Abstract
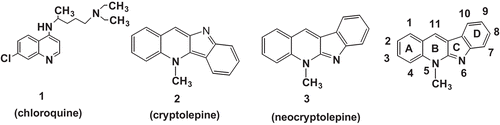
Context: The cryptolepines originate from the roots of the climbing shrub Cryptolepis sanguinolenta (Lindi) Schitr (Periplocaeae) which is used in Central and West Africa in traditional medicine for the treatment of malaria.
Objectives: Evaluation for the first time of a series of chloro- and aminoalkylamino derivatives of neo- and norneocryptolepines for potential schistosomicidal and molluscicidal activities.
Materials and methods: A series of chloro- and aminoalkylamino substituted neo- and norneocryptolepine derivatives were synthesized. They were tested in vitro against viable Schistosoma mansoni Sambon mature worms in culture medium with fetal serum and antibiotics and in dechlorinated water against the snail vector Biomphalaria alexandrina Ehrenberg. Active compounds were further subjected to determination of their IC50 values.
Results: Results showed that six neocryptolepine and two norneocryptolepine derivatives had in vitro schistosomicidal activity on Egyptian and Puerto Rican strains of S. mansoni. The most effective derivative (2-chloro-5-methyl-N-(2-morpholin-4-ethyl)-5H-indolo[2,3b]quinoline-11-amine) has IC50 and IC90 1.26 and 4.05 µM and 3.54 and 6.83 µM with the Egyptian and Puerto Rican strains of Schistosoma, respectively. All eight derivatives showed molluscicidal activity against the vector snail B. alexandrina. The most active compound (2-chloro-11-(4-methylpiperazin-1-yl)-6H-indolo [2,3-b] quinoline) has LC50 0.6 and LC90 3.9 ppm after 24 h.
Discussion and conclusions: The findings demonstrate that introducing chloro- and aminoalkylamino side chain initiated both schistosomicidal and molluscicidal activities in these derivatives. The structure–activity relationship of this series of compounds is discussed.
Introduction
Schistosomiasis is the second most prevalent disease in the world after malaria, with about 200 million human beings infected in 74 countries. It is estimated that 20 million of them have serious forms of the disease or related disability, and that 200,000 people die from the disease every year (CitationWorld Health Organization, 2002). Chemotherapeutic measures have been the mainstay in the control of this disease (CitationFenwick & Webster, 2006). Since 1970, praziquantel has become the drug of choice against the three major human species of schistosomes (Schistosomatidae), Schistosoma mansoni Sambon, S. hematobium (Bilharz) and S. japonicum (Katsurada) (CitationGönnert & Andrews, 1977; Citationpica-Mattoccia 2004; CitationDoenhoff & Pica-Mattoccia, 2006). It is a relatively safe, orally administered drug that leads to reducing the prevalence of schistosomiasis (CitationSouthgate et al., 2005). Consequently, a targeted as well as mass drug administration program presently relies heavily on this drug for the control of schistosome-induced morbidity. However, with only one drug of choice for treatment and the possibility of development of parasite resistance (CitationIsmail et al., 1999; CitationDoenhoff et al., 2002; CitationBotros & Bennett, 2007), the present situation is dangerous. Therefore, there is a real need for discovery of newer drugs. To reach this objective, the first step could be by testing compounds for antischistosomal activity on mature worms in culture (in vitro) to isolate potentially effective ones. This should be followed by testing top active compounds in vivo. Moreover, it should be useful to test these compounds for other biological activities, especially molluscicidal activity against snail vector of the parasite.
The present work has been carried out as part of our ongoing program for developing novel antimalarial drugs which are based on a natural product. This is isolated from the roots of the climbing shrub Cryptolepis sanguinolenta (Lindi) Schitr (CitationAlajarin et al., 1997) that is used in Central and West Africa in traditional medicine for the treatment of malaria (CitationWright, 2005).
As far as can be ascertained, cryptolepine 2 (CitationWright, 2007) and neocryptolepine 3 (CitationJonckers et al., 2002; CitationEl Sayed et al., 2009) have never been reported to exhibit antischistosomal activity ().This initiated searching for analogues of both compounds as potential antischistosomal agents. Side chains were incorporated at different core positions which proved to be an important feature for the antimalarial activity.
Materials and methods
Chemical
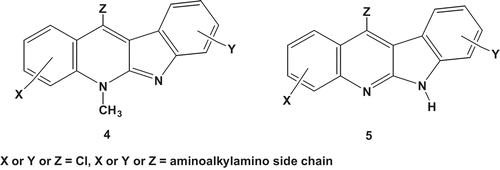
The source of the neocryptolepine core used in this study is the roots of the climbing shrub C. sanguinolenta (Lindi) Schitr (Periplocaeae). A series of neo- and norneocryptolepines, (4 and 5 respectively, ) with various basic aminoalkyamino side chains at different core position were synthesized ( and ). This was performed according to methods previously described by CitationShi et al. (1999) and CitationEl Sayed et al. (2009). The aminoalkylamino- or aminoalkyl group was chosen because of their indispensable importance for the antimalarial drug chloroquine. The active compounds were further subjected to determination of their IC50 and IC90 values. The starting materials used here were either commercially available or prepared. Anhydrous THF (tetrahydrofuran), toluene and dioxane were obtained from Sigma-Aldrich (St. Louis, MO) or Acros. Moisture-sensitive reactions were carried out under nitrogen or an argon atmosphere. Analytical thin-layer chromatography was performed on silica gel 60 F254 (Merck, Whitehouse Station, NJ). Column chromatography was carried out on silica gel 60 (230–400 mesh, Merck). Characterization of all compounds was done with 1H-NMR and mass spectrometry. 1H-NMR spectra were recorded on a 400 MHz.Bruker Avance DRX-400 spectrometer with NMR shifts being expressed in ppm downfield from internal TMS. ES Mass spectra were obtained from an Esquire 3000 plus iontrap mass spectrometer from Bruker Daltonics. Purity was verified using two diverse HPLC systems using respectively a mass and UV-detector. Water (A) and ACN (B), were used as eluents. LC-MS spectra were recorded on an Agilent 1100 Series HPLC system using a Alltech Prevail C18 column (2.1, 50 mm, 3 μm) coupled with an Esquire 3000plus as MS detector and a 5–100% B, 20-min gradient was used with a flow rate of 0.2 mL/min. 0.1% formic acid was added to solvent A and B. Reversed phase HPLC was run on a Gilson instrument equipped with an Ultrasphere ODS column (4.6, 250 mm, 5 μm). A 10–100% B, 35-min gradient was used with a flow rate of 1 mL/min. 0.1% of trifluoroacetic acid was added to solvent A and B. 214 nm was used as wavelength. Flash chromatography was carried out using Flash Master II (Jones Chromatography, Lakewood, CO) using Merck silica gel 60 (230–400 mesh).
Table 1. In vitro schistosomicidal activity of neocryptolepine (5-methyl-5H-indolo[2,3- b]quinoline) derivatives on adult Schistosoma mansoni worms (results after 5 days exposure).
Table 2. In vitro schistosomicidal activity of norneocryptolepine (quinindoline, 6H-indolo [2,3-b]quinoline) derivatives for Schistosoma mansoni worms (results after 5 days exposure).
General procedure for synthesis of the new neocryptolepine analogues
A round-bottom flask was charged with chloroindoloquinoline (0.5 mmol), the appropriate amine (0.75 mmol), and NaOtBu (67.30 mg, 0.7 mmol) followed by dry toluene (1 mL) in air. Subsequently, the flask was flushed with Ar for a few minutes under magnetic stirring. A stock solution (1 mL) of Pd-catalyst (4 mol%) was added via a syringe and the flask was flushed with Ar for an additional 3 min. The resulting mixture was heated at reflux (oil bath temperature: 105–110°C) for 2–24 h under magnetic stirring and an argon atmosphere. After cooling to room temperature dichloromethane (DCM, 25 mL) was added and the suspension filtered over a path of celite and rinsed with DCM (30 mL). The solvent was removed under reduced pressure and the residue purified by flash chromatography using DCM-2.0 N-ammonia in MeOH (90:10) as the eluent to yield title compounds g [N-(5-methyl-5H-indolo[2,3-b]quinolin-2-yl)-N-(1-phenylethyl)amine] and h {5-methyl-2-(4-morpholinyl)-5H-indolo[2,3-b]quinoline}.
Analytical and spectra data
Preparation of 4 mol% stock solution of the catalyst: A 250 mL bottle was charged with Pd (OAc)2 as Pd(0) source (89.8 mg, 0.4 mmol), and DCPB [2-(dicyclohexylphosphanyl)biphenyl] or DTPB [2-(di-t-butylphosphanyl)biphenyl] as ligand (0.8 mmol) and toluene (10 mL) in air. Subsequently, the bottle was flushed with Ar for 10 min under magnetic stirring. The stock solutions were stored under an Ar atmosphere. When DTPB was used as ligand for the catalyst the stock solution was stirred for 16 h prior to its use.
N-(5-methyl-5H-indolo[2,3-b]quinolin-2-yl)-N-(1-phenylethyl) amine 4 g
Yield: 74 mg (42%), 1H NMR (CDCl3) δ 1.61 (d, 3H, J = 6.8 Hz), 4.3 (s, 3H), 4.6 (m, 1H), 7.17 (m, 2H), 7.26 (m, 1H), 7.35 (m, 2H), 7.43 (m, 2H), 7.51 (m, 1H), 7.51 (m, 1H), 7.54 (d, 1H, J = 9.8 Hz), 7.7 (s, 1H, J = 8 Hz), 7.95 (d, 1H, J = 7.6 Hz), 8.29 (s, 1H). HPLC: 214 nm tr 21.78 min. 100%. LC/MS: tr 14.5 min. 100%. MS (ESI): m/z = 352 (M+1).
5-methyl-2-(4-morpholinyl)-5H-indolo[2,3-b]quinoline 4 h
Yield: 124 mg (78%), 1H NMR (CDCl3) δ 3.21 (m, 4H), 3.92 (m, 4H), 4.27 (s, 3H), 7.18 (m, 1H), 7.28 (m, 1H), 7.39 (m, 1H), 7.51 (m, 1H), 7.59 (d, 1H, J = 9.2 Hz), 7.7 (d, 1H, J = 7.6 Hz), 7.98 (d, 1H, J = 7.6 Hz), 8.37 (s, 1H). HPLC: 214 nm: tr 16.39 min. 100%. LC/MS tr 12 min. 95%. MS (ESI): m/z = 318 (M+1).
In vitro schistosomicidal bioassay
The schistosomicidal bioassay used here followed the main procedure previously described by CitationYousif et al. (2007) and CitationRamirez et al. (2007).Thus, the parasite material was S. mansoni mature worms of two strains (Egyptian and Puerto Rican strains) maintained at the Schistosome Biological Supply Centre (SBSC), Theodor Bilharz Research Institute (TBRI), Cairo, Egypt. The mature worms were obtained from hamsters (Mesocricetus auratus) (Waterhouse, 1839) percutaneously infected with cercariae 7 weeks earlier. They were obtained by perfusion using citrated saline and the recovered worms were washed from blood in small sieves (20 µm mesh) by phosphate buffer. Worms were washed three times with the culture medium, which is used for the assay under a sterilized laminar flow chamber. The culture medium is RPMI 1640 + l-glutamine + 20% fetal calf serum + antibiotics (300 µg streptomycin + 300 IU penicillin + 160 µg gentamycin per ml). The bioassay was carried out using 24-well tissue culture plates. Stock solutions 5 mg/ml of compounds were prepared in 100% dimethyl sulfoxide (DMSO) immediately before being used or stored at −20°C. Successive dilutions were made using DMSO and water (1:1). Three pairs of worms, males and females equally, were used for each test (well), and two replicates were set up. Exposure of worms to a standard concentration of 5 µg/ml of each compound was made for 5 days at 37°C ± 0.5°C in 5% CO2 incubator. A pure medium and medium with 0.5% DMSO were used as negative control while praziquantel was used as a reference drug. Worms were examined for their viability using a stereomicroscope and those not showing motility for 1 min were considered dead. The mortality rate of worms was calculated after 5 days exposure. Compounds showing activity in the primary screen were retested (secondary screen) using the same technique by successive descending dilutions (five dilutions) of the solution. Two replicates were used for six worms in each and the mortality of worms was determined in each case. IC50 and IC90 values were calculated using the statistical program SPSS version 7.5.
Molluscicidal tests
Adult Biomphalaria alexandrina (Ehrenberg) (Planorbidae) snails, the intermediate host of S. mansoni in Egypt, were collected from the irrigation system in the Nile Delta and maintained in the laboratory for 3 weeks before being used. The efficacy of the compounds was primarily determined against the snails using the standard method. Thus, 1 L of dechlorinated water with a concentration 5 ppm of each compound was prepared and ten snails were added. They were maintained in the solution for 24 h at 25°C ± 1°C. After the exposure period, the snails were washed thoroughly with dechlorinated water and maintained in fresh water for another 24 h for recovery. In each case, two replicates were carried out and two groups of snails were used as negative control. The conventional molluscicide (niclosamide) at the same concentration was used as positive control. The compounds showing molluscicidal activity were retested by the same method using descending concentrations for LC50 and LC90 determination by SPSS statistical program.
Results and discussion
The synthetic strategy of neocryptolepine and norneocryptolepine compounds was based on the amination of chlorosubstituted compounds obtained through Graebe-Ullman condensation (CitationPeczynska-Czoch et al., 1994; CitationKaczmarek et al., 1988). This method was used for synthesis of neocryptolepines with substitutions on the A or D ring (2-, 3-, 8- and 9 substitution).
The chloroquine-derived N1, N1-diethyl-1,4-pentanediamine was firstly used as basic side chain. This chain was introduced on 2-, 3- and 9-chloroneocryptolepines with a palladium-catalyzed amination reaction using a Buchwald-Hartwig amination (CitationSheng & Hartwig, 2008; CitationZim & Buchwald, 2003). A series of neo- and norneocryptolepines were also prepared with various aminoalkylamino groups in position 11 and a chlorine atom at the 2-position by following the procedure previously reported by CitationEl Sayed et al. (2009) starting from 1H-methyl indole-3-carboxylate and aniline derivatives. The analytical and spectral data of the newly synthesized neocryptolepine compounds g and h agreed very well with the proposed structures (cf. material and methods section). All other derivatives of neo-and norneocryptolepines revealed analytical and spectral data consistent with those reported by CitationEl Sayed et al. (2009). The synthesized compounds used for biological screening are listed in and .
Schistosomicidal activity
The schistosomicidal (primary bioassay using Egyptian strain) showed that 6 neocryptolepine compounds out of 16 and 2 norneocryptolepines out of 9 exhibited 100% worm mortality at the concentration used (5 μg/ml) after 5 days. IC50 and IC90 of the schistosomicidally effective neocryptolepine were lowest in compounds k and I being 1.26 and 4.05 μM and 1.77 and 4.55 μM for S. mansoni Egyptian strain respectively. IC50 and IC90 were 3.54 μM and 6.83 µM and 3.29 and 5.57 μM for the Puerto Rican strain, respectively, thus showing more sensitivity of Egyptain than Puerto Rican strain. However, the efficacy of these compounds is still less than that of the reference drug Praziquantel which has IC50 and IC90 0.6 and 1.08 μM for the Egyptian strain and 0.89 and 1.5 μM for the Puerto Rican strain respectively (). The structure–activity relationship studies revealed that all mono- and dichlorosubstituted neocryptolepines 4a and 4b and norneocryptolepines 5a and 5b exhibited no antischistosomal activity ( and ). In general, introduction of the aminoalkylamino side chain into the indoloquinoline core in combination with the chlorine atom at the A ring resulted in significant increases in the antischistosomal activity as shown in compounds 4i-p for neocryptolepines () and 5c and 5e-i for norneocryptolepines (). However, the activity is completely lost by switching the position of the aminoalkylamino side chain from 11 to 3 or 9 position with the absence of chlorine at the A ring, as in compounds 4d and 4e, respectively (). In case of norneocryptolepine series compounds 5g and 5i with piperazinyl moiety as side chain were the most active compounds (). The absence of activity of chloroneocryptolepines 4a-b and chloronorneocryptolepine 5a-b, which have no basic side chain, gives credence to the view that an aminoalkylamino side chain is important for antischistosomal activity. The possibility to obtain potent derivatives with dibasic side chains is a potential lead worthy of exploration. Further work will be directed toward the synthesis and evaluation of more neocryptolepines with this structural form.
Molluscicidal activity
Concerning the molluscicidal activity seven compounds (six neocryptolepines and one norneocryptolepine) provided 100% snail mortality at the tested concentration of 5 ppm (). The LC50 and LC90 for these compounds ranged between 0.63–3.9 ppm and 0.91–4.8 ppm, respectively. The norneocryptolepine compound (g) gave the highest effect since LC50 and LC90 were 0.63 ppm and 0.91 ppm, respectively. However, comparing these results with the activity of the conventional molluscicides, niclosamide, and the latter compound shows a better activity. The observed LC50 and LC90 values for niclosamide were 0.2 ppm and 0.6 ppm, respectively.
Table 3. Molluscicidal activity of substituted neo- and norneocryptolepines on Biomphalaria alexandrina snails (results after 24 h).
Declaration of interest
The authors report no conflicts of interest.
References
- Alajarin M, Molina P, Vidal A. (1997). Formal total synthesis of the alkaloid cryptotackieine (neocryptolepine). J Nat Prod, 60, 747–748.
- Bilharz T, Bir Beity Zur. (1852). Helminthology. Zsc f wiss Zoo, 4, 53–72.
- Botros S, Bennett J. (2007). Praziquantel resistance. Expert Opin Drug Discov, 2, 535–540.
- Doenhoff MJ, Kusel JR, Coles GC, Cioli D. (2002). Resistance of Schistosoma mansoni to praziquantel: Is there a problem? Trans R Soc Trop Med Hyg, 96, 465–469.
- Doenhoff MJ, Pica-Mattoccia L. (2006). Praziquantel for the treatment of schistosomiasis: Its use for control in areas with endemic disease and prospects for drug resistance. Expert Rev Anti Infect Ther, 4, 199–210.
- El Sayed I, Van der Veken P, Steert K, Dhooghe L, Hostyn S, Van Baelen G, Lemière G, Maes BU, Cos P, Maes L, Joossens J, Haemers A, Pieters L, Augustyns K. (2009). Synthesis and antiplasmodial activity of aminoalkylamino-substituted neocryptolepine derivatives. J Med Chem, 52, 2979–2988.
- Fenwick A, Webster JP. (2006). Schistosomiasis: Challenges for control, treatment and drug resistance. Curr Opin Infect Dis, 19, 577–582.
- Gönnert R, Andrews P. (1977). Praziquantel, a new board-spectrum antischistosomal agent. Z Parasitenkd, 52, 129–150.
- Ismail M, Botros S, Metwally A, William S, Farghally A, Tao LF, Day TA, Bennett JL. (1999). Resistance to praziquantel: Direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am J Trop Med Hyg, 60, 932–935.
- Jonckers TH, van Miert S, Cimanga K, Bailly C, Colson P, De Pauw-Gillet MC, van den Heuvel H, Claeys M, Lemière F, Esmans EL, Rozenski J, Quirijnen L, Maes L, Dommisse R, Lemière GL, Vlietinck A, Pieters L. (2002). Synthesis, cytotoxicity, and antiplasmodial and antitrypanosomal activity of new neocryptolepine derivatives. J Med Chem, 45, 3497–3508.
- Kaczmarek L, Balicki R, Nantka-Namirski P, Peczynska-Czoch W, Mordarski M. (1988). Cancerostatics, VI. Synthesis and antineoplastic properties of some benzo-iso-alpha-carbolines. Arch Pharm (Weinheim), 321, 463–467.
- Peczynska-Czoch W, Pognan F, Kaczmarek L, Boratynski J. (1994). Synthesis and structure-activity relationship of methyl-substituted indolo[2,3-b]quinolines: Novel cytotoxic, DNA topoisomerase II inhibitors. J Med Chem, 37, 3503–3510.
- Pica-Mattoccia L, Cioli D. (2004). Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int j Parasitol, 34, 527–533.
- Sheng Q, Hartwig FG. (2008). Amination of heteroaryl and aryl halide. Org Lett, 10, 4109–4112.
- Shi C, Zhang Q, Wang KK. (1999). Biradicals from thermolysis of N-[2-(1-alkynyl)phenyl]-N’-phenylcarbodiimides and their subsequent transformations to 6H-indolo[2,3-b]quinolines. J Org Chem, 64, 925–932.
- Southgate VR, Rollinson D, Tchuem Tchuenté LA, Hagan P. (2005). Towards control of schistosomiasis in sub-Saharan Africa. J Helminthol, 79, 181–185.
- Wright CW. (2007). Recent developments in naturally derived antimalarials: Cryptolepine analogues. J Pharm Pharmacol, 59, 899–904.
- Wright CW. (2005). Plant derived antimalarial agents: New leads and challenges. Phytochem Rev, 4, 55–61.
- World Health Organization. (2002). Prevention and control of schistosomiasis and soil-transmitted helminthiasis. Report of a WHO Expert Committee. Geneva, Switzerland: World Health Organization.
- Ramirez B, Bickle Q, Yousif F, Mouries MA, Nwaka S.(2007). Schistosome challenge in compound screening. Exp Opin Drug Discov, 2, 1–9.
- Yousif F, Hifnawy MS, Soliman G, Boulos L, Labib Th Mahmoud, S, Ramzy F, Yousif M, Hassan I, Mahmoud K,El- Hallouty SM, EL-Gendy M, Gohar L, EL-Manawaty M, EL-Menshawi BS. (2007).Large-scale in vitro screening of Egyptian native and cultivate plants for schistosomicidal activity. Pharm Biol, 45, 501–510.
- Zim D, Buchwald SL. (2003). An air and thermally stable one- component catalyst for the amination of aryl chlorides. Org Lett, 5, 2413–2415.