Abstract
Context: Currently there has been an increased global interest to identify antioxidant compounds for use in preventive medicine and the food-industry that are pharmacologically potent and have low or no side effects. As plants produce significant amount of antioxidants to prevent oxidative stress, they represent a potential source of new compounds with antioxidant activity.
Objective: The current study was designed to evaluate the methanol extract of Artemisia absinthium Linn. (Asteraceae; MAB) for its in vitro free-radical scavenging effects using different classical assays, and in vivo antioxidant activity using global cerebral ischemia and reperfusion (I/R)-induced oxidative stress in mice.
Materials and methods: The in vitro scavenging activity was studied on the superoxide anions, hydrogen peroxide, hydroxyl, nitric oxide radical, and reducing power. Further, in the in vivo studies, the animal model of global cerebral I/R was established by occluding the bilateral carotid artery for 15 min followed by 24-h reperfusion. The thiobarbituric acid reactive substances (TBARS) concentration, superoxide dismutase (SOD) activity and glutathione (GSH) content were determined by colorimetric assays.
Results: In the in vitro assays, methanol extract of A. absinthium showed significant (p < 0.05) superoxide anion, hydrogen peroxide, hydroxyl and nitric oxide radical scavenging activities, and significant reducing power. Furthermore, in the in vivo studies, oral administration of MAB (100 or 200 mg/kg) inhibited cerebral I/R-induced oxidative stress by decreasing TBARS, and restoring levels of SOD and GSH.
Conclusion: The results indicated that A. absinthium possess potent antioxidant properties, and may be used as a protective agent against disorders associated with oxidative stress.
Introduction
Reactive oxygen species (ROS) readily attack and induce oxidative damage to various biomolecules including proteins, lipids, lipoproteins, and DNA. This oxidative damage is a crucial etiological factor implicated in several chronic human diseases, namely cardiovascular diseases, rheumatism, diabetes mellitus, cerebrovascular diseases, and cancer (CitationSabir & Rocha, 2008; CitationYang et al., 2010). Based on a growing interest in free-radical biology and the lack of effective therapies for most chronic diseases, a study of the usefulness of antioxidants in protection against these diseases is warranted. Antioxidants are chemical substances that reduce or prevent oxidation. They have the ability to counteract the damaging effects of free radicals in tissues and thus are believed to protect against cancer, cerebrovascular diseases, arteriosclerosis, heart disease, and several other diseases (CitationBandyopadhyay et al., 2007).
Many antioxidant compounds, naturally occurring in plant sources have been identified as free-radical scavengers (CitationVerma et al., 2010). A number of synthetic antioxidants, such as 2- and 3-tert-butyl-4-methoxyphenol (butylated hydroxyanisole; BHA) and tert-butylhydroquinone have been added to foodstuffs but because of toxicity issues, their use is being questioned. Therefore, attention has been directed towards the development and isolation of natural antioxidants from plant sources. Crude extracts of spices, herbs, and other plant materials rich in polyphenolics are increasingly of interest to the pharmaceutical and food industry because they have the capacity to retard oxidative degradation and thereby improve the quality of pharmaceutical products, and nutritional value of food (CitationAmarowicz et al., 2004).
Many studies have shown that phenolic compounds display antioxidant activity as a result of their capacity to scavenge free radicals (CitationSeyoum et al., 2006; CitationYang et al., 2010). Phenolic compounds can also act as antioxidants by chelating metal ions, preventing radical formation and improving the antioxidant endogenous system. Therefore, the search for natural antioxidants of plant origin has gained momentum in recent years.
Artemisia absinthium Linn. (Asteraceae), commonly known as wormwood, is an aromatic, perennial undershrub growing naturally in Europe, North America, and Asia. Traditionally, wormwood has been used as an antiseptic, antispasmodic, anticancer, febrifuge, cardiac stimulant, for the restoration of declining mental function and inflammation of the liver, and to improve memory (CitationKoul, 1997; CitationWake et al., 2000).
Phytochemically, A. absinthium has been reported to possess essential oil, absinthin, anabsin, anabsinthin, artabsin, and matricin; resins, lactones, and organic acids (CitationOmer et al., 2007). Wormwood also contains flavonoids such as quercetin, rutin (CitationRice-Evans et al., 1996; CitationLee et al., 2004), and other flavonoid glycosides (isoquercitrin, quercitin-3-O-β-d-glucoside, quercitin-3-O-rhamnoglucoside, isorhamnetin-3-O-rhamnoglucoside, isorhamnetin-3-glucoside), and phenolic acids such as chlorogenic, syringic, coumaric, salicylic, and vanillic acids (CitationKordali et al., 2005) are probably involved in the mechanism of free-radical scavenging activity. These pharmacophores have been shown to possess potent antioxidant and free-radical scavenging activity, and anti-inflammatory activity (CitationKordali et al., 2005).
Pharmacological reports revealed that A. absinthium enhance the cognitive ability as evidenced by its nicotinic and muscarinic receptor activity in homogenates of human cerebral cortical membranes (CitationWake et al., 2000). Hexane-, chloroform-, and water-soluble extracts of the plant exhibited antipyretic activity against subcutaneous yeast injections in rabbits. Moreover, it has been reported that methanol extract of this plant enhanced neurite outgrowth induced by nerve growth factor and PC12D cells (CitationLi & Ohizumi, 2004). Recently, the authors have shown that A. absinthium exhibited significant neuroprotective effects against ischemia and reperfusion insult in rats (CitationKundan & Anupam, 2010).
The aim of the this study was to evaluate the in vitro and in vivo antioxidant and free-radical scavenging activity, total phenolic and flavonoid content of methanol extract of A. absinthium, by using a number of classical assays, and to assess whether A. absinthium could be a source of natural antioxidant for pharmaceutical applications.
Materials and methods
Plant materials
Aerial parts of the plant A. absinthium were procured from Himalaya Herbs Stores, Saharanpur, Uttar Pradesh, India. Identity of the plant material was authenticated by Dr. B. Singh, scientist at National Institute of Science Communication and Information Resources (NISCAIR), New Delhi, India. A voucher specimen of A. absinthium has been deposited in the Herbarium-cum-Museum of the University Institute of Pharmaceutical Sciences, Panjab University, Chandigarh, India. The dried aerial parts of the plant were milled to a fine powder using an electric blender. The methanol extract was prepared by extracting 100 g of powdered plant material using Soxhlet apparatus (20 h). Thereafter, the resulting methanol extract was reduced in vacuo (40°C), freeze-dried and stored at 4°C until used. The extract obtained was called MAB.
Chemicals
Nitroblue tetrazolium (NBT), phenazine methosulfate (PMS), 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB), BHA, butylated hydroxy toluene (BHT), l- ascorbic acid, nicotinamide adenine dinucleotide (NADH), gallic acid (GA), α-tocopherol, 1,1,3,3-tetraethoxypropane, trichloroacetic acid (TCA) and thiobarbituric acid (TBA) were obtained from Sigma–Aldrich, Germany. Chloral hydrate was obtained from Reidel–deHaen, Germany. All other chemicals used were analytical grade and were obtained from Merck.
Standardization of extract
Determination of total phenolic content
The total phenolic content of MAB was analyzed according to the Folin–Ciocalteu method as described by CitationCliffe et al. (1994). In brief, MAB was well mixed with 2.5 mL of distilled water, and then 0.5 mL of the Folin–Ciocalteu stock reagent and 1.0 mL of Na2CO3 reagent (75 g/L) were added to the mixture. They were then incubated at room temperature for 30 min. The mixture absorbance was spectrophotometrically (Beckman DU 640B, Nyon, Switzerland) measured at 765 nm.
Determination of total flavonoid content
The total flavonoid content of MAB was determined according to colorimetric method as described by CitationZou et al. (2004). In brief, 0.5 mL of sample solution was mixed with 2 mL of distilled water and subsequently with 0.15 mL of 5% NaNO2 solution. After 6 min of incubation, 0.15 mL of 10% AlCl3 solution was added and then allowed to stand for 6 min, followed by adding 2 mL of 4% sodium hydroxide solution to the mixture. Immediately after water was added to the sample to bring the final volume to 5 mL, the mixture was thoroughly mixed and allowed to stand for another 15 min. The mixture absorbance was determined at wavelength 510 nm.
In vitro assays
Superoxide anion scavenging activity
Superoxide anion scavenging activity of the extract was validated according to the method described by CitationLiu et al. (1997). Superoxide radical is generated in PMS-NADH systems by oxidation of NADH and assayed by the reduction of NBT. In this experiment, the superoxide radical was generated in 3 mL of Tris–HCl buffer (16 mM, pH 8.0) containing 1 mL of NBT (50 μM), 1 mL NADH (78 μM) and sample solutions of extract (25–75 μg/mL) in water were mixed. The reaction was started adding 1 mL of 10 M PMS to the mixture. The reaction mixture was incubated at 25°C for 5 min, and the absorbance was recorded at 560 nm against blank samples. GA was used as a control. The percentage inhibition of superoxide anion radical generation was calculated using the following formula:
where A0 was the absorbance of the control, and A1 was the absorbance in the presence of the sample of MAB and standards.
Hydroxyl radical scavenging activity
The scavenging capacity for hydroxyl radical was measured according to the modified method of CitationRajeshwar et al. (2005). The assay was performed by adding 0.1 mL ethylenediaminetetraacetic acid, 0.01 mL of FeCl3, 0.1 mL hydrogen peroxide, 0.36 mL of deoxyribose, 1.0 mL of test solutions (10–75 µg/mL) dissolved in distilled water, 0.33 mL of phosphate buffer (50 mM, pH 7.4), and 0.1 mL of ascorbic acid in sequence. The mixture was then incubated at 37°C for 1 h. A 1.0 mL portion of the incubated mixture was mixed with 1.0 mL of 10% TCA and 1.0 mL of 0.5% TBA to develop the pink chromogen, which was measured at 532 nm. BHT was used as standard.
Nitric oxide scavenging activity
Nitric oxide scavenging activity was measured by the spectrophotometric method (CitationMadan et al., 2005). Sodium nitroprusside (5 mmol) in phosphate-buffered saline was mixed with a control without the test compound, but with an equivalent amount of methanol. Test solution of increasing concentrations (10–75 µg/ mL) were dissolved in methanol and incubated at 25°C for 30 min. After 30 min, 1.5 mL of the incubated solution was diluted with 1.5 mL of Griess reagent (1% sulfanilamide, 2% phosphoric acid, and 0.1% naphthyl ethylenediamine dihydrochloride). The absorbance of the chromophore formed during the diazotization of the nitrite with sulfanilamide and the subsequent coupling with naphthylethylene diamine dihydrochloride was measured at 546 nm.
Hydrogen peroxide scavenging activity
The ability of the MAB to scavenge hydrogen peroxide was determined according to the method of CitationRuch et al. (1989). A solution of hydrogen peroxide (4 mM) was prepared in phosphate buffer (pH 7.4). Hydrogen peroxide concentration was determined spectrophotometrically at 230 nm. Increasing concentrations of MAB (10–75 µg/ mL) in distilled water was added to hydrogen peroxide solution (0.6 mL, 40 mM). Absorbance of hydrogen peroxide at 230 nm was determined after 10 min against a blank solution containing in phosphate buffer without hydrogen peroxide. The percentage of scavenging of hydrogen peroxide of MAB and standard compounds:
where A0 was the absorbance of the control, and A1 was the absorbance in the presence of the sample of MAB and standards.
Reducing power assay
The reducing power of MAB was determined according to method of CitationOyaizu (1986). Briefly, increasing concentrations of the extract and the standard compound (BHT) in 1 mL of distilled water were mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of a potassium ferric cyanide solution (1%, w/v). The mixture was incubated in a water bath at 50°C for 20 min. Then, 2.5 mL of a TCA solution (10%, w/v) was added, and the mixture was then centrifuged at 3000g for 10 min. A 2.5 mL aliquot of the upper layer was combined with 2.5 mL of distilled water and 0.5 mL of a ferric chloride solution (0.1%, w/v), and the absorbance was measured at 700 nm. Increased absorbance of the reaction mixture correlates with greater reducing power.
In vivo biochemical assays
Animals and experimental protocols
Male Swiss albino mice (20–30 g) were maintained on standard environmental conditions, and fed with standard rodent diet and tap water ad libitum. They were housed in the institutional animal house and maintained on a 12-h light/dark cycle regulated at 22 ± 2°C room temperature. All studies were performed in accordance with the guidelines on regulation of scientific experiments on animals, as adopted and promulgated by the Animals Ethics Committee of L.R. Institute of Pharmacy.
A total of four groups of seven mice each were employed in the present study. The first group was sham operated (mice were subjected to surgical procedure, a thread was passed below both carotid arteries but the arteries were not occluded after 15 min, thread was removed and the animal was sutured back and allowed to recover for 24 h). A second group served as control {mice were orally administered vehicle [simple syrup i.p. + Tween 80 (5%), 10 mL/kg] 1 h before subjecting to 15-min global cerebral ischemia by bilateral carotid artery occlusion (BCAO) followed by reperfusion for 24 h}. Third and fourth groups of mice received 100 mg kg−1 and 200 mg kg−1 doses of MAB, respectively. The vehicle and extracts were administered orally, 60 min before subjecting them to global cerebral ischemia.
Bilateral carotid arteries occlusion
Animals were subjected to BCAO under chloral hydrate anesthesia (400 mg/kg, i.p.). A cotton thread was passed below each carotid artery. Pulling the ends of thread with constant weight induced global cerebral ischemia. After 15 min of global cerebral ischemia, weight on the thread was removed to allow the reflow of blood through carotid arteries. The incision was sutured back in layers (CitationHimory et al., 1990). Temperature was maintained at 37°C throughout the surgical procedure.
Estimation of TBA reactive substance
After 24 h-reperfusion, animals were sacrificed and brains were removed, weighted, minced and suspended in a buffer containing 30 mM Tris–HCl and 2.5 mM CaCl2 (pH 7.6). The above mixture was homogenized, and the homogenate was centrifuged at 750g to separate cellular debris. The supernatant was accurately divided into two parts. Both portions were centrifuged at 8200g to obtain mitochondrial fraction. One was utilized for determination of thiobarbituric acid reactive substances (TBARS) (CitationYagi, 1982) and the other one was employed for protein estimation (CitationLowry et al., 1951).
Superoxide dismutase
Superoxide dismutase (SOD) activity was measured by method of CitationKakkar et al. (1984). The mice brains were homogenized in ice-cold sodium pyrophosphate buffer (pH 8.3) in a ratio of 50 mg/mL; 200 μL of this homogenate was used for the assay. The inhibition by SOD of reduction of NBT to blue-colored chromogen in the presence of PMS and NADH was measured at 560 nm. One unit of enzyme activity was defined as the enzyme concentration required inhibiting the absorbance at 560 nm of chromogen production by 50% in 1 min under assay condition, and expressed as specific activity in unit of SOD/min/mg protein.
Reduced glutathione
Glutathione (GSH) in the brain was determined by the method of CitationJollow et al. (1974). 1.0 mL of PMS (10% w/v) was precipitated with 1.0 mL of sulfosalicylic acid (4%). The samples were kept at 4°C for at least 1 h and, then, subjected to centrifugation at 1200g for 15 min at 4°C. The assay mixture contained 0.1 mL of PMS (10%, w/v), 2.7 mL of phosphate buffer (0.1 M, pH 7.4) and 0.2 mL DTNB (40 mg/10 mL phosphate buffer, 0.1 M, pH 7.4) in a total volume of 3.0 mL. The yellow color developed was read immediately at 412 nm. The enzyme activity was calculated as nM DTNB oxidized/min/mg of protein.
Statistical analysis
The results are presented as means ± SD. All parameters of in vitro assays were analyzed using Student’s t-test. Whereas, the data of in vivo biochemical assays were statistically analyzed using one-way analysis of variance followed by post hoc Tukey’s multiple range test. p < 0.05 Was considered significant.
Results
Extract yield, total phenolic, and flavonoid content
The methanol extract yield was 12.26% (w/w). The total phenolic content was estimated to be 123 ± 0.82 mg GA equivalents per gram of extract from triplicate measurements. The total flavonoid content was expressed in milligrams of rutin equivalents per gram of extract. The total flavonoids content was 24 ± 0.96 mg rutin equivalents per gram of extract from triplicate measurements.
In vitro observations of MAB
Scavenging of superoxide anion radical
In PMS-NADH-NBT system, superoxide anion derived from dissolved oxygen by PMS-NADH coupling reaction reduces NBT. The decrease of absorbance at 560 nm with antioxidants indicates the consumption of superoxide anion in the reaction mixture. shows the percentage inhibition of superoxide radical generation at different concentrations (25–100 µg/mL) of MAB compared with the same concentration of GA (standard). MAB exhibited concentration-dependent scavenging activities against superoxide anion radicals generated in PMS-NADH systems. MAB showed significant (p < 0.05) superoxide radical scavenging activity (78.1%), at 100 μg/mL concentration.
Table 1. Superoxide anion radical scavenging activity of MAB and GA by the PMS-NADH-NBT method.
Scavenging of hydrogen peroxide
Hydrogen peroxide scavenging activity of MAB is shown in and compared with that of α-tocopherol as standard. The results indicate that MAB is capable of scavenging hydrogen peroxide in a concentration-dependent manner. From the different concentrations of MAB and α-tocopherol tested for scavenging activity, the EC50 value was found to be 106.41 and 18.52 µg/mL, respectively.
Table 2. Nitric oxides (NO) scavenging and hydrogen peroxide (H2O2) scavenging effects of MAB, BHA and α-tocopherol.
Scavenging of hydroxyl radical
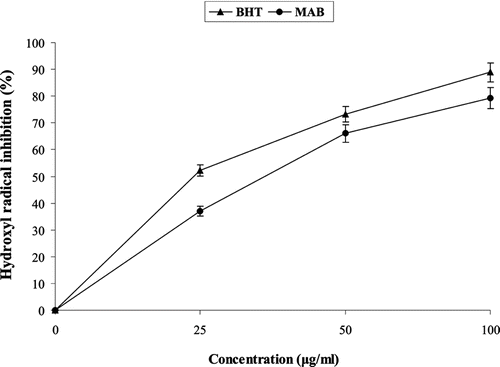
MAB exhibited significant (p < 0.05) hydroxyl radical scavenging activity in a concentration-dependent manner in the range of 25–100 µg/mL in the reaction mixture with 79.3% scavenging at a concentration of 100 µg/mL ().
Scavenging of nitric oxide
Nitric oxide was generated from sodium nitroprusside and measured by Greiss reaction. MAB showed nitric oxide scavenging activity between 25 and 200 µg/mL in a concentration-manner (EC50 = 180.71 µg/mL; ). The extract showed a moderate nitric oxide scavenging activity. The percentage of inhibition was increased with increasing concentration of the extract. BHA (EC50 = 26.51 µg/mL) was used as a positive control for comparison.
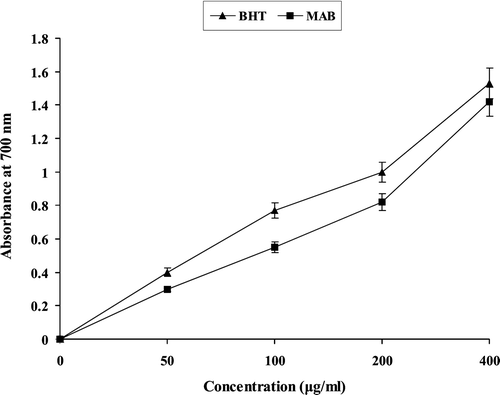
Reducing power
shows the reductive capability of MAB compared to BHT. The reducing power of MAB increased with increasing concentration. At the concentrations of 50, 100, 200 µg/mL of MAB showed lower activities than BHT, and these differences were statistically significant (p < 0.05). But at 400 µg/mL concentration of MAB the significant (p < 0.05) reducing power was shown.
In vivo observations of MAB
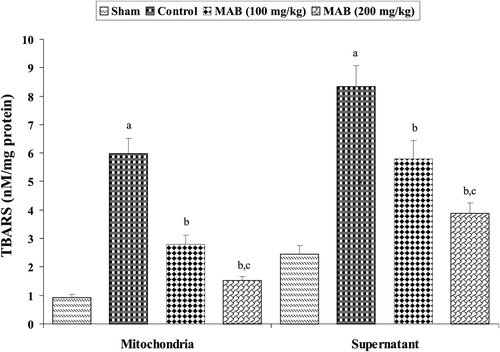
TBARS concentration in brain mitochondrial and supernatant fractions was significantly (p < 0.05) elevated in mouse ischemic brain due to ischemia-reperfusion (I/R) insult (). Pre-treatment with MAB significantly (p < 0.05) decreased the elevated TBARS concentration in brain mitochondrial and supernatant fractions as compared to control group.
Figure 3. Effect of methanol extract of Artemisia absinthium (MAB) on mitochondrial and supernatant thiobarbituric acid reactive substances formation in mice subject to global cerebral ischemia followed by reperfusion. Each column represents the mean ± SD, n = 7; a = p < 0.05 versus sham; b = p < 0.05 versus control; c = p < 0.05 versus 100 mg/kg, p.o., of the extract. TBARS, thiobarbituric acid reactive substances.
SOD and GSH showed a significant (p < 0.01) decrease in the control group versus the respective sham group. Pre-treatment with MAB markedly reversed the alterations in biochemical parameters brought about by I/R. The values were almost restored to near normal levels with no significant differences versus the sham group. SOD and GSH were significantly (p < 0.01) elevated in the MAB (200 mg/kg) treated animals subjected to BCAO and reperfusion injury as compared to control group ().
Table 3. Effect of methanol extract of Artemisia absinthium (MAB) on superoxide dismutase (SOD) activity and reduced glutathione (GSH) content during cerebral post-ischemic reperfusion (15 min BCAO and 24-h reperfusion).
Discussion
Oxidative stress, in which ROS like superoxide, hydrogen peroxide, hydroxyl radical, singlet oxygen, and nitrogen species are generated, is one of the earliest responses to stress. Superoxide anions are the most common free radicals, generated in a variety of biological systems and the concentration of superoxide anions increases under conditions of oxidative stress (CitationLee et al., 2002). These anions are produced endogenously by flavoenzymes-like xanthine oxidase, which converts hypoxanthine to xanthine and subsequently to uric acid in I/R (CitationBlaszczyk et al., 1994). The significant decrease in the concentration of the superoxide anion radicals was observed in this study due to the scavenging ability of MAB.
Hydrogen peroxide can be formed in vivo by many oxidized enzymes such as SOD. It can cross membranes and may slowly oxidize a number of compounds. Hydrogen peroxide itself is not very reactive, but it can sometimes be toxic to cell because it may give rise to hydroxyl radical in the cells (CitationIlhami et al., 2004). Levels of hydrogen peroxide at or below about 20–50 mg seem to have limited cytotoxicity to many cell types. Thus, removing hydrogen peroxide as well as superoxide anion is very important for protection. The EC50 value for scavenging activity was 106.41 µg/mL.
Hydroxyl radicals are extremely ROS, capable of modifying almost every molecule in the living cells. They have the capacity to cause strand damages in DNA leading to carcinogenesis, mutagenesis, and cytotoxicity. Moreover, hydroxyl radicals are capable of the quick initiation of lipid peroxidation process as by abstracting hydrogen atoms from unsaturated fatty acids (CitationAruoma, 1998). In the present observation, MAB exhibited significant (p < 0.05) hydroxyl radical scavenging activity in a dose dependent manner.
The reducing properties are generally associated with the presence of reductones, which have been shown to exert antioxidant action by breaking the free-radical chain by donating a hydrogen atom (CitationPin-Der-Duh, 1998). Reductones are also reported to react with certain precursors of peroxide, thus preventing peroxide formation. The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity. Our data on the reducing power of MAB suggest that it is likely to contribute significantly towards the observed antioxidant effect. Like the antioxidant activity, the reducing power of MAB increased with increasing amount of sample. No significant difference was observed between MAB and BHT.
In addition to ROS, nitric oxide is also implicated in inflammation, cancer and other pathological conditions. Nitric oxide was generated from sodium nitroprusside and measured by Greiss reaction. Scavengers of nitric oxide compete with the oxygen, leading to reduced production of nitric oxide (CitationGovindarajan et al., 2003). The extract showed a moderate nitric oxide scavenging activity. The percentage of inhibition was increased with increasing concentration of the extract.
TBARS, SOD, and GSH were estimated as an index to assess the severity of oxidative damage in the brain tissue, and also the effect of A. absinthium on the reversal of the damage produced by BCAO. All these parameters were markedly reversed and restored to near normal levels in the groups pre-treated with MAB. Free radicals are well investigated in the development of I/R-induced cerebral injury (CitationReynolds et al., 2007). ROS produces malondialdehyde (MDA), an end product of lipid peroxidation. MDA reacts with TBA and is, thus, estimated as TBARS (CitationDib et al., 2002). Therefore, MDA was estimated using TBARS assay to estimate extent of ROS.
The over production of free radicals can be detoxified by endogenous antioxidants causing their cellular stores to be depleted (CitationAhmad et al., 2006). GSH is considered a central component in the antioxidant defenses of cells. It acts both to directly detoxify ROS and as a substrate for various peroxidises. Moreover, it is well evidenced that SOD activity in serum is reduced in stroke patients, and replacement of antioxidant activity could be beneficial in the acute treatment of cerebral ischemia (CitationSpranger et al., 1997). In the control groups SOD and GSH activity was significantly reduced. Pre-treatment with MAB significantly prevented MCAO-induced decline in SOD and GSH activity.
A. absinthium has been reported to contain flavonoids (CitationZheng, 1994; CitationRice-Evans et al., 1996; CitationLee et al., 2004), thymol and carvacrol as well as other phenolic compounds (CitationKordali, et al., 2005). These pharmacophores have been shown to possess potent antioxidant and free-radical scavenging activity (CitationAlessandra et al., 2003). In the present study, high levels of phenolic and flavonoid contents were estimated.
Conclusion
In conclusion, the present study indicates that A. absinthium possesses potent in vitro and in vivo antioxidant activity and free-radical scavenging capacity which could be due to the presence of phenols and flavonoids in the extract. These antioxidant activities could contribute, at least partly, to the therapeutic benefits of certain traditional claims of A. absinthium. In addition, MAB exhibited neuroprotection as is evident from the reduction of lipid peroxidation (decreased level of TBARS) and restoration of endogenous antioxidant (GSH and SOD) system. This suggests A. absinthium may be used as protective agent against disorders associated with oxidative stress.
Declaration of interest
Authors are grateful to the L.L.R. Educational Trust, which runs the L.R. Institute of Pharmacy, Solan, Himachal Pradesh, India, for providing funds to carry out the present investigation. The authors alone are responsible for the content and writing of the paper.
References
- Ahmad S, Yousuf S, Ishrat T, Khan MB, Bhatia K, Fazli IS, Khan JS, Ansari NH, Islam F. (2006). Effect of dietary sesame oil as antioxidant on brain hippocampus of rat in focal cerebral ischemia. Life Sci, 79, 1921–1928.
- Alessandra B, Gelsomina F, Ivano M, Francesco DS, Franca T, Nunziatina DT. (2003). Antioxidant and free radical scavenging activity of flavonol glycosides from different Aconitum species. J Ethnopharmacol, 86, 63–67.
- Amarowicz R, Pegg RB, Rahimi-Moghaddam P, Barl B, Weil JA. (2004). Free radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem, 84, 551–562.
- Aruoma OI. (1998). Free radicals oxidative stress and antioxidants in human health and disease. J Am Oil Chem Soc, 175, 199–212.
- Bandyopadhyay M, Chakraborty R, Raychaudhuri U. (2007). A process for preparing a natural antioxidant enriched dairy product (Sandesh). Food Technol, 40, 842–851.
- Blaszczyk J, Kedziora J, Luciak M, Sibinska E, Trznadel K, Pawlicki L. (1994). Effect of morphine and naloxone on oxidative metabolism during experimental renal ischemia and reperfusion. Exp Nephrol, 2, 364–370.
- Cliffe S, Fawer MS, Maier G, Takata K, Ritter G. (1994). Enzyme assays for the phenolic content of natural juices. J Agr Food Chem, 42, 1824–1828.
- Dib M, Garrel C, Favier A, Robin V, Desnuelle C. (2002). Can malondialdehyde be used as a biological marker of progression in neurodegenerative disease? J Neurol, 249, 367–374.
- Govindarajan R, Rastogi S, Vijayakumar M, Shirwaikar A, Rawat AK, Mehrotra S, Pushpangadan P. (2003). Studies on the antioxidant activities of Desmodium gangeticum. Biol Pharm Bull, 26, 1424–1427.
- Himori N, Watanabe H, Akaike N, Kurasawa M, Itoh J, Tanaka Y. (1990). Cerebral ischemia model with conscious mice. Involvement of NMDA receptor activation and derangement of learning and memory ability. J Pharmacol Methods, 23, 311–327.
- Ilhami G, Sukru B, Ahmet A, Mahfuz E, Emin B. (2004). In vitro antioxidant properties of morphine. Pharmacol Res, 49, 59–66.
- Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. (1974). Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology, 11, 151–169.
- Kakkar P, Das B, Viswanathan PN. (1984). A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys, 21, 130–132.
- Kordali S, Cakir A, Mavi A, Kilic H, Yildirim A. (2005). Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three Turkish artemisia species. J Agric Food Chem, 53, 1408–1416.
- Koul MK. (1997). Medicinal Plants of Kashmir and Ladakh Temperate and Cold Arid Himalaya. New Delhi: Indus Publishing Company, 102.
- Kundan SB, Anupam S. (2010). Neuroprotective effect of Artemisia absinthium L. on focal ischemia and reperfusion-induced cerebral injury. J Ethnopharmacol, 129, 403–409.
- Lee HG, Kim H, Oh WK, Yu KA, Choe YK, Ahn JS, Kim DS, Kim SH, Dinarello CA, Kim K, Yoon DY. (2004). Tetramethoxy hydroxyflavone p7F downregulates inflammatory mediators via the inhibition of nuclear factor kappaB. Ann N Y Acad Sci, 1030, 555–568.
- Lee JC, Kim HR, Kim J, Jang YS. (2002). Antioxidant property of an ethanol extract of the stem of Opuntia ficus-indica var. saboten. J Agric Food Chem, 50, 6490–6496.
- Li Y, Ohizumi Y. (2004). Search for constituents with neurotrophic factor-potentiating activity from the medicinal plants of paraguay and Thailand. Yakugaku Zasshi, 124, 417–424.
- Liu F, Ooi VE, Chang ST. (1997). Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci, 60, 763–771.
- LOWRY OH, ROSEBROUGH NJ, FARR AL, RANDALL RJ. (1951). Protein measurement with the Folin phenol reagent. J Biol Chem, 193, 265–275.
- Madan MP, Raghavan G, Ajay KSR, Palpu P. (2005). Free radical scavenging potential of Saussarea costus. Acta Pharmaceut, 55, 297–304.
- Omer B, Krebs S, Omer H, Noor TO. (2007). Steroid-sparing effect of wormwood (Artemisia absinthium) in Crohn’s disease: a double-blind placebo-controlled study. Phytomedicine, 14, 87–95.
- Oyaizu M. (1986). Studies on products of browning reaction prepared from glucoseamine. Jpn J Nutr, 44, 307–315.
- Pin-Der-Duh X. (1998). Antioxidant activity of burdock (Arctium lappa Linne): Its scavenging effect on free-radical and active oxygen. J Am Oil Chem Soc, 75, 455–461.
- Rajeshwar Y, Senthil KGB, Malay AG, Mazumder UK. (2005). Studies on in vitro antioxidant activities of methanol extract of Mukuna pruriens (Fabaceae) Sedds. Eur Bull Drug Res, 13, 131–138.
- Reynolds A, Laurie C, Mosley RL, Gendelman HE. (2007). Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol, 82, 297–325.
- Rice-Evans CA, Miller NJ, Paganga G. (1996). Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med, 20, 933–956.
- Ruch RJ, Cheng SJ, Klaunig JE. (1989). Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis, 10, 1003–1008.
- Sabir SM, Rocha JBT. (2008). Water-extractable phytochemicals from Phyllanthus niruri exhibit distinct in vitro antioxidant and in vivo hepatoprotective activity against paracetamol-induced liver damage in mice. Food Chem, 111, 845–851.
- Seyoum A, Asres K, El-Fiky FK. (2006). Structure-radical scavenging activity relationships of flavonoids. Phytochemistry, 67, 2058–2070.
- Spranger M, Krempien S, Schwab S, Donneberg S, Hacke W. (1997). Superoxide dismutase activity in serum of patients with acute cerebral ischemic injury. Correlation with clinical course and infarct size. Stroke, 28, 2425–2428.
- Verma AR, Vijayakumar M, Rao CV, Mathela CS. (2010). In vitro and in vivo antioxidant properties and DNA damage protective activity of green fruit of Ficus glomerata. Food Chem Toxicol, 48, 704–709.
- Wake G, Court J, Pickering A, Lewis R, Wilkins R, Perry E. (2000). CNS acetylcholine receptor activity in European medicinal plants traditionally used to improve failing memory. J Ethnopharmacol, 69, 105–114.
- Yagi K. (1982). Assay for serum lipid peroxide level and its clinical significance. In: Yagi K, ed. Lipid Peroxides in Biology and Medicine. New York: Academic Press, 232–342.
- Yang Q, Pan X, Kong W, Yang H, Su Y, Zhang L, Zhang Y, Yang Y, Ding L, Liu G. (2010). Antioxidant activities of malt extract from barley (Hordeum vulgare L.) toward various oxidative stress in vitro and in vivo. Food Chem, 118, 84–89.
- Zheng GQ. (1994). Cytotoxic terpenoids and flavonoids from Artemisia annua. Planta Med, 60, 54–57.
- Zou Y, Lu Y, Wei D. (2004). Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J Agric Food Chem, 52, 5032–5039.