Abstract
Context: Ligusticum chuanxiong Hort (LC; Umbelliferae) is an effective medical plant, which has been extensively applied for many years to treat various diseases with other Chinese herbal medicines. Although a considerable amount of scientific research was reported on LC in the last decade, it is currently scattered across various publications. The present review comprises the chemical and pharmacological research on LC in the last decade.
Objective: The objective of this review is to bring together most of the scientific research available on LC and evaluate its effects and mechanisms.
Methods: The information for 82 cases included in this review was compiled using major databases such as Medline, Elsevier, Springer, Pubmed, and Scholar.
Results: The compounds contained in LC can be divided into five kinds, essential oil (EO), alkaloids, phenolic acids, phthalide lactones, and other constituents. A great deal of pharmacological research has been done, which mainly focuses on cardiovascular and cerebrovascular effects, antioxidation, neuroprotection, antifibrosis, antinociception, antiinflammation, and antineoplastic activity.
Conclusion: A large number of pharmacological and chemical studies during the last 10 years have demonstrated the vast medicinal potential of LC. It is still very clear that LC is a plant with widespread use now and also with extraordinary potential for the future. The documents strongly support the view that LC has beneficial therapeutic properties and indicates its potential as an effective adaptogenic herbal remedy.
Introduction
Ligusticum chuanxiong Hort (LC), family Umbelliferae, is also called Ligusticum wallichii Franchat. It is mainly distributed in Sichuan province (China) and first recorded in the Divine Husbandman’s Classic of the Materia Medica (Shen Nong Ben Cao Jing). The rhizome of LC is warm in property and pungent in flavor, with functions of promoting the circulation of the blood and qi, expelling wind, and alleviating pain, which has high medicinal value. LC has long been used as a traditional Chinese medicine in folk remedies and is widely applied in food preparation as a health protection. Its major chemical components include essential oil (EO), alkaloids, phenolic acids, phthalide lactones, and other constituents, which display vasorelaxation, antiinflammation, antioxidant, antiproliferation, and other activities in both in vitro and in vivo studies. The aim of this article is to summarize and review the published scientific information about this important Chinese medicinal plant in last decade for further study.
Chemical constituents
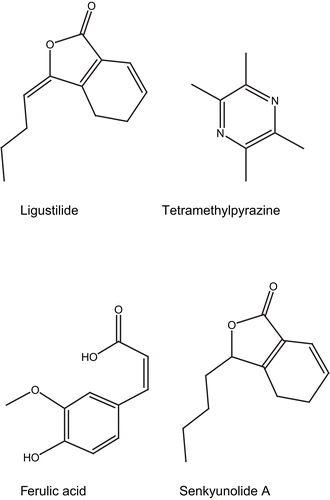
Chemical studies of LC have established a foundation of pharmacological research. Many compounds are isolated from this plant (mostly from its rhizomes), including EO, alkaloids, phenolic acids, phthalide lactones, and other constituents. These compounds are listed in and the chemical structure of ligustilide, tetramethylpyrazine (TMP), ferulic acid (FA), and senkyunolide A is showed in .
Table 1. Compounds isolated from Ligusticum chuanxiong.
Pharmacology
There have been an increasing number of researchers focusing on the pharmacological activity of LC. This article in this section describes the main pharmacological effects in the last decade.
Effects on cardiovascular and cerebrovascular diseases
Effects on atherosclerosis
LC plays an active role in prevention and therapy of atherosclerosis, which decreases the levels of the serum cholesterol, lowers density lipoprotein, relieves the extent of atherosclerosis, and reduces the red cell deformability in rabbits subjected to experimental atherosclerosis (CitationWang et al., 1995). The extract of LC and Angelica sinensis (Apiaceae; ELCAS) can inhibit proliferation and protein synthesis, and increase nitric oxide (NO) production of vascular smooth muscle cell (VSMC) in a dose- and time-dependent manner. ELCAS also markedly inhibits VSMC proliferation by arresting G(1) to S progression, which may be associated with NO production (CitationHou et al., 2005). Chuanxiongzine, also called TMP or ligustrazine, can significantly inhibit the proliferation of VSMC in a dose- and time-dependent manner. The inhibitory effect can be realized via down regulating the expression of proliferating cell nuclear antigen (PCNA) and c-myc (CitationLi et al., 2007). TMP can decrease angiotensin II (Ang II)-induced VSMC proliferation through repression of nuclear factor-κB (NF-κB) activation and BMP-2 reduction (Ren et al., Citation2007b). TMP also restrains Ang II-induced DNA synthesis, ET-1 mRNA levels, and the secretion of endothelin-1 (ET-1, a potent vasopressor synthesized by endothelial cells both in culture and in vivo), suppresses Ang II-increased NAD(P)H oxidase activity, intracellular reactive oxygen species (ROS) levels, and the ERK phosphorylation (CitationWong et al., 2007).
Some investigators observed the combination therapy of Red Paeonia and Rhizoma chuanxiong (RC, rhizome of LC) for atherosclerosis. RC can increase the thickness of fibre cap, lower the levels of total cholesterol and the serum triglyceride (TC), down regulate the lipid-core/plaque area ratio, and reduce the macrocytic infiltration. In addition to the effects mentioned above, Xiongshao Capsule (XC, a mixture of chuanxingols and paeoniflorins) can also raise the levels of high-density lipoprotein cholesterol (HDL-C) and lower TC/HDL-C ratio, reduce inflammatory reaction, and enlarge the collagen area in plaque. The combination of Red Paeonia and RC shows a more evident effect on atherosclerosis (CitationXu et al., 2007). The active components of Red Paeonia and RC definitely delay the genesis and development of atherosclerosis (Zhang et al., Citation2009a), lower the serum levels of matrix metalloproteinase-3 (MMP-3) and MMP-9, suppress the expression of MMP-3 and cluster of differentiation antigen 40 ligand (CD40L) in plaque, and decrease the blood content of total cholesterol. These might be associated with the role of XC stabilizing the atherosclerotic plaque (Zhang et al., Citation2009b).
Effects on vasodilatation
TMP has a direct vasodilatation effect on isolated aortic rings from Wistar rats. Only the inhibitors for potassium channel specific to small conductance calcium-activated potassium [SK(Ca)] channel or ATP-sensitive potassium [K(ATP)] channel inhibit the action of TMP, but the TMP-induced relaxation is reversed by the inhibitor of soluble guanylyl cyclase in a way similar to that of K(ATP) channel blockade. The obtained results indicate that TMP-induced vasodilatation is related to the opening of SK(Ca) and K(ATP) channels (CitationTsai et al., 2002). TMP also decreases strain-induced ET-1 secretion, ROS formation, and phosphorylation of extracellular signal-regulated kinases (ERK) 1/2, and attenuates the strain-induced activity of the activator protein-1 reporter. These findings indicate that TMP represses strain-induced ET-1 gene expression, in part by interfering with the ERK1/2 pathway via attenuation of ROS formation (CitationBi et al., 2005). In addition to TMP, ligustilide and butylidenephthalide also inhibit the vasoconstrictions induced by norepinephrine bitartrate (NE) and calcium chloride (CaCl2), and the relaxing effect of ligustilide is more potent than that of butylidenephthalide (CitationLiang et al., 2005). Ligustilide and senkyunolide A display the vasorelaxation activity in contradiction to various contractile agents in rat isolated aorta (CitationChan et al., 2007). Ligustilide and FA have a vasodilatation effect on rabbit isolated basilar artery rings, and the combination vasodilatation effect of two constituents are better than any single constituent (CitationLi et al., 2009).
Butylidenephthalide (BDPH) has an antianginal effect without changing blood pressure in conscious rats. The relaxant effects of BDPH are evaluated by using isolated dog coronary artery (CA), femoral vein (FV), femoral artery (FA), and mesenteric artery (MA; CitationKo et al., 2002). The potency order of BDPH to these blood vessels is FV > CA > FA ≥ MA. The results show the higher potencies of BDPH on FV and CA than on FA and MA. After administration of BDPH in vivo, the venous return is reduced and the coronary flow is increased without affecting the arterioles, such as MA, the main determinant of blood pressure. Therefore BDPH is useful in the treatment of angina pectoris without changing blood pressure. BDPH-mediated vasorelaxation comprises both endothelium-dependent (NO) and independent components. BDPH acts through an inhibitory mechanism downstream to I-type voltage-operated and prostanoid TP receptor-operated Ca2+ channels operating late in the contractile pathway (CitationChan et al., 2006). BDPH is often prescribed together with NO donors for treating coronary heart diseases. They interacted synergistically under 9,11-dideoxy-9α, 11α-methanoepoxyprostaglandin H2 (U-46619)-induced tone. BDPH-SNP (sodium nitroprusside) synergism becomes greater with increasing U-46619 concentrations where Ca2+ sensitization contributes more significantly, and less when U-46619 is replaced with phenylephrine where participation of Ca2+ sensitization is minimal, and remained intact in the absence of external Ca2+. This interaction is related to an enhancement of the effectiveness of SNP in producing relaxation undertone induced mainly by Ca2+ sensitization, but the synergistic relaxation between BDPH and SNP in rat isolated aorta is not required for the regulation of Ca2+ influx (CitationChan et al., 2009).
Effects on thrombus formation
Li et al. demonstrated the inhibitory effect of TMP on platelet thrombus formation. They found that TMP inhibits shear-induced platelet aggregation under relatively high shear rate, platelet activation, and microparticle release, demonstrating platelet thrombus formation on the collagen and von Willebrand factor (vWF) surface at high shear rates without significant influences on those occurring under relatively low shear rates. Because platelet thrombus formation occurring under high shear rates is known to be mediated by the interaction between vWF and platelet receptor proteins GP Ib[α] and GP IIb/IIIa, TMP possibly exerts antiplatelet effects by inhibiting the vWF-mediated process of platelet thrombus formation (CitationLi et al., 2001, Citation2004). LC phthalides has a protective effect on focal cerebral ischemia in rats, which decreases the infarct size and behavior deficit scores, inhibits the thrombus formation and platelet aggregation, and ameliorated hemorrheological parameters in a dose-dependent manner (CitationTian et al., 2005).
Effects on myocardial ischemia/reperfusion (I/R) injury
Chuanxiong-pathalide A pretreatment protects the endothelial function from the injury caused by ischemia and reperfusion (CitationGao et al., 2005). The mixture of aqueous extracts from Salviae miltiorrhizae (Labiatae) and Rhizoma chuanxiong can reduce myocardial ischemia/reperfusion (I/R) injury (CitationZhang et al., 2010).
Angiogenic effects
CitationMeng et al. (2006, Citation2008) observed the angiogenic effects of LC. Research results show that LC affects vascular endothelial growth factor (VEGF) expression in rat myocardial infarction, promote endothelial cell proliferation, and stimulate quantity of vessels in the chick embryo chorioallantoic membrane model.
Effects on stroke
Stroke is a major health-care problem and is one of the leading causes of death and serious long-term disability. Prevention of stroke is considered an important strategy. LC has been traditionally used in China in the treatment and prevention of stroke. FA isolated from LC can reduce cerebral infarct area and neurological deficit score, and the mechanism is related to inhibition of superoxide radicals, intercellular adhesion molecule-1 (ICAM-1) and NF-κB expression in transient middle cerebral artery occlusion (MCAo) rats (CitationCheng et al., 2008). CitationYang et al. (2010) also demonstrated the effects and safety of chuanxiong preparations in preventing stroke in high-risk adults.
Other effects
LC could activate blood flow and remove blood stasis for dairy cow mastitis (CitationLu et al., 2008). TMP is one of the active principles contained in LC, which has been used to treat vascular disorders in China. TMP attenuates the increase in cultured vascular smooth muscle (A7r5) cells produced by vasopressin (1 μM) or phenylephrine (1 μM), and inhibits changes in membrane potential elicited by KCl (20 mM) or phenylephrine (1 μM) in a concentration-dependent manner. The decrease in [Ca2+] in A7r5 cells is mediated mainly by opening of small conductance calcium-activated potassium [SK(Ca)] channel and/or ATP-sensitive potassium [K(ATP)] channel (CitationWong et al., 2003). FA could inhibit blood coagulation and erythrocyte agglutination, which is immobilized on silk fibroin (SF) by graft polymerization, and the anticoagulant activity of modified SF is improved significantly. Although SF surface composition is altered by FA, its β-sheet conformation is not disturbed (CitationWang et al., 2008).
Antioxidant effects
TMP could decrease and scavenge free radical generation in kainate-induced excitotoxicity in rat hippocampus (CitationShih et al., 2002). CitationLi et al. (2010) also demonstrated TMP neuroprotection against kainate-induced excitotoxicity in vitro and in vivo. TMP partly alleviates kainate-induced status epilepticus in rats and prevents and rescues neuronal loss in the hippocampal CA3 but not the CA1 region. The partial prevention and rescue of neuronal loss by TMP attribute to the preservation of the structural and functional integrity of mitochondria, evidenced by maintaining the mitochondrial membrane potential, ATP production, and complex I and III activities. Stabilization of mitochondrial function is related to that TMP functions as a reductant/antioxidant to quench ROS, block lipid peroxidation, and protect enzymatic antioxidants such as glutathione peroxidase and glutathione reductase. Therefore, TMP may protect against oxidative brain injury by stabilization of mitochondrial function through quenching of ROS.
Active oxygen free radical (OFR) increases abnormally in myocardial tissue in rats with ischemic myocardial damage, whereas overproduction of OFR can induce damage of heart tissue (CitationWang et al., 2003). The ethanol extract of LC could lower the increased OFR level close to normal, thereby alleviating the myocardial damage. The extract of LC and Angelica sinensis (ELCAS) protects human umbilical vein endothelial cells (ECV304) against hydrogen peroxide damage, suppresses the production of ROS, increases the phosphorylation of ERK, and promotes eNOS expression. These observations indicate that ELCAS protects ECV304 cells against hydrogen peroxide damage by enhancing the antioxidative ability, activating ERK and eNOS signaling pathway (CitationHou et al., 2004). The herbal extract of LC could delay the aging of human umbilical endothelial cells (HUVECs) irrespective of whether they are induced by angiotensin II or not, because it can down regulate the expression of nicotinamide adenine dinucleotide phosphate-oxidase (NAD(P)H oxidase), subunit-p47phox through angiotensin type I receptor (AT1 R), and further reduces the superoxide anion production (Yang et al., Citation2009a, Citation2009b). The major polysaccharides obtained from LC include LC A, B, C (LCA, LCB, and LCC), all of which exhibit antioxidation and cytotoxicity. LCB displays the highest antioxidant and cytotoxic activities among them (CitationYuan et al., 2008). The production of OH significantly increases in retinal ischemia/reperfusion-induced alterations. FA, one active ingredient of Ligusticum walliichi (chuanxiong), could attenuate OH production and protect against retinal ischemia, which possibly acts as an OH scavenger (CitationChao et al., 2008). The EO from LC possesses antioxidant activity (CitationJeong et al., 2009). It inhibits the migration of damaged DNA induced by ultraviolet radiation b (UV-B), decreases p21 expression, and increases cyclin D1 expression as apoptosis-regulatory genes. These results suggest that the EO of LC may exert inhibitory effects on DNA damage and cell apoptosis induced by UVB through its high free radical-scavenging ability. Besides, TMP also attenuates gentamicin-induced oxidative stress and apoptotic injury in rat renal tubular cells (RTCs; CitationJuan et al., 2007). Heme oxygenase-1 (HO-1) contributes to protection by TMP against gentamicin-induced apoptosis in murine RTCs through antioxidation and antiinflammation (CitationSue et al., 2009). Two senkyunolide isomers from chuanxiong extract, senkyunolide-H and its stereoisomer senkyunolide-I, also induce HO-1 to inhibit the formation of ROS and lipid peroxidation in human liver hepatocellular carcinoma cells (HepG2) and enhance the cellular resistance to hydrogen peroxide-induced oxidative damage. Notably, heme oxygenase inhibitor tin protoporphyrin IX (SnPP) significantly suppresses the antioxidant activity of senkyunolide stereoisomers. Thus, senkyunolide-H and -I attenuate oxidative damage via activation of HO-1 pathway (CitationQi et al., 2010).
Neuroprotective effects
TMP has the protective effect on kainate-induced excitotoxicity in rat hippocampus, which is associated with its protection of mitochondria, decrease in free radical generation, and scavenging of free radicals (CitationShih et al., 2002; CitationLi et al., 2010). The addition of the neurotoxin can cause significant cell death and reduction of cell proliferation in PC12 cells, and elevate activation of caspase-3, the key enzyme for activation of the cellular apoptotic cascade. LC could prevent the toxicity to some degree and inhibit the activation of caspase-3 (CitationJia et al., 2005). The butanol extract of LC (LC-BuOH) protects neuronal-like pheochromocytoma (PC12) cells from serum deprivation-induced apoptosis. The protective mechanism is carried out possibly through a protein kinase A (PKA)/cyclic-AMP response element-binding protein (CREB)-dependent pathway, where CREB is a downstream target of PKA and a nuclear transcription factor known for neuroprotective mechanism (CitationLin et al., 2007). The protective effect is also related to mitogen-activated protein kinases (MAPKs) and LC-BuOH suppressed c-JUN N-terminal kinase (JNK)/p38 phosphorylation (CitationLin et al., 2009). FA has an effect on neurological deficit scores and cerebral infarct area, which is related to the inhibition of superoxide radicals, ICAM-1, and NF-κB expression in transient MCAo rats (CitationCheng et al., 2008). Chuanxiong Chatiao pulvis improves the motor deficit in mice induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and attenuates MPTP-induced dopaminergic neurodegeneration in mice, and the neuroprotective effect might be associated with its strong antioxidant capacity in vivo (CitationShu et al., 2009). Besides, TMP enhances the proliferation and differentiation of neural stem cells (NSCs) into neurons of rat after hypoxia in vitro (or in hypoxic condition), which is related to the phosphorylation of ERK and p38 (CitationTian et al., 2010).
Antifibrotic effects
Hepatic stellate cell (HSC) plays a central role in hepatic fibrosis. Suppression of HSC activation and induction of HSC apoptosis have been proposed as therapeutic strategies for the treatment and prevention of liver fibrosis. LC has antiproliferative and proapoptotic activities in HSC (CitationChor et al., 2005). CitationLin et al. (2006) observed that LC significantly inhibits platelet-derived growth factor (PDGF)-activated HSC proliferation possibly through induction of apoptosis. They also investigated the effects of a combination regimen of Salvia miltiorrhiza (Lamiaceae; S), LC (L) and Glycyrrhiza glabra (Fabaceae; G) in rats with hepatic fibrosis. SLG exerts antifibrotic effects in rats with dimethylnitrosamine (DMN)-induced hepatic fibrosis. Two phthalides, Z,Z’-6,8’,7,3’-diligustilide (1) and levistolide A (2) from LC significantly abrogate PDGF-BB-induced proliferation in both rats and human HSC lines, which is related to inhibition of cell cycle and induction of apoptosis. They might be potential antifibrotic drugs for the treatment and prevention of hepatic fibrosis (CitationLin et al., 2006, Citation2008; CitationLee et al., 2007).
EO extracted from rhizomes of LC could be an effective cure for human hypertrophic scar which is characterized by abnormal proliferation of fibroblasts, an overproduction of collagen, and excessive deposition of extracellular matrix. EO could impair mitochondrial membranes, elicit caspase-3 activation, and induce apoptosis in human hypertrophic scar fibroblasts (HSFs). EO-induced apoptosis is at least partially carried out via destruction of the intracellular antioxidant system and elicitation of excessive ROS accumulation in HSFs (CitationWu et al., 2010). Besides, EO also inhibits hypertrophic scarring in rabbit ears. The levels of transforming growth factor-β1 (TGF-β1), collagen I and collagen III are evidently decreased and the MMP-1 level is markedly increased in the scar tissue. Scar elevation index (SEI) is also significantly reduced. Immunohistochemical findings exhibit significant amelioration of the scar tissue (CitationWu et al., 2010).
Antinociceptive effects
TMP could elevate the threshold of thermal nociception in rats. It significantly prolongs the withdrawal latency of ipsilateral hindpaw to noxious heating in the rat, inhibits high-voltage gated calcium current of dorsal root ganglion (DRG) neuron, and decreases tetrodotoxin (TTX)-resistant sodium current in a relatively selective and dose-dependent manner. These results demonstrate that TMP has the effects of inhibiting the high-voltage gated calcium current and TTX-resistant sodium current of DRG neuron in the rat (CitationBie et al., 2006). LC can treat headache, and the volatile oil from LC (CXVO) is likely to be the mainly active ingredient in curing headache. The investigation of CitationPeng et al. (2009) shows that CXVO has central analgesic and sedative effects and the effect of synergism with sodium pentobarbital to prolong the sleeping time in mice. The effect on pain threshold of rabbits exposed to hot radiation proves the direct therapeutic effect of CXVO on treating headache. Besides, CXVO inhibits c-fos gene expression in the brain tissue, raises the levels of 5-hydroxytryptamine (5-HT), endothelin (ET), and reduces the calcitonin gene-related protein (CGRP) level in plasma, which is possibly related to the mechanism of analgesic action of CXVO. The EO from LC is demonstrated to have faster onset of action as well as better analgesic and sedative efficacy after nasal administration than oral administration (CitationGuo et al., 2010). LC has been used to treat dysmenorrheal. The extract of Radix angelica and Rhizoma chuanxiong (1.5:1) shows the strongest effect on the uterine tissue by reducing the writhing times, increasing NO concentration, and reducing calcium ion (Ca2+) concentration, suggesting that the extract can treat primary dysmenorrhea in women (Wang et al., Citation2010a).
Antiinflammatory and antibacterial effects
LC aqueous extract has antipruritic and antiinflammatory effects in mice (CitationDai et al., 2002). Two phthalide lactones from LC, Z-ligustilide and senkyunolide A, are identified and characterized as inhibitors of lipopolysaccharide (LPS)-induced TNF-α production in monocytes. Two phthalides exhibit significant suppressive effects on TNF-α-mediated NF-κB activation in reporter gene assays. The results suggest that Z-ligustilide and senkyunolide A may have potential applications in the treatment of inflammation and related diseases based on their inhibitory activity on TNF-α production and TNF-α bioactivity (CitationLiu et al., 2005). TMP is used clinically to treat asthma as an assistant therapy of glucocorticoid. It could significantly lower the level of interleukin-4 (IL-4) in bronchoalveolar lavage fluid (BALF) and the expression of GATA-3 protein (GATA binding protein-3) in lung and also increase the levels of interferon-gamma (IFN-γ) and T-β (T-box expressed in cells) in asthmatic rats, resulting in a decreased percentage of eosinophils (EOS) in BALF and ameliorated airway inflammatory cell infiltration in ovalbumin (OVA)-induced asthmatic rats. TMP possibly inhibits OVA-induced airway inflammation by modulating key master switches GATA-3 and T-bet that result in reversing the Th2 cytokine (contained IL-4, IL-6, IL-10, and IL-13) patterns in asthma (CitationXiong et al., 2007). Some researchers isolated senkyunolide A and Z-ligustilide using chromatographic and spectrometric methods, which could inhibit the production of proinflammatory mediators in lipopolysaccharide (LPS)-stimulated murine BV-2 microglial cells and human peripheral blood monocyte–derived macrophages. In addition, both compounds protect Neuro-2a cells from neuroinflammatory toxicity induced by the conditioned culture media produced by LPS-stimulated BV-2 cells (CitationOr et al., 2010).
The aqueous extract of LC possesses anti-Helicobacter pylori action. Infection by Helicobacter pylori has been ascertained to be an important etiologic impetus leading usually to chronic active gastritis and gastric ulcer with growing incidences worldwide. LC may be used to treat ulcer diseases (CitationLi et al., 2005). Ligustilide and butylidene phthalide could enhance the inhibitory effects of ketoconazole and itraconazole on Trichophyton species, indicating that there is a synergistic or additive antifungal activity between the antibiotic and ligustilide or butylidene phthalide (CitationSim & Shin, 2008).
Antineoplastic effects
Chinese herbal medicine 1023 Recipe (contain LC) could block cancer transformation of experimental oral precancerous lesion, and the possible reason is that 1023 Recipe induces precancerous lesions to differentiate into normal tissues (CitationChen et al., 2004). PCH4, a derivative of n-butylidenephthalide, has antitumor effects on oral squamous cell carcinoma (OSCC) via induction of tumor cell apoptosis. The antitumor mechanism is that PCH4 promotes Nur77 (a potential target gene in OSCC cells) translocation from the nucleus to the cytoplasm and induces cell apoptosis in OSCC cells. PCH4 may serve as a potential anti-tumor drug for OSCC therapy (CitationLiu et al., 2010). Some membrane transporters in liver, such as P-glycoprotein, multidrug resistance (MDR)-associated protein 2 (MRP2), MRP3, and MRP5 can lead to a complex MDR to antineoplastic agents. TMP could reverse the multidrug resistance of BEL-7402/ADM cells and down regulated the expression of these transporters (Wang et al., Citation2010d).
Progesterone-like activity
Riligustilide, one of dimeric phthalides isolated from LC, displays a weak progesterone-like activity, whereas another dimeric phthalide compound, (3Z’)-(3Z’R,6’R,3R,6R,7R)-3,8-dihydro-6.6’,7.3a’-diligustilide, is a potent and specific activator of progesterone receptor. The in vivo progestogenic activity of LC extract in male Sprague-Dawley rats probably has utility for progesterone-replacement therapy (Lim et al., Citation2006a). These bioactive phthalides and their parent extracts may have utility for the treatment of conditions requiring progesterone action (Lim et al., Citation2006b). In addition, Jiantai liquid (JTL; contain LC) could promote the development of endometrium and improve the embryo implantation in mice with embryo implantation dysfunction (EID) via regulating the levels of estrogen receptor (ER) and progesterone receptor (PR) protein and gene expression (CitationLiu et al., 2004).
Antipyretic effects
Fever, or pyrexia, occurs when the body reaches a temperature above what is considered “average.” It usually results from microbes such as bacteria or viruses triggering the body’s defense mechanisms. An antipyretic is a type of medication that can prevent or reduce fever by lowering body temperature from a raised state. They will not affect normal body temperature if the patient does not have a fever. The EO of LC has an antipyretic effect on endotoxin (ET) fever in the rabbits and the mechanism may be related to monoamine neurotransmitters (CitationZhulun et al., 2003).
Effects on caries
Dental caries, also known as tooth decay or cavity, is a disease where bacterial processes damage hard tooth structure (enamel, dentin, and cementum). These tissues progressively break down, producing dental caries (cavities, holes in the teeth). LC could effectively inhibit the adherence of Streptococcus mutans to salivary acquired pellicle, and may be used as an effective natural medicine for the prevention of caries (CitationXiao et al., 2004).
Effects on endothelial dysfunction in type 2 diabetes
Radix astragali (Legumisae) and Rhizoma Ligustici chuanxiong have protective effects on endothelial dysfunction in type 2 diabetic patients with microalbuminuria. In these patients, impaired endothelial-dependent vasodilation (EDV), elevated plasma plasminogen activator inhibitor type I (PAI-I) activity, and increased C reactive protein (CRP) and malonic aldehyde (MDA) concentration at baseline are observed. After 6 months treatment, Radix astragali and Rhizoma Ligustici chuanxiong significantly decrease the activity of PAI-1, the levels of MDA and CRP and their urinary albumin-to-creatinine ratio, and evidently improve EDV. But there are no significant changes in the intima-media thickness (IMT) and endothelial-independent vasodilation (EIV). The mixed treatment of Radix astragali and Rhizoma Ligustici chuanxiong possibly decreases urinary albumin excretion and improves endothelial dysfunction in type 2 diabetic patients with microalbuminuria. The mechanism may be related with the effects of Radix astragali and Rhizoma Ligustici chuanxiong on antiinflammation, antioxidation, and alleviation of the hypofibrinolytic/prothrombotic state (CitationLu et al., 2005).
Chondroprotective effects
TMP could attenuate the IL-1β-induced cartilage and chondrocyte destruction in a dose-dependent manner. It decreases glycosaminoglycan (GAG) degradation and MMP-3 mRNA production, and enhances tissue inhibitor of metalloproteinase 1 (TIMP-1) mRNA production in cartilage explants. TMP also increases the cell viability in chondrocytes and inhibits the chondrocytes apoptosis through suppression of ROS production, maintenance of mitochondrial membrane potential, and down regulation of caspase-3 activity. These results demonstrate that TMP possesses the cartilage and chondroprotective effect (CitationJu et al., 2010).
Effects on lifespan of Caenorhabiditis elegans
Caenorhabiditis elegans (Rhabditidae) is unsegmented, vermiform, and bilaterally symmetrical, with a cuticle integument, four main epidermal cords, and a fluid-filled pseudocoelomate cavity. Members of the species have many of the same organ systems as other animals. In the wild, they feed on bacteria that develop on decaying vegetable matter. LC extract significantly extends the mean lifespan and the maximum lifespan of Caenorhabiditis elegans. It could up regulate the expression of hsp-70 and skn-1, but down regulate the expression of akt-2 and tub-1. The underlying molecular mechanism is related to genes of Insulin/Insulin-like growth factor 1 (IGF-1) signaling pathway and dietary restriction system. In the progress of aging stages of Caenorhabiditis elegans, the expression of age-1, daf-2, let-363 increases, functioning as aging-promoting genes; the expression of ins-18, let-60, sir-2.1, sod-3 decreases, functioning as longevity genes; LC extract extends the lifespan through inhibiting the expression of these aging-promoting genes and increasing the expression of longevity genes (Wang et al., Citation2010b, Citation2010c).
Other effects
TMP, vanillic, and chrysophol could completely combine with cardiac muscle membrane acceptors, whereas FA could not (CitationZhang et al., 2004). TMP probably acts on an acceptor and vanillic acts on β1 acceptor. TMP could relax isolated corpus cavernosum strips and increase intracavernous pressure (ICP) of rabbits in vivo, which may be partly mediated by the inhibition of cAMP phosphodiesterase or cGMP phosphodiesterase (CitationXiao et al., 2010).
Conclusion
LC has been used as a traditional Chinese medicine in China for thousands of years. Many extracts and single compounds obtained from LC have been found to possess various pharmacological effects on organs and systems such as brain, blood, cardiovascular and nervous systems, and to display numerous bioactivities such as antioxidation, neuronprotection, antiinflammation, and antinociception, indicating marked adaptogenic properties of this plant.
However, further study is necessary. Although various bioactivities of extracts or compounds obtained from LC are substantiated by using laboratory animals or cells, few molecular mechanisms of action are known, which will make against further clinical application of LC. In addition, when a drug is used in clinical practice, its security is especially important. Unfortunately, there are also few toxicological evaluations reported on these extracts or compounds. However, the data obtained from animals or cells may be inconsistent with the human situation. There exist gaps between experimental study and clinical practice, which need to be bridged to exploit the full medicinal potential of LC. To develop more new drugs with good therapeutic effects and few side effects for clinic, the above-mentioned problems need to be resolved.
The documents summarized above strongly support the view that LC has beneficial therapeutic properties indicating its potential as an effective adaptogenic herbal remedy. The prospect of increasing production of LC raises expectations for increased utilization opportunities for this plant. Recent years, some pharmacological activities of LC such as antioxidant, neuroprotective, antiinflammatory, antinociceptive, antiproliferative, proapoptotic, and antifibrotic effects make significant contributions to human health. It is very clear that LC is a plant with tremendous widespread use now and also with extraordinary potential for the future. Nevertheless, some problems are also needed to be further resolved.
Declaration of interest
This work was supported by the Open Research Fund of State Key Laboratory Breeding Base of Systematic research, development and Utilization of Chinese Medicine Resources and by a grant from the Key Programs for Basic Research of Shanghai Committee of Science and Technology (Grant No. 08JC1405700).
References
- Bi WF, Yang HY, Liu JC, Cheng TH, Chen CH, Shih CM, Lin H, Wang TC, Lian WS, Chen JJ, Chiu HC, Chang NC. (2005). Inhibition of cyclic strain-induced endothelin-1 secretion by tetramethylpyrazine. Clin Exp Pharmacol Physiol, 32, 536–540.
- Bie BH, Chen Y, Zhao ZQ. (2006). Ligustrazine inhibits high voltage-gated Ca(2+) and TTX-resistant Na(+) channels of primary sensory neuron and thermal nociception in the rat: A study on peripheral mechanism. Neurosci Bull, 22, 79–84.
- Chan SS, Cheng TY, Lin G. (2007). Relaxation effects of ligustilide and senkyunolide A, two main constituents of Ligusticum chuanxiong, in rat isolated aorta. J Ethnopharmacol, 111, 677–680.
- Chan SS, Choi AO, Jones RL, Lin G. (2006). Mechanisms underlying the vasorelaxing effects of butylidenephthalide, an active constituent of Ligusticum chuanxiong, in rat isolated aorta. Eur J Pharmacol, 537, 111–117.
- Chan SS, Jones RL, Lin G. (2009). Synergistic interaction between the Ligusticum chuanxiong constituent butylidenephthalide and the nitric oxide donor sodium nitroprusside in relaxing rat isolated aorta. J Ethnopharmacol, 122, 308–312.
- Chang XL, Jiang ZY, Ma YB, Zhang XM, Tsim KW, Chen JJ. (2009). Two new compounds from the roots of Ligusticum chuanxiong. J Asian Nat Prod Res, 11, 805–810.
- Chang XL, Ma YB, Zhang XM, Jiang ZY, Chen JJ. (2007). Studies on chemical constituents of rhizomes of Ligusticum chuanxiong. China J Chin Mat Med, 32, 1533–1536.
- Chao HM, Lin DE, Chang Y, Hsu WM, Lee SM, Lee FL, Chi CW, Pan WH, Liu TY, Lui WY, Ho LT, Kuo CD, Chan CC, Chao FP. (2008). Ferulic acid, but not tetramethylpyrazine, significantly attenuates retinal ischemia/reperfusion-induced alterations by acting as a hydroxyl radical scavenger. J Ocul Pharmacol Ther, 24, 461–472.
- Chen ZL, Guan YQ, Chen X, Chen XL, Chen JC. (2004). Effect of Chinese herbal medicine 1023 Recipe in blocking cancer transformation of experimental precancerous lesion and its mechanism. Chin J Integr Med, 2, 281–284.
- Cheng CY, Ho TY, Lee EJ, Su SY, Tang NY, Hsieh CL. (2008). Ferulic acid reduces cerebral infarct through its antioxidative and anti-inflammatory effects following transient focal cerebral ischemia in rats. Am J Chin Med, 36, 1105–1119.
- Chor SY, Hui AY, To KF, Chan KK, Go YY, Chan HL, Leung WK, Sung JJ. (2005). Anti-proliferative and pro-apoptotic effects of herbal medicine on hepatic stellate cell. J Ethnopharmacol, 100, 180–186.
- Dai Y, But PP, Chan YP, Matsuda H, Kubo M. (2002). Antipruritic and antiinflammatory effects of aqueous extract from Si-Wu-Tang. Biol Pharm Bull, 25, 1175–1178.
- Gao W, Liang RX, Xiao YQ, Yang HJ. (2005). Protective effect of the pretreatment with Chuanxiong-phthalide A on the vascular endothelial cells impaired by the ischemia and reperfusion in isolated rats hearts. China J Chin Mat Med, 30, 1448–1451.
- Guo J, Duan JA, Tang Y, Jia N, Li X, Zhang J. (2010). Fast onset of action and the analgesic and sedative efficacy of essential oil from Rhizoma chuanxiong after nasal administration. Pharmazie, 65, 296–299.
- Hou YZ, Zhao GR, Yang J, Yuan YJ, Zhu GG, Hiltunen R. (2004). Protective effect of Ligusticum chuanxiong and Angelica sinensis on endothelial cell damage induced by hydrogen peroxide. Life Sci, 75, 1775–1786.
- Hou YZ, Zhao GR, Yuan YJ, Zhu GG, Hiltunen R. (2005). Inhibition of rat vascular smooth muscle cell proliferation by extract of Ligusticum chuanxiong and Angelica sinensis. J Ethnopharmacol, 100, 140–144.
- Jeong JB, Ju SY, Park JH, Lee JR, Yun KW, Kwon ST, Lim JH, Chung GY, Jeong HJ. (2009). Antioxidant activity in essential oils of Cnidium officinale makino and Ligusticum chuanxiong Hort and their inhibitory effects on DNA damage and apoptosis induced by ultraviolet B in mammalian cell. Cancer Epidemiol, 33, 41–46.
- Jia RR, Gou YL, Ho LS, Ng CP, Tan NH, Chan HC. (2005). Anti-apoptotic activity of Bak Foong Pills and its ingredients on 6-hydroxydopamine-induced neurotoxicity in PC12 cells. Cell Biol Int, 29, 835–842.
- Ju XD, Deng M, Ao YF, Yu CL, Wang JQ, Yu JK, Cui GQ, Hu YL. (2010). The protective effect of tetramethylpyrazine on cartilage explants and chondrocytes. J Ethnopharmacol, 132, 414–420.
- Juan SH, Chen CH, Hsu YH, Hou CC, Chen TH, Lin H, Chu YL, Sue YM. (2007). Tetramethylpyrazine protects rat renal tubular cell apoptosis induced by gentamicin. Nephrol Dial Transplant, 22, 732–739.
- Ko WC, Liao CC, Shih CH, Lei CB, Chen CM. (2002). Relaxant effects of butylidenephthalide in isolated dog blood vessels. Planta Med, 68, 1004–1009.
- Lee TF, Lin YL, Huang YT. (2007). Studies on antiproliferative effects of phthalides from Ligusticum chuanxiong in hepatic stellate cells. Planta Med, 73, 527–534.
- Li L, Yang H, Xiao Y, Zhang C, Wang Y. (2009). Effect of Chuanxiong Fangfeng Baizhi prescription on isolated rabbit basilar artery. China J Chin Mat Med, 34, 1415–1417.
- Li M, Handa S, Ikeda Y, Goto S. (2001). Specific inhibiting characteristics of tetramethylpyrazine, one of the active ingredients of the Chinese herbal medicine “Chuanxiong,” on platelet thrombus formation under high shear rates. Thromb Res, 104, 15–28.
- Li M, Zhao C, Wong RN, Goto S, Wang Z, Liao F. (2004). Inhibition of shear-induced platelet aggregation in rat by tetramethylpyrazine and salvianolic acid B. Clin Hemorheol Microcirc, 31, 97–103.
- Li QX, Liao YH, Zhang HL, Jiang YY, Wang YF. (2007). Inhibitory effect and mechanism of ChUanxiOngzine on multiplication. J Nanjing Med Univ, 21, 82–85.
- Li SY, Jia YH, Sun WG, Tang Y, An GS, Ni JH, Jia HT. (2010). Stabilization of mitochondrial function by tetramethylpyrazine protects against kainate-induced oxidative lesions in the rat hippocampus. Free Radic Biol Med, 48, 597–608.
- Li Y, Xu C, Zhang Q, Liu JY, Tan RX. (2005). In vitro anti-Helicobacter pylori action of 30 Chinese herbal medicines used to treat ulcer diseases. J Ethnopharmacol, 98, 329–333.
- Li YH, Peng SL, Zhou Y, Yu KB, Ding LS. (2006). Two new phthalides from Ligusticum chuanxiong. Planta Med, 72, 652–656.
- Liang MJ, He LC, Yang GD. (2005). Screening, analysis and in vitro vasodilatation of effective components from Ligusticum chuanxiong. Life Sci, 78, 128–133.
- Lim LS, Shen P, Gong YH, Lee LS, Yong EL. (2006a). Dynamics of progestogenic activity in serum following administration of Ligusticum chuanxiong. Life Sci, 79, 1274–1280.
- Lim LS, Shen P, Gong YH, Yong EL. (2006b). Dimeric progestins from rhizomes of Ligusticum chuanxiong. Phytochemistry, 67, 728–734.
- Lin YL, Hsu YC, Chiu YT, Huang YT. (2008). Antifibrotic effects of a herbal combination regimen on hepatic fibrotic rats. Phytother Res, 22, 69–76.
- Lin YL, Lee TF, Huang YJ, Huang YT. (2006). Inhibitory effects of Ligusticum chuanxiong on the proliferation of rat hepatic stellate cells. J Gastroenterol Hepatol, 21, 1257–1265.
- Lin YL, Lee YC, Huang CL, Lai WL, Lin YR, Huang NK. (2007). Ligusticum chuanxiong prevents rat pheochromocytoma cells from serum deprivation-induced apoptosis through a protein kinase A-dependent pathway. J Ethnopharmacol, 109, 428–434.
- Lin YL, Wang GJ, Huang CL, Lee YC, Liao WC, Lai WL, Lin YJ, Huang NK. (2009). Ligusticum chuanxiong as a potential neuroprotectant for preventing serum deprivation-induced apoptosis in rat pheochromocytoma cells: Functional roles of mitogen-activated protein kinases. J Ethnopharmacol, 122, 417–423.
- Liu L, Ning ZQ, Shan S, Zhang K, Deng T, Lu XP, Cheng YY. (2005). Phthalide lactones from Ligusticum chuanxiong inhibit lipopolysaccharide-induced TNF-alpha production and TNF-alpha-mediated NF-kappaB activation. Planta Med, 71, 808–813.
- Liu PY, Sheu JJ, Lin PC, Lin CT, Liu YJ, Ho LI, Chang LF, Wu WC, Chen SR, Chen J, Harn YC, Lin SZ, Tsai CH, Chiou TW, Harn HJ. (2010). Expression of Nur77 induced by an n-butylidenephthalide derivative promotes apoptosis and inhibits cell growth in oral squamous cell carcinoma. Invest New Drugs, doi:10.1007/s10637-010-9518-z
- Liu YJ, Huang GY, Yang MW, Gong P, Lu F. (2004). Effect of jiantai liquid on expression of estrogen and progesterone receptors in uterus of mice with embryo implantation dysfunction induced by mifepristone. China J Chin Mat Med, 24, 816–819.
- Lu Y, Hu YL, Kong XF, Wang DY. (2008). Selection of component drug in activating blood flow and removing blood stasis of Chinese herbal medicinal formula for dairy cow mastitis by hemorheological method. J Ethnopharmacol, 116, 313–317.
- Lu ZM, Yu YR, Tang H, Zhang XX. (2005). The protective effects of Radix Astragali and Rhizoma Ligustici chuanxiong on endothelial dysfunction in type 2 diabetic patients with microalbuminuria. J Sichuan Univ (Medical Science Edition), 36, 529–532.
- Meng H, Guo J, Sun JY, Pei JM, Wang YM, Zhu MZ, Huang C. (2008). Angiogenic effects of the extracts from Chinese herbs: Angelica and Chuanxiong. Am J Chin Med, 36, 541–554.
- Meng H, Zhu MZ, Guo J, Sun JY, Pei JM, Huang C. (2006). The study on angiogenesis activity of danggui, chuanxiong and danshen. J Chin Med Mater, 29, 574–576.
- Miao C, Wu S, Luo B, Wang J, Chen Y. (2010). A new sesquiterpenoid from Ligusticum chuanxiong Hort. Fitoterapia, 81, 1088–1090.
- Or TCT, Yang CLH, Law AHY, Li JCB, Lau ASY. (2010). Isolation and identification of anti-inflammatory constituents from Ligusticum chuanxiong and their underlying mechanisms of action on microglia. Neuropharmacology, doi:10.1016/j.neuropharm.2010.12.002
- Peng C, Xie X, Wang L, Guo L, Hu T. (2009). Pharmacodynamic action and mechanism of volatile oil from Rhizoma Ligustici chuanxiong Hort. on treating headache. Phytomedicine, 16, 25–34.
- Qi H, Siu SO, Chen Y, Han Y, Chu IK, Tong Y, Lau AS, Rong J. (2010). Senkyunolides reduce hydrogen peroxide-induced oxidative damage in human liver HepG2 cells via induction of heme oxygenase-1. Chem Biol Interact, 183, 380–389.
- Ren DC, Yang NY, Qian SH, Xie N, Zhou XM, Duan JA. (2007a). Chemical study on aerial parts of Ligusticum chuanxiong. Chin Tradit Herb Drugs, 32, 1418–1420.
- Ren XY, Ruan QR, Zhu DH, Zhu M, Qu ZL, Lu J. (2007b). Tetramethylpyrazine inhibits agiontensin II-induced nuclear factor-kappaB activation and bone morphogenetic protein-2 downregulation in rat vascular smooth muscle cells. Acta Physiol Sin, 59, 339–344.
- Shih YH, Wu SL, Chiou WF, Ku HH, Ko TL, Fu YS. (2002). Protective effects of tetramethylpyrazine on kainate-induced excitotoxicity in hippocampal culture. Neuroreport, 13, 515–519.
- Shu D, He J, Chen J. (2009). Neuroprotective effects and mechanisms of Chuanxiong Chatiao pulvis against MPTP-induced dopaminergic neurotoxicity in mice model of Parkinson’s disease. Chin Tradit Herb Drugs, 34, 2494–2497.
- Sim Y, Shin S. (2008). Combinatorial anti-Trichophyton effects of Ligusticum chuanxiong essential oil components with antibiotics. Arch Pharm Res, 31, 497–502.
- Sue YM, Cheng CF, Chang CC, Chou Y, Chen CH, Juan SH. (2009). Antioxidation and anti-inflammation by haem oxygenase-1 contribute to protection by tetramethylpyrazine against gentamicin-induced apoptosis in murine renal tubular cells. Nephrol Dial Transplant, 24, 769–777.
- Tian JW, Fu FH, Jiang WL, Wang CY, Sun F, Zhang TP. (2005). Protective effect of Ligusticum chuanxiong phthalides on focai cerebral ischemia in rats and its related mechanism of action. Chin Tradit Herb Drugs, 30, 466–468.
- Tian Y, Liu Y, Chen X, Zhang H, Shi Q, Zhang J, Yang P. (2010). Tetramethylpyrazine promotes proliferation and differentiation of neural stem cells from rat brain in hypoxic condition via mitogen-activated protein kinases pathway in vitro. Neurosci Lett, 474, 26–31.
- Tsai CC, Lai TY, Huang WC, Liu IM, Cheng JT. (2002). Inhibitory effects of potassium channel blockers on tetramethylpyrazine-induced relaxation of rat aortic strip in vitro. Life Sci, 71, 1321–1330.
- Wang H, Tang Y, Guo J, Ding A, Li W, Jiang W, Duan J. (2010a). Antidysmenorrheic effects of Radix angelica and Rhizoma chuanxiong with different proportions and preparation methods on dysmenorrhea model mice. Chin Tradit Herb Drugs, 35, 892–895.
- Wang J, Liu XR, Shi YM. (1995). Observation of the preventive and therapeutic effect of Chuanxiong on the atherosclerosis of rabbits. Chin J Integr Med, 1, 52–55.
- Wang S, Gao Z, Chen X, Lian X, Zhu H, Zheng J, Sun L. (2008). The anticoagulant ability of ferulic acid and its applications for improving the blood compatibility of silk fibroin. Biomed Mater, 3, 044106.
- Wang WX, Gu M, Jiang XL. (2002). Studies on chemical constituents of the rhizomae of Ligusticum chuanxiong. Chin Tradit Herb Drugs, 33, 4–5.
- Wang X, Wang D, Li L, Niu X. (2010b). Effect of Ligusticum chuanxiong extract on lifespan of Caenorhabditis elegans and its underlying molecular mechanisms. Chin Tradit Herb Drugs, 35, 1042–1045.
- Wang X, Wang D, Li L, Niu X. (2010c). Expression changes of age-related genes in different aging stages of Caenorhabiditis elegans and the regulating effects of Chuanxiong extract. Chin Tradit Herb Drugs, 35, 1599–1602.
- Wang XB, Wang SS, Zhang QF, Liu M, Li HL, Liu Y, Wang JN, Zheng F, Guo LY, Xiang JZ. (2010d). Inhibition of tetramethylpyrazine on P-gp, MRP2, MRP3 and MRP5 in multidrug resistant human hepatocellular carcinoma cells. Oncol Rep, 23, 211–215.
- Wang ZY, Qian RQ, Guan SH. (2003). Study on free radical mechanism of guanxin II in antagonizing ischemic myocardial damage. Chin J Integr Med, 23, 363–366.
- Wong KL, Chan P, Huang WC, Yang TL, Liu IM, Lai TY, Tsai CC, Cheng JT. (2003). Effect of tetramethylpyrazine on potassium channels to lower calcium concentration in cultured aortic smooth muscle cells. Clin Exp Pharmacol Physiol, 30, 793–798.
- Wong KL, Wu KC, Wu RS, Chou YH, Cheng TH, Hong HJ. (2007). Tetramethylpyrazine inhibits angiotensin II-increased NAD(P)H oxidase activity and subsequent proliferation in rat aortic smooth muscle cells. Am J Chin Med, 35, 1021–1035.
- Wu JG, Ma L, Zhang SY, Zhu ZZ, Zhang H, Qin LP, Wei YJ. (2011). Essential oil from rhizomes of Ligusticum chuanxiong induces apoptosis in hypertrophic scar fibroblasts. Pharm Biol, 49, 86–93.
- Wu JG, Wei YJ, Ran X, Zhang H, Nian H, Qin LP. (2010). Inhibitory effects of essential oil from rhizomes of Ligusticum chuanxiong on hypertrophic scarring in the rabbit ear model. Pharm Biol, doi:10.3109/13880209.2010.542761
- Xiao HJ, Wang T, Chen J, Fan LC, Yin CP, Liu JH, Gao X. (2010). Chuanxiongzine relaxes isolated corpus cavernosum strips and raises intracavernous pressure in rabbits. Int J Impot Res, 22, 120–126.
- Xiao Y, Liu TJ, Huang ZW, Zhou XD, Li JY. (2004). The effects of natural medicine on adherence of Streptococcus mutans to salivary acquired pellicle. J Sichuan Univ (Med Sci Ed), 35, 687–689.
- Xiao YQ, Li L, You XL, Taniguchi M, Baba K. (2002). Studies on chemical constituents of the rhizomae of Ligusticum chuanxiong. Chin Tradit Herb Drugs, 27, 519–522.
- Xiong L, Fang ZY, Tao XN, Bai M, Feng G. (2007). Effect and mechanism of ligustrazine on Th1/Th2 cytokines in a rat asthma model. Am J Chin Med, 35, 1011–1020.
- Xu H, Wen C, Chen KJ. (2007). Study on the effect of rhizoma Chuanxiong, radix paeoniae rubra and the compound of their active ingredients, Xiongshao Capsule, on stability of atherosclerotic plaque in ApoE(-/-) mice. Chin J Integr Med, 27, 513–518.
- Yang J, Lei Y, Cui W, Fang S, Chen K. (2009a). Mechanisms of delay endothelial cell replicative senescence by extracts from Panax ginseng, Panax notoginseng and Ligusticum chuanxiong. Chin Tradit Herb Drugs, 34, 1544–1548.
- Yang J, Lei Y, Fang SP. (2009b). Study on acting mechanism of extracts from ginseng, notoginseng and chuanxiong for delaying the aging of endothelial cells induced by angiotensin II. Chin J Integr Med, 29, 524–528.
- Yang X, Zeng X, Wu T. (2010). Chuanxiong preparations for preventing stroke. Cochrane Database Syst Rev, CD006765.
- Yuan J, Zhang Z, Fan Z, Yang J. (2008). Antioxidant effects and cytotoxicity of three purified polysaccharides from Ligusticum chuanxiong Hort. Carbohyd Polym, 74, 822–827.
- Zhang DW, Liu JG, Feng JT, Zhang L, Yang XP, Shi DZ, Liang XM. (2010). Effects of effective components compatibility of aqueous extracts of Salviae miltiorrhizae and Rhizoma Chuanxiong on rat myocardial ischemia/reperfusion injury. Chin Crit Care Med, 22, 109–112.
- Zhang L, Jiang YR, Guo CY, Wu CF, Chen KJ, Yin HJ. (2009a). Effects of active components of Red Paeonia and Rhizoma chuanxiong on angiogenesis in atherosclerosis plaque in rabbits. Chin J Integr Med, 15, 359–364.
- Zhang L, Xue M, Ma XJ. (2009b). Effect of the active components of red paeonia and rhizoma chuanxiong on matrix metalloproteinases in rabbits with atherosclerosis. Chin J Integr Med, 29, 514–518.
- Zhang YN, Yue XF, Zhang ZQ. (2004). Study on the interactions between four components in Ligusticum chuanxiong rhizome and acceptors on cardiac muscle membrane. China J Chin Mat Med, 29, 660–662.
- Zhulun L, Jinrong Y, Rong F, Jun S. (2003). The antipyretic effect of the essential oil of Ligusticum chuanxiong Hort. on endotoxin-induced fever and hypothalamic content of 5-HT DA in rabbits. Pharmacol Clin Chin Mat Med, 19, 17–19.