation of SOD, molecular oxygen acts as an electron acceptor, producing superoxide radical and hydrogen peroxide. Consequently, SOD is considered to be amongst the important biological sources of superoxide radicals (CitationMontoro et al., 2005). For survival purposes, organisms are equipped with several antioxidant enzyme systems that prevent oxygen cytotoxicity such as SOD and CAT (CitationOkada et al., 1999). Joloo did not show any significant effect on antioxidants as indicated in the result of this 28-day study but may exhibit some activities if the study is prolonged.
Both gross and histopathologic examination did not reveal any treatment-related significant changes at all doses used as shown by normal lobular architectures, portal spaces containing arterioles, venules and bile duct. Creatinine is a compound derived from protein and eliminated by the kidney. The highest value of creatinine observed in the Joloo treated groups, 1.84 ± 0.03, is within the reference values of 0.39–2.29 mg dL−1 (CitationMatsuda et al., 2000). Moreover, the histopathologic analysis did not reveal any alteration in the kidney morphology. The renal cortex, renal corpuscles and urinary tubules were all preserved indicating the safety of Joloo. It may be possible that the kidneys were protected due to the presence of some important phytochemicals such as flavonoids and anthraquinones in Joloo as observed from our preliminary studies.
Hematopoietic indices have been reported to be very sensitive to toxic compounds and serve as important index of physiologic and pathologic status for both animals and humans (Rosidah et al., 2009). It is likely that the decrease in RBC associated with increased doses of Joloo may be an indication of anemia or disturbances in erythropoiesis, which could indicate early signs of toxicity. Leucocytes that are known to fight against and destroy microorganisms and other foreign compounds were not adversely affected by Joloo. They are therefore responsible for congenital immune response (CitationKonan et al., 2007). Thus, the lack of adverse effect on WBC, neutrophils and PCV shows that Joloo does not exhibit any adverse effect on the defense mechanism of the body.
Conclusion
The oral administration of Joloo to rats for 28 days at 400 and 800 mg kg−1 did not result in death. In addition, no adverse effects were observed in the body and organ weights of rats and in the values of biochemical analytes. Histopathologic abnormalities were also not observed. Thus Joloo may be regarded safe, especially at 400 and 800 mg kg−1, within the period studied. It is therefore suggested that Joloo be subjected to further long-term studies.
Declaration of interest
The authors declare that there are no present or future conflicts of interest in the preparation of this manuscript.
References
- Ayandele A, Adebiyi A. (2007). The phytochemical analysis and anti-microbial screening of extract of Olax subscorpioidea. Afri J Biotechnol, 6, 868–870.
- Asres K, Bucar F, Kartnig T, Witvrouw M, Pannecouque C, De Clercq E. (2001). Antiviral activity against human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2) of ethnobotanically selected Ethiopian medicinal plants. Phytother Res, 15, 62–69.
- Burkill HM. (1985). Useful Plants of West Tropical Africa. Royal Botanic Gardens, Kew, Vol. 3, p. 960.
- Burkill HM. (1995). Useful plants of West Tropical Africa. Royal Botanic Gardens, Kew, Vol. 3, p. 857.
- Burkill HM. (1997). Useful Plants of West Tropical Africa. Royal Botanic Gardens, Kew, Vol. 4, p. 969.
- Burkill HM. (2000). The Useful Plants of West Tropical Africa. Royal Botanic Gardens, Kew, Vol. 5, p. 686.
- El-Beshbishy HA. (2008). Aqueous garlic extract attenuates hepatitis and oxidative stress induced by galactosamine/lipoploysaccharide in rats. Phytother Res, 22, 1372–1379.
- Konan NA, Bacchi EM, Lincopan N, Varela SD, Varanda EA. (2007). Acute, subacute toxicity and genotoxic effect of a hydroethanolic extract of the cashew (Anacardium occidentale L.). J Ethnopharmacol, 110, 30–38.
- Kubavat JB, Asdaq SM. (2009). Role of Sida cordifolia L. leaves on biochemical and antioxidant profile during myocardial injury. J Ethnopharmacol, 124, 162–165.
- Matsuda H, Tanaka A, Itakura A. (2000). Immunology and hematology. In: Krinke GJ, ed. The Laboratory Rat. London: Academic Press, pp. 437–446.
- Mbagwu HO, Anene RA, Adeyemi OO. (2007). Analgesic, antipyretic and anti-inflammatory properties of Mezoneuron benthamianum Baill (Caesalpiniaceae). Nig Q J Hosp Med, 17, 35–41.
- Montoro P, Braca A, Pizza C, De Tommasi N. (2005). Structure antioxidant activity relationships of flavonoids isolated from different plant species. Food Chem, 92, 349–355.
- Odugbemi T. (2006). Outlines and Pictures of Medicinal Plants from Nigeria. 1st ed.University of Lagos Press, p. 283.
- Okada F, Nakai K, Kobayashi T, Shibata T, Tagami S, Kawakami Y, Kitazawa T, Kominami R, Yoshimura S, Suzuki K, Taniguchi N, Inanami O, Kuwabara M, Kishida H, Nakae D, Konishi Y, Moriuchi T, Hosokawa M. (1999). Inflammatory cell-mediated tumour progression and mini satellite mutation correlate with the decrease of antioxidative enzymes in murine fibrosarcoma cells. Brit J Cancer, 79, 377–385.
- Oloyede A, Okpuzor J, Omidiji O, Mbagwu H. (2008). A pharmacological evaluation of a herbal cocktail. Int J Pharmacol 4, 196–201.
- Oloyede A, Okpuzor J, Omidiji O. (2009). Cytological and toxicological properties of a decoction used for managing tumors in Southwestern Nigeria. Pak J Biol Sci, 12, 383–387.
- Rosidah Mun F, Amirin S, Mariam A, Gabriel A, Mohamed Z. (2009). Toxicology evaluation of standardized methanol extract of Gynura procumbens. J Ethnopharmacol, 123, 244–249.
- Sellers RS, Morton D, Michael B, Roome N, Johnson JK, Yano BL, Perry R, Schafer K. (2007). Society of Toxicologic Pathology position paper: organ weight recommendations for toxicology studies. Toxicol Pathol, 35, 751–755.