Abstract
Objective: Saffron Crocus sativus L. (Iridaceae) is known for anticancer properties. However, limited effort has been made to correlate these effects to the active ingredients of saffron. In the present study, cytotoxic effects of crocin, the major coloring compound in saffron, and its nanoliposomal form for better cellular delivery are investigated.
Methods: HeLa and MCF-7 cells were cultured and exposed to crocin (1, 2, and 4 mM) and liposomal crocin (0.5 and 1 mM). The 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was performed to assess cytotoxicity. Apoptotic cells were determined using propidium iodide (PI) staining of DNA fragmentation by flow cytometry.
Results: MTT assay revealed a remarkable and concentration-dependent cytotoxic effect of crocin on HeLa and MCF-7 cells in comparison with non-malignant cell line (L929). Crocin liposomal forms (IC50 values after 48 h: 0.61, 0.64, and 1.2 mM) showed enhanced cytotoxic effect compared with the crocin (IC50 after 48 h: 1.603 mM) in HeLa cells. Crocin and its liposomal form induced a sub-G1 peak in flow cytometry histogram of treated cells indicating apoptosis is involved in this toxicity. Liposomal encapsulation enhances apoptogenic effects of crocin on cancerous cells.
Conclusion: It might be concluded that crocin and its liposomes could cause cell death in HeLa and MCF-7 cells, in which liposomal encapsulation improved cytotoxic effects. They could be also considered as a promising chemotherapeutic agent in cancer treatment in future.
Introduction
Saffron is the dry stigmas of the plant Crocus sativus L. (Iridaceae) (CitationAbdullaev, 1993). Although it is currently used as a spice and food colorant, traditional medicines have used saffron in the treatment of numerous diseases including cough, colic, insomnia, chronic uterine hemorrhage, cardiovascular disorders, and tumors (CitationAbdullaev and Espinosa-Aguirre, 2004). Crocin is a constituent of C. sativus, which is responsible for the color of the saffron. It has been reported that crocin has different properties including antiplatelet (CitationLiakopoulou-Kyriakides and Skubas, 1990), neuron-protecting (CitationAbe and Saito, 2000), antiatherogenic (CitationHe et al., 2005), antioxidant (CitationChen et al., 2008), aphrodisiac properties (CitationHosseinzadeh et al., 2008), hypolipidemic effect by inhibiting pancreatic lipase, leading to the malabsorption of fat and cholesterol (CitationSheng et al., 2006), and anxiolytic-like effect in the rat (CitationPitsikas et al., 2008). This natural carotenoid protects the brain against excessive oxidative stress and constitutes a potential therapeutic candidate in transient global cerebral ischemia (CitationZheng et al., 2007). Several studies have showed that crocin has anticarcinogenic and antitumor activities (CitationFernndez, 2006; CitationZhao et al., 2008). Crocin possesses significant antiproliferation effects on human colorectal cancer cells (CitationAung et al., 2007). This carotenoid can induce the significant alteration of gene expression profile of T24 (transitional cell carcinoma of bladder) cell. It is suggested that the antitumor effects of crocin are medicated at least in part by regulating the cell cycle controlling gene expression (CitationLv et al., 2008). The mechanisms of this antitumor activity may down-regulate the expression of Bcl-2, Survivin, Cyclin D1 and up-regulate the expression of Bax (CitationZhao et al., 2008).
Crocin has been proposed as the one of the antitumor ingredient of saffron. On the other hand, an interesting property of liposomes is their natural ability to target tumor site (CitationGoyal et al., 2005; CitationTorchilin, 2005). Leaky tumor microvasculature facilitates the accumulation of different drugs in the tumor tissues. This ability is known as the enhanced permeability and retention (EPR) effect. Nanosized liposomes can rapidly enter tumor sites from the blood vessels but these nanocarriers are kept in the bloodstream by the intact vasculature in the other tissues (CitationAndresen et al., 2005; CitationFarokhzad and Langer, 2009). In the present study, we decided to make nanoliposomes of crocin to improve the antitumor effect. As the cytotoxicity mechanisms of crocin are still unclear, the role of apoptosis was evaluated for both crocin and liposomal crocin.
Methods
Materials
Standard crocin was obtained from Fluka. Distearoylphosphatidylcholine (DSPC), dioleoylphosphatidylethanolamine (DOPE), dipalmitoylphosphatidylcholine (DPPC), egg phosphatidylcholine (EPC), and distearoylglycerophosphoglycerol (DSPG) were purchased from Avanti Polar Lipids . Cholesterol, chloroform, and methanol were ordered from Merck (Darmstadt, Germany). All other materials and solvents were of analytical grade.
Cell culture
The human cervical adenocarcinoma cell line (HeLa), human breast cancer cell line (MCF-7), and mouse fibroblast cell line (L929) as non-malignant cell lines were obtained from Pasteur Institute (Tehran, Iran). They were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin (100 U/mL), streptomycin (100 µg/mL) at 37°C in an atmosphere of 95% humidified air and 5% carbon dioxide.
Preparation of liposomes containing crocin
Liposomal crocin was prepared from lipids and cholesterol by dehydration and rehydration (DRV) method (CitationMalaekeh-Nikouei and Davies, 2009). In brief, liposomes were first prepared by solvent evaporation method and extruded 11 times by thermobarrel extruder (Northernlipids, Canada) through 100-nm polycarbonate filter. To 1 mL of this liposome (phospholipid concentration = 32 µmol/mL), 1 mL crocin aqueous solution was added. After freezing of this mixture by acetone and dried ice, freezed liposome was vacuum-dried in the freeze-drier for 12 h. The dried material was rehydrated by distilled water and shaked for 10 min to form liposomal suspension. Non-entrapped complex was separated by centrifugation at 14,000 rpm for 30 min and liposomes were washed twice with the same process. To prepare nanosized liposomes, liposomes were extruded repeatedly through 100-nm polycarbonate membranes at 45°C. Liposomal formulation was passed at least 11 times through the polycarbonate membrane to produce liposomes with uniform size. The composition of liposomal formulations was summarized in .
Table 1. Composition and molar ratio of different liposomal formulations containing crocin.
Unincorporated crocin was removed three times by centrifugation at 100,000 g for 20 min. For determination of crocin concentration in the purified liposomes, aliquots of liposomes were dissolved in 20% Triton X-100 solution and the optical density was measured at 440 nm and compared with the standard curve.
Size analysis of liposomes
The mean diameter and particle size distribution of liposomes were determined using Zetasizer (NanoZS, Malvern, UK) after suitable dilution.
MTT test
The viability of cultured cells was determined by assaying the reduction of 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) to formazan. In brief, HeLa and L929 cell lines were seeded at an initial density of 5 × 103 cells/well. MCF-7 cells were seeded 10 × 103 cells/well, in a 96-well plate for 24 h. HeLa cells were then incubated with fresh medium containing concentrations (1, 2, 3, and 4 mM) of crocin and (0.14, 0.28, 0.42, 0.57, and 0.7 mM) of liposomal formulation I, (0.25, 0.5, 0.75, 1, 1.2 mM) of liposomal formulation II, and (1.1, 2.2, and 3.3 mM) of liposomal formulation III for 24, 48, and 72 h, respectively. The concentrations of crocin for L929 cells were 3, 5, 7, and 9 mM and for liposomal crocin were as the same of HeLa cell experiment. After incubation period, MTT was added into each well in final concentration of 0.5 mg/mL. The cells were then incubated at 37°C for 4 h. Then the insoluble formazan was collected and dissolved in dimethyl sulfoxide (DMSO, 0.5%). The absorption was measured at 570 nm (620 nm as a reference) in an ELISA reader (Start Fax-2100, UK) (CitationTavakkol-Afshari et al., 2008).
Propidium iodide staining
Apoptotic cells were determined by staining using the propidium iodide (PI) method described elsewhere (CitationZhang et al., 2004; CitationMousavi et al., 2009). In brief, HeLa cells were adhered overnight in a 24-well plate at the initial density of 2 × 105 cells/well, and then treated with crocin and its nanoliposomes with the same concentrations for 24 h. Floating and adherent cells were then harvested and incubated overnight at 4°C in the dark with 750 µL of a hypotonic buffer (50 µg/mL PI in 0.1% sodium citrate plus 0.1% Triton X-100) before flow cytometric analysis.
Statistical analysis
Data were expressed as mean ± SD. Test was performed using one-way ANOVA followed by Tukey–Kramer post-hoc test for multiple comparisons. The P-value <0.05 was considered to be statistically significant.
Results
shows the results of mean size and encapsulation efficiency of different liposomal formulations prepared in the present study. Mean diameters of the different liposomes containing crocin ranged between 150 and 195 nm. The highest encapsulation efficiency was achieved by liposomal formulation III.
Table 2. Mean size and encapsulation efficiency of different liposomal formulations containing crocin (mean ± SD, n = 3).
Effects on cell viability
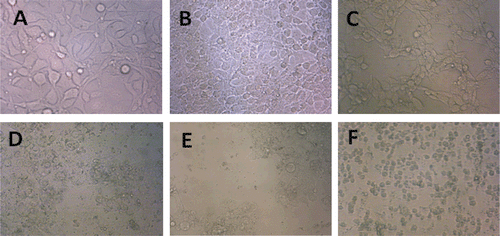
Malignant cells (HeLa and MCF-7) and L929 (as non-malignant control cells) were incubated with various concentrations of crocin (1, 2, and 4 mM) and liposomal crocin (0.5 and 1 mM) for 24, 48, and 72 h. Crocin and liposomal crocin decreased cell viability in malignant cells but not in non-malignant cells, in a concentration-dependent manner ( and ). This toxicity was consistent with morphologic changes including reduction in cell volume and rounding (). Doses inducing 50% cell growth inhibition (IC50) against HeLa, MCF-7, and L929 cells are presented in . Empty liposomes (without crocin) were also tested and there was no difference in cell viability in comparison with control.
Table 3. Doses inducing 50% cell growth inhibition (IC50) of crocin and its nanoliposomes against HeLa, MCF-7, and L929 cell lines.
Figure 1. Effect of crocin on cell viability of HeLa (A), MCF-7 (B), and L929 (C) cells. Cells were treated with different concentrations of crocin for 24, 48, and 72 h. Viability was quantitated by 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Results are mean ± SD (n = 3). The asterisks are indicator of statistical differences obtained separately at different time points compared with their controls shown in figure as *P < 0.05, **P < 0.01, and ***P < 0.001.
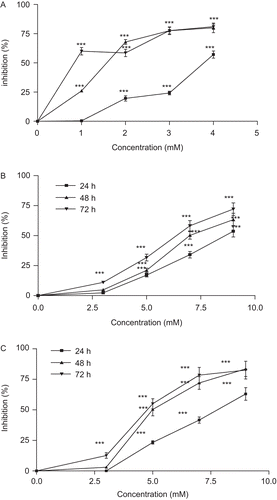
Figure 2. Effect of crocin liposome 1 on cell viability of HeLa (A), MCF-7 (B), and L929 (C) cells. Cells were treated with different concentrations of crocin liposome 1 for 24, 48, and 72 h. Viability was quantitated by 3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Results are mean ± SD (n = 3). The asterisks are indicator of statistical differences obtained separately at different time points compared with their controls shown in figure as *P < 0.05, **P < 0.01, and ***P < 0.001.
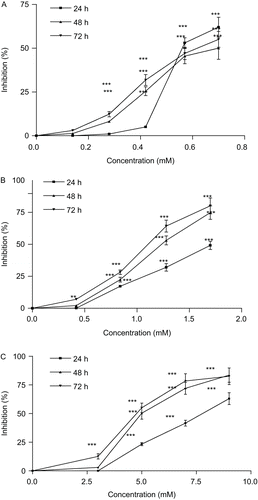
Figure 3. Morphological changes of crocin and liposomal crocin in MCF-7 and HeLa cells. HeLa and MCF-7 control cells (A and B), 48 h after treatment with 1 mM of crocin (C and D) and liposomal crocin (E and F). Control cells (A and B) remained untreated although receiving an equal volume of the solvent.
Role of apoptosis
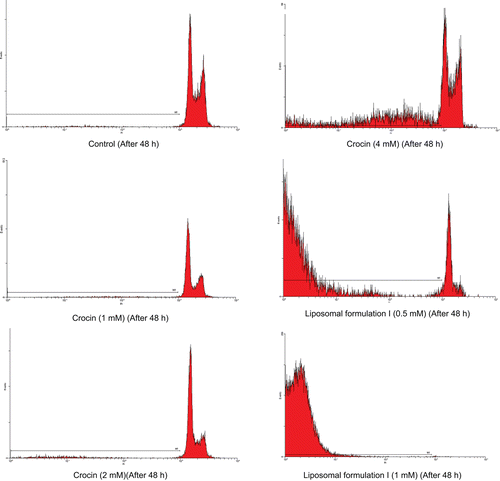
The proportion of apoptotic cells was measured with PI staining of DNA fragmentation by flow cytometry. Crocin (1, 2, and 4 mM) and liposomal crocin (0.5 and 1 mM) induced a sub-G1 peak (one of the reliable biochemical markers of apoptosis) in flow cytometry histogram of treated cells compared with control indicating apoptotic cell death is involved in induced toxicity (). The percent of apoptotic cells in HeLa cells is shown in .
Table 4. The percent of apoptotic cells in HeLa cells after 48 h of crocin and its liposomal forms.
Figure 4. Flow cytometry histograms of apoptosis assays by propidium iodide (PI) method in HeLa cells after 24 and 48 h. HeLa cells were treated with (1, 2, and 4 mM) of crocin and (0.5 and 1 mM) of crocin nanoliposomes for 24 and 48 h. Sub-G1 peak as an indicative of apoptotic cells was induced in crocin and nanoliposome-treated but not in control cells.
Discussion
In different studies, the improvement of the pharmacokinetic properties and the pharmacological effects of liposomal drugs were reported (CitationChonn and Cullis, 1995; CitationFahr et al., 1995; CitationMalaekeh-Nikouei et al., 2008). In the present study, the results show that the liposomal formulations containing crocin have the better cytotoxic effects compared with free crocin.
It is obvious that the carrier is an important factor for drug efficiency. Also, longer half-life of the drug in the bloodstream and better uptake by cancer cells were reported after using liposomal preparations (CitationAndresen et al., 2005). The results of MTT test could show that crocin more effectively reached inside the cancer cells after encapsulation in liposomes. Liposomes interact with cells by different mechanisms such as adsorption, fusion, and endocytosis (CitationPagano and Weinstein, 1978; CitationKamps and Scherphof, 2003). These interactions largely related to different parameters such as the composition, size, and liposome charge (CitationTorchilin, 2005). In this study, liposomal formulation I showed the best inhibitory effects. These fusogenic liposomes were composed of DOPE (CitationChonn and Cullis, 1995, Citation1998; CitationKono et al., 2000; CitationKunisawa et al., 2001). Using of DOPE in the liposomal formulation could change the structure of the liposome from bilayer to hexagonal, and then this conformation follows by transfer of liposome contents to cytoplasm (CitationPagano and Weinstein, 1978; CitationKamps and Scherphof, 2003). Previously, we used fusogenic liposomes for delivery of CyA to T cells. Our results showed the enhancement of immunosuppressive effects of cyclosporine A using these fusogenic liposomes (CitationMalaekeh-Nikouei et al., 2008).
On the other hand, the size of prepared liposomes was <200 nm. As it was mentioned before, nanoparticles with a diameter up to 400 nm can accumulate in the tumor interstitium. A possible explanation for the high level of selectivity observed with these nanocarriers would be the EPR phenomena. Vascular system in tumors is irregular in shape, dilated, and contains endothelial cells with multiple defects that create “leaks.” This phenomenon makes it easier to deliver particulate delivery systems to the tumor site, which cannot normally leave the blood vessels but can extravasate into the tumor through these pores (CitationFarokhzad and Langer, 2009).
In the present study, crocin-induced apoptosis was involved in induction of cell death. Apoptosis is a gene-regulated phenomenon, which is induced by many chemotherapeutic agents in cancer treatment (CitationHersey and Zhang, 2001; CitationDebatin et al., 2002). It is characterized by distinct morphological features including chromatin condensation, cell and nuclear shrinkage, membrane blebbing, and oligonucleosomal DNA fragmentation (CitationGreen and Reed, 1998). In our study, apoptosis was determined by detection of sub-G1 peak. It has been reported that DNA fragmentation creates small fragments of DNA that can be eluted following incubation in a hypotonic phosphate–citrate buffer. When stained with a quantitative DNA-binding dye such as PI, cells that have lost DNA will take up less stain and will appear to the left of the G1 peak. The induction of apoptosis in the tumor cells is considered very useful in the management and therapy as well as in the prevention of cancer (CitationZhang et al., 2004). A wide variety of natural substances have been recognized to have the ability to induce apoptosis in various tumor cells (CitationTripathi et al., 2005). Apoptogenic effects of crocin in HeLa and MCF-7 cells have not been shown previously.
Conclusions
Altogether, the present study shows toxicity of crocin in MCF-7 and HeLa cell line with involvement of apoptosis or programmed cell death. Liposome encapsulation improved cytotoxic effects of crocin. They could be also considered as a promising chemotherapeutic agent in cancer treatment in future.
Acknowledgement
This work was supported financially by a research grant from the Vice Chancellor for Research of Mashhad University of Medical Sciences, Mashhad, Iran.
Declaration of interest
The authors declared that there is no conflict of interests in this study.
References
- Abdullaev FI. (1993). Biological effects of saffron. Biofactors, 4, 83–86.
- Abdullaev FI, Espinosa-Aguirre JJ. (2004). Biomedical properties of saffron and its potential use in cancer therapy and chemoprevention trials. Cancer Detect Prev, 28, 426–432.
- Abe K, Saito H. (2000). Effects of saffron extract and its constituent crocin on learning behaviour and long-term potentiation. Phytother Res, 14, 149–152.
- Andresen TL, Jensen SS, Jørgensen K. (2005). Advanced strategies in liposomal cancer therapy: problems and prospects of active and tumor specific drug release. Prog Lipid Res, 44, 68–97.
- Aung HH, Wang CZ, Ni M, Fishbein A, Mehendale SR, Xie JT, Shoyama CY, Yuan CS. (2007). Crocin from Crocus sativus possesses significant anti-proliferation effects on human colorectal cancer cells. Exp Oncol, 29, 175–180.
- Chen Y, Zhang H, Tian X, Zhao C, Cai L, Liu Y, Jia L, Yin HX, Chen C. (2008). Antioxidant potential of crocins and ethanol extracts of Gardenia jasminoides ELLIS and Crocus sativus L.: a relationship investigation between antioxidant activity and crocin contents. Food Chem, 109, 484–492.
- Chonn A, Cullis PR. (1995). Recent advances in liposomal drug-delivery systems. Curr Opin Biotechnol, 6, 698–708.
- Chonn A, Cullis PR. (1998). Recent advances in liposome technologies and their applications for systemic gene delivery. Adv Drug Deliv Rev, 30, 73–83.
- Debatin KM, Poncet D, Kroemer G. (2002). Chemotherapy: targeting the mitochondrial cell death pathway. Oncogene, 21, 8786–8803.
- Fahr A, Holz M, Fricker G. (1995). Liposomal formulations of cyclosporin A: influence of lipid type and dose on pharmacokinetics. Pharm Res, 12, 1189–1198.
- Farokhzad OC, Langer R. (2009). Impact of nanotechnology on drug delivery. ACS Nano, 3, 16–20.
- Fernndez J-A. (2006). Anticancer properties of saffron, Crocus sativus Linn. Adv Phytomed, 2, 313–330.
- Goyal P, Goyal K, Vijaya Kumar SG, Singh A, Katare OP, Mishra DN. (2005). Liposomal drug delivery systems—clinical applications. Acta Pharm, 55, 1–25.
- Green DR, Reed JC. (1998). Mitochondria and apoptosis. Science, 281, 1309–1312.
- He SY, Qian ZY, Tang FT, Wen N, Xu GL, Sheng L. (2005). Effect of crocin on experimental atherosclerosis in quails and its mechanisms. Life Sci, 77, 907–921.
- Hersey P, Zhang XD. (2001). How melanoma cells evade trail-induced apoptosis. Nat Rev Cancer, 1, 142–150.
- Hosseinzadeh H, Ziaee T, Sadeghi A. (2008). The effect of saffron, Crocus sativus stigma, extract and its constituents, safranal and crocin on sexual behaviors in normal male rats. Phytomedicine, 15, 491–495.
- Kamps JAAM, Scherphof GL. (2003). Liposome in biological system. In: Torchilin VP, Weissig V, eds., Liposomes. Oxford, USA: Oxford University Press, pp. 267–301.
- Kono K, Iwamoto M, Nishikawa R, Yanagie H, Takagishi T. (2000). Design of fusogenic liposomes using a poly(ethylene glycol) derivative having amino groups. J Control Release, 68, 225–235.
- Kunisawa J, Nakagawa S, Mayumi T. (2001). Pharmacotherapy by intracellular delivery of drugs using fusogenic liposomes: application to vaccine development. Adv Drug Deliv Rev, 52, 177–186.
- Liakopoulou-Kyriakides M, Skubas AI. (1990). Characterization of the platelet aggregation inducer and inhibitor isolated from Crocus sativus. Biochem Int, 22, 103–110.
- Lv CF, Luo CL, Ji HY, Zhao P. (2008). [Influence of crocin on gene expression profile of human bladder cancer cell lines T24]. Zhongguo Zhong Yao Za Zhi, 33, 1612–1617.
- Malaekeh-Nikouei B, Davies N. (2009). Double loading of cyclosporine A in liposomes using cyclodextrin complexes. PDA J Pharm Sci Technol, 63, 139–148.
- Malaekeh-Nikouei B, Jaafari MR, Tabassi SA, Samiei A. (2008). The enhancement of immunosuppressive effects of cyclosporine A on human T-cells using fusogenic liposomes. Colloids Surf B Biointerfaces, 67, 238–244.
- Mousavi SH, Tavakkol-Afshari J, Brook A, Jafari-Anarkooli I. (2009). Role of caspases and Bax protein in saffron-induced apoptosis in MCF-7 cells. Food Chem Toxicol, 47, 1909–1913.
- Pagano RE, Weinstein JN. (1978). Interactions of liposomes with mammalian cells. Annu Rev Biophys Bioeng, 7, 435–468.
- Pitsikas N, Boultadakis A, Georgiadou G, Tarantilis PA, Sakellaridis N. (2008). Effects of the active constituents of Crocus sativus L., crocins, in an animal model of anxiety. Phytomedicine, 15, 1135–1139.
- Sheng L, Qian Z, Zheng S, Xi L. (2006). Mechanism of hypolipidemic effect of crocin in rats: crocin inhibits pancreatic lipase. Eur J Pharmacol, 543, 116–122.
- Tavakkol-Afshari J, Brook A, Mousavi SH. (2008). Study of cytotoxic and apoptogenic properties of saffron extract in human cancer cell lines. Food Chem Toxicol, 46, 3443–3447.
- Torchilin VP. (2005). Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov, 4, 145–160.
- Tripathi YB, Tripathi P, Arjmandi BH. (2005). Nutraceuticals and cancer management. Front Biosci, 10, 1607–1618.
- Zhang XD, Gillespie SK, Hersey P. (2004). Staurosporine induces apoptosis of melanoma by both caspase-dependent and -independent apoptotic pathways. Mol Cancer Ther, 3, 187–197.
- Zhao P, Luo CL, Wu XH, Hu HB, Lv CF, Ji HY. (2008). [Proliferation apoptotic influence of crocin on human bladder cancer T24 cell line]. Zhongguo Zhong Yao Za Zhi, 33, 1869–1873.
- Zheng YQ, Liu JX, Wang JN, Xu L. (2007). Effects of crocin on reperfusion-induced oxidative/nitrative injury to cerebral microvessels after global cerebral ischemia. Brain Res, 1138, 86–94.
