Abstract
Context: Millettia barteri (Benth.) Dunn (Fabaceae) is an African medicinal plant used in folk medicine to treat many diseases. This species, as well as other Mellettia species, has been of interest to researchers because of their wide range of traditional uses.
Objective: Phytochemical, antimicrobial and 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH)-radical scavenging investigations of the hexane and EtOAc extracts of the stem bark of M. barteri were carried out here for the first time.
Materials and methods: The isolation of compounds was done through silica gel column chromatography and their structures were established using spectroscopic analysis, especially, 1D NMR in conjunction with 2D experiments (COSY, HMQC and HMBC), and physical data compared with literature values. The broth micro dilution method was used for antimicrobial test while DPPH radical scavenging assay was used for antioxidant test.
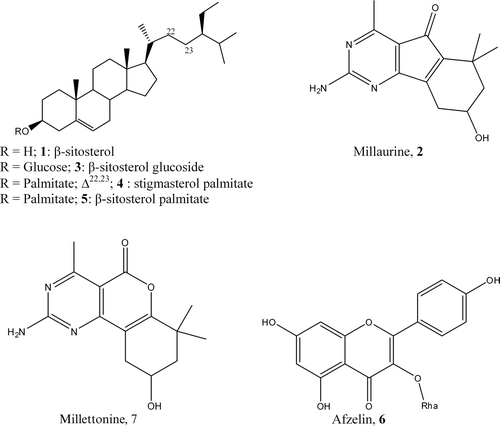
Results: Seven compounds, including two guanidine alkaloids: millaurine (2) and milletonine (7); one flavonoid: afzelin (6); four sterols: β-sitosterol (1), β-sitosterol glucoside (3), mixture of stigmasterol (4) and β-sitosterol (5) palmitates have been isolated from stem bark of hexane and ethyl acetate extracts of M. barteri. These extracts showed antimicrobial activity on the set of germs tested with minimum inhibitory concentration values varying from 64 to 512 µg/mL, as well as antioxidant activity (IC50 62.74 and 77.23 µg/mL). Compounds 2 and 7, tested for the first time, demonstrated antimicrobial and antioxidant activities.
Discussion and conclusions: The present study clearly demonstrated that M. barteri and some of its isolates possess antimicrobial and antioxidant properties and may act as potential antioxidant for biological systems susceptible to free radical-mediated reactions.
Introduction
The genus Millettia (Fabaceae) includes more than 200 species found in Africa, Asia, America and Australia (CitationBanzouzi et al., 2008). Seventeen species of this genus have been identified in Cameroon (CitationVivien & Faure, 1986). The decoctions of the stem and root bark of various Millettia species are used in traditional medicine as a vermifuge (CitationSofowora et al., 1982), to heal heart disorders, broncho-pneumonia, rheumatism, headaches and back pains (CitationBouquet, 1969). Various parts of Millettia barteri (Benth.) Dunn are traditionally used to treat different diseases. The maceration of its twigs is used as a purgative and rectal injection; the decoction of stem bark is drunk or applied in pomade in the treatment of feverish aches, cough, and dysmenorrhea. The bark root decoction is drunk in case of cardiac pains (CitationBanzouzi et al., 2008). To the best of our knowledge, no phytochemical study has been reported on M. barteri. Chemical investigations of different parts of Millettia species have led to the isolation of various secondary metabolites, alkaloids (CitationKamnaing et al., 1994; CitationNgamga et al., 1993, Citation1994, Citation2007), steroids (CitationHui et al., 1973), terpenoids (CitationHui et al., 1973; CitationUchiyama et al., 2003), coumarins (CitationKhalid et al., 1983; CitationPalazzino et al., 2003; CitationYankep et al., 1998), flavonoids (CitationBaruah et al., 1984; Islam et al., 1980; CitationMahmoud & Waterman, 1985) and isoflavonoids (CitationGaleffi et al., 1997; CitationKamnaing et al., 1999). Some of the flavonoids and isoflavonoids isolated possess insecticidal, piscicidal, and molluscicidal properties (CitationSinghal et al., 1982). In this paper, we describe the isolation and identification of seven compounds from M. barteri and their antimicrobial and antioxidant activities.
Materials and methods
General procedure
NMR spectra were measured on Bruker Avance DRX 500 and Brucker Ultrashield Plus 600 spectrometers at 500 and 600 MHz for 1H, and 125 and 150 MHz for 13C NMR, with TMS as internal standard; chemical shift are given in δ values (ppm). MS analyses were performed using a QTOF Premier (Waters, Milford, MA) equipped with a nanoelectrospray ionization source. The instrument was operated in positive ion mode, performing full-scan analysis over the m/z range 50–1990 at 1 spectra/s. Melting points were determined on a Microscope Reichert and are uncorrected. Column chromatography was run on Merck silica gel 60 (0.063–0.200 mm) and Sephadex LH-20 while TLC was carried out on silica gel GF254 precoated plates with detection accomplished by visualizing with a UV lamp at 254 and 365 nm, followed by spraying with 50% H2SO4 and then heating at 100°C.
Plant material
The stem bark of M. barteri was collected at Sa’ a, Centre region of Cameroon in May 2010. It was identified by Mr. Mezilli at the National Herbarium, Yaoundé, where a voucher specimen n°26631/SRF Cam describing the plant is deposited.
Extraction and isolation
Dried and ground stem bark of M. barteri (4.3 kg) was extracted three times with methanol (3 × 7 L) at room temperature for 1 week. Filtration and evaporation of solvent under reduced pressure gave a brown residue (275 g). This extract was successively partitioned with hexane and ethyl acetate to yield 50 and 40 g of extracts, respectively.
Hexane extract (20 g) was subjected to silica gel column chromatography eluted with a gradient system of hexane/EtOAc and EtOAc/MeOH. Each of 193 fractions (50 mL) was collected and combined on the basis of TLC profile (A-X). Series T, fractions: 120–146 (4 g) resulting from elution with EtOAc, was subjected to SiO2 gel column chromatography eluted with CH2Cl2/MeOH gradient system. The 43 fractions obtained were combined on the basis of TLC profile. A yellowish powder was formed in subfractions 15–21 obtained with CH2Cl2/MeOH (95/5); its filtration and recrystalization yielded 15.10 mg of millaurine 2 (CitationNgamga et al., 1993). Series P, fractions: 75–84 (1.5 g) obtained with hexane/EtOAc: 50/50 was purified on a silica gel column with a gradient of hexane/EtOAc and monitored by TLC. 39 fractions were obtained and combined on the basis of TLC analyses. 13.08 mg of β-sitosterol glucoside 3 (CitationHisashi et al., 1990) were obtained from subfractions 18–30 eluted with hexane/EtOAc: 55/45. 12.24 mg of β-sitosterol 1 (CitationHisashi et al., 1990) crystallized from series G, fractions: 23–26, obtained with hexane/EtOAc: 85/15.
In addition, 19 g of EtOAc extract was subjected to silica gel column eluted with a gradient system of hexane/EtOAc and EtOAc/MeOH; 263 fractions of 50 mL each were collected and combined on the basis of TLC profile (A-W). Series L, fractions: 93–99 (3 g) obtained with hexane/EtOAc (1-1) was rechromatographed on SiO2 column and 100 fractions of 25 mL each were collected. Subfractions 28–36 were purified on SiO2 column eluted with hexane/EtOAc: 45/55 and Sephadex LH-20 eluted with MeOH to yield 2 mg of Afzelin, kaempferol 3-O-α-l-rhamnoside 6 (CitationKimiko et al., 1983). Series O, fractions: 118–133, was obtained with hexane/EtOAc: 25/75. Left at room temperature for 24 h, millettonine 7 (CitationKamnaing et al., 1994) crystallized. It was filtered and recrystalized to give 31.30 mg of pure whitish powder. Series J, fractions: 75–83 (0.8 g), obtained with hexane/EtOAc: 3/2, afforded after two series of SiO2 gel column chromatography eluted with hexane/EtOAc gradients, a mixture of stigmasterol 4 and β-sitosterol 5 palmitates (5.21 mg) (CitationTian et al., 2009). The chemical structures of the isolated compounds are shown in .
Biological tests
Antimicrobial activity
Microorganisms
The microorganisms used in this study consisted of three bacteria (Staphylococcus aureus ATCC25922, Pseudomonas aeruginosa ATCC27853, Salmonella typhi ATCC6539) and two Candida species (Candida albicans ATCC9002 and Candida parapsilosis ATCC22019); all of which are reference strains obtained from the American Type Culture Collection. Also, included was one strain of Cryptococcus neoformans IP95026 obtained from the Pasteur Institute (IP, Paris-France). The bacterial and yeast strains were grown at 35°C and maintained on nutrient agar (NA, Conda, Madrid, Spain) and Sabouraud Dextrose Agar (SDA, Conda) slants, respectively.
Determination of minimum inhibitory concentration and minimum microbicidal concentration
Minimum inhibitory concentrations (MICs) were determined by broth micro dilution method as described by CitationNyaa et al. (2009) with slight modifications. The test samples were first of all dissolved in dimethylsulfoxide (DMSO). The solution obtained was then added to Mueller Hinton Broth (MHB) for bacteria or Sabouraud Dextrose Broth (SDB) for yeasts to give a final concentration of 2048 µg/mL. This was serially diluted two fold to obtain concentration ranges of 0.50–2048 µg/mL. Each concentration (100 µL) was added in each well (96-wells microplate) containing 95 µL of MHB or SDB and 5 µL of inoculum for final concentrations varying from 0.25 to 1024 µg/mL. The inoculum was standardized at 1.50 × 106 CFU/mL by adjusting the optical density to 0.1 at 600 nm JENWAY 6105 UV/Vis spectrophotometer. The final concentration of DMSO in each well was less than 1% [preliminary analyses with 1% (v/v) DMSO do not inhibit the growth of the test organisms]. The negative control well consisted of 195 μL of appropriate medium (MHB for bacteria and SDB for yeasts) and 5 μL of the standard inoculum. The plates were covered with the sterile lid, then agitated to mix the contents of the wells using a plate shaker and incubated at 35°C for 24 h (for bacteria) or for 48 h (for yeasts). The assay was repeated thrice. The MICs of samples were determined by adding 50 µL of a 0.2 mg/mL p-iodonitrotetrazolium violet solution followed by incubation at 35°C for 30 min. Viable micro-organisms reduced the yellow dye to a pink color. MICs were defined as the lowest sample concentrations that prevented this change in color indicating a complete inhibition of microbial growth.
For the determination of minimum microbicidal concentrations (MMCs), a portion of liquid (5 µL) from each well that showed no growth of microorganism was plated on Mueller Hinton Agar or Sabouraud Dextrose Agar and incubated at 35°C for 24 h (for bacteria) or 35°C for 48 h (for yeasts). The lowest concentrations that yielded no growth after this sub-culturing were taken as the MMCs (CitationNyaa et al., 2009). Ciprofloxacin (Sigma-Aldrich, Steinheim, Germany) and nystatin (Merck, Darmstadt, Germany) for bacteria and yeasts, respectively, were used as positive controls.
Antioxidant assay: DPPH assay method
The free radical scavenging activity of the crude extract as well as their isolated compounds was evaluated as described by CitationMensor et al. (2001) with slight modifications. The test samples, prior dissolved in DMSO (Sigma), were mixed with a 20 mg/L 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) methanol solution to give final concentrations of 10, 50, 100, 500 and 1000 µg/mL. After 30 min at room temperature, the absorbance values were measured at 517 nm and converted into percentage of antioxidant activity as follows:
Ascorbic acid was used as a standard control. The inhibition ratio was converted in probits. The Probit values were plotted against the logarithmic values of concentrations of the test samples and a linear regression curve was established in order to determined the IC50 (µg/mL); being the amount of sample necessary to decrease by 50% the absorbance of DPPH. All the analyses were carried out in triplicate and the results were expressed as the mean ± standard deviation (SD) and compared using Waller-Duncan test. A value of p < 0.05 was considered statistically significant.
Results
The air-dried stem bark of M. barteri was extracted with MeOH. The resulting extract was partitioned with hexane and ethyl acetate. Both extracts underwent antimicrobial (antibacterial and antifungal) and antioxidant assays revealing MICs from 64 to 512 µg/mL and IC50 from 62.74–77.23 µg/mL. These extracts were subjected to successive column chromatography to yield compounds 1-7 including two guianadine alkaloids: millaurine (2) (mp 248–249°C, Rf: 0.40) and millettonine (7) (mp 169–170°C, Rf: 0.53); one flavonoid: afzelin (6) (mp 213–214°C, Rf: 0.46); four sterols: β-sitosterol (1) (mp 146–147°C, Rf: 0.33), β-sitosterol glucoside (3) (mp 261–263°C, Rf: 0.30), a mixture of stigmasterol 4 and β-sitosterol 5 palmitates (Rf: 0.37).
The antimicrobial activity of hexane and EtOAc extracts, as well as compounds 1, 2, 3 and 7 are presented in . The hexane and EtOAc extracts have shown both antibacterial and antifungal activities on the set organisms tested with MIC values varying from 64–512 µg/mL. The EtOAc extract was more active (MIC = 64–256 µg/mL) than the hexane extract (MIC = 128–512 µg/mL) suggesting that the active principles might be more concentrated in the EtOAc extract. Compound 2 was the most active compound (MIC = 4–32 µg/mL) against bacteria and fungi species while compound 7 (MIC = 8–128 µg/mL) was less active but more than compound 1 (MIC = 64–256 µg/mL) and compound 3 (MIC = 64–256 µg/mL). However, the antimicrobial activity of all tested compounds was lower than that of the controls (ciprofloxacin and nystatin).
Table 1. Antimicrobial activity (in μg/mL) of hexane and EtOAc extracts from Millettia barteri and isolated compounds.
The above extracts and some isolated compounds were also evaluated for their antioxidant activity using DPPH assay method. As shown for antimicrobial activity, the EtOAc extract (IC50 = 62.74 µg/mL) was also more active than the hexane extract (IC50 = 77.23 µg/ mL). Furthermore, compound 2 (IC50 = 48.01 µg/ mL) was more active than compound 7 (242.68 µg/mL). No antioxidant activity was noticed for compounds 3 and 1.
Discussion
The crude extracts and all the isolated compounds showed antimicrobial activities on at least one microorganism. Such a finding supports the traditional uses of this plant in the treatment of infectious diseases. The antimicrobial properties of some individual flavonoids, sterols and alkaloids of plant origin were documented (CitationRahman et al., 2009; CitationTamokou et al., 2009). The antimicrobial activities varied with the bacterial and fungal species. These variations may be due to genetic differences between the microorganisms. It was also found that the MMC values obtained were generally less than four fold greater than the MICs () on the corresponding microbial species, suggesting that a cidal effect of the hexane and EtOAc extracts and some of the isolated compounds could be expected on most of the tested microorganisms (CitationTamokou et al., 2009).
In the DPPH radical scavenging assay, the extracts and compounds 2 and 7 were active with IC50 lower than that of L-ascorbic acid used as reference drug (IC50 = 0.96 µg/mL). Phenolic or nitrogenous compounds as millaurine (2) and millettonine (7) have been reported to function as potent antioxidants by virtue of their hydrogen-donating and metal-chelating properties (CitationHall & Cuppett, 1997; CitationPietta et al., 1998). Therefore, the presence of these compounds could be responsible for the antioxidant activity found in the hexane and EtOAc extracts. Biological activities of millaurine (2) and millettonine (7) are reported here for the first time. No biological test was done with afzelin (6), stigmasterol (4) and β-sitosterol (5) palmitates due to their small quantity. The antimicrobial properties of afzelin (6), stigmasterol (4) and β-sitosterol (5) palmitates as well as the antioxidant activity of afzelin (6) are documented (CitationChung et al., 1996; CitationLiu et al., 2007; CitationTamokou et al., 2011).
This study can be considered as very promising in the perspective of new drugs discovery from plant sources, when considering the medical importance of tested microorganisms as well as the high level of neurodegenerative diseases associated with oxidative stress. P. aeruginosa has emerged as one of the most problematic Gram-negative pathogens, with the alarmingly high antibiotics resistance rates (CitationSavafi et al., 2005). Even with the most effective antibiotic against this pathogen, namely carbapenems (imipenem and meropenem), the resistance rates were detected as 15–20.4% amongst 152 P. aeruginosa strains (CitationTamokou et al., 2009). S. aureus is a major cause of community and hospital-associated infection with the attributed mortality around 7–10% (CitationBowersox, 2007) and about 2% of patients in Cameroon, are infected by Staphylococcus spp. (CitationTamokou et al., 2011). Each year, some 500,000 patients in American hospitals contract a staphylococcal infection (CitationBowersox, 2007). These pathogens were found to be sensitive to the crude extract and isolated compounds. About 77% of immune-deficient patient death is attributable to microscopic fungi amongst which Candida species and C. neoformans (CitationMohammad et al., 1999; CitationTamokou et al., 2011). In addition, 75% of women would have had at least one episode of vulvo-vaginitis candidiasis during their lives, which is considered as the second most common form of vaginitis after bacterial vaginosis (Fidel et al., 1999). Typhoid fever caused by S. typhi continues to be a mark public health problem in developing countries in general and in Sub-Saharan Africa in particular (CitationTamokou et al., 2011). Generally, at least three samples tested (i.e., EtOAc extract, compounds 2 and 7) in this study prevented the growth of all microbial strains.
Conclusions
The overall results of this study clearly demonstrated that M. barteri possesses antimicrobial and antioxidant properties. However, further pharmacological and toxicological investigations need to be done in order to confirm or infirm this hypothesis.
Acknowledgments
We thank Mr. Mezilli (National Herbarium of Cameroun) for the collection and the identification of the plant.
Declaration of interest
Havyarimana Léopold is grateful to the Government of Burundi for financial assistance throughout his stay in Cameroon.
References
- Banzouzi JT, Prost A, Rajemiarimiraho M, Ongoka. (2008). Traditional uses of the African Millettia species (Fabaceae). Int J Bot, 4, 406–420.
- Baruah P, Barua NC, Sharma RP, Baruah JN, Kulanthaivel P, Herz W. (1984). Flavonoids from Millettia pulchra. Phytochemistry, 23, 443–447.
- Bouquet A. (1969). Féticheurs et Médecins traditionnels du Congo Brazzaville. Mémoire O.R.S.T.O.M. 36, Paris.
- Bowersox J. (2007). Experimental Staph vaccine broadly protective in animal studies NIH, 1999-05-27. Retrieved on 2007-07-28.
- Chung YT, Kim AM, Jones DA. (1996). Antioxidative activity of flavonoids isolated from Jindalree flowers (Rhododendron muronulatum Turz.). Agr Chem Biotechnol, 39, 320–326.
- Fidel P, Lynch ME, Sobel JD. (1993). Candida-specific Th1-type responsiveness in mice with experimental vaginal candidiasis. Infect Immun 61, 4201–4207.
- Galeffi C, Rasoanaivo P, Federici E, Palazzino G, Nicoletti M, Rasolondratovo B. (1997). Two prenylated isoflavanones from Millettia pervilleana. Phytochemistry 45, 189–192.
- Hall CA, Cupett SL. (1997). Structure activities of natural antioxidants, In: Hudson BJL, (eds), Antioxidant Methodology In Vitro Concepts. Elsevier Applied Science, London, pp. 1–18.
- Hisashi K, Noriko S, Akiko H, Haruo O. (1990). Sterol glucosides from Prunella vulgaris. Phytochemistry, 29, 2351–2355.
- Hui WH, Chain WS, Leung HK. (1973). Triterpenoids and sterols from three Millettia species. Phytochemistry 12, 474–475.
- Islam A, Gupta RK, Krishnamurti M. (1977). Furano chalcone and prenylated flavanones from Millettia ovalifolia seeds. Phytochemistry 19, 1558–1559.
- Kamnaing P, Fanso free SNY, Nkengfack AE, Folefoc G, Fomum ZT. (1994). Millettonine, a guanidine alkaloid from Millettia laurentii. Phytochemistry, 36, 1561–1562.
- Kamnaing P, Fanso Free SNY, Nkengfack AE, Folefoc G, Fomum ZT. (1999). An isoflavan-quinone and a flavonol from Millettia laurentii. Phytochemistry, 51, 829–833.
- Khalid SA, Waterman PG. (1983). Thonningine-A and thonningine-B: Two 3-phenylcoumarins from the seeds of Millettia thonningii. Phytochemistry, 22, 1001–1003.
- Kimiko N, Mikiko T, Toshiaki T, Toshihiro N. (1983). Four kaempferol glycosides from leaves of Cinnamomum sieboldii. Phytochemistry, 22, 2831–2833.
- Liu Y, Murakami N, Ji H, Abreu P, Zhang S. (2007). Antimalarial flavonol glycosides from Euphorbia hirta. Pharm Biol, 45, 278–281.
- Mahmoud EN, Waterman PG. (1985). Flavonoids from the stem bark of Millettia hemsleyana. Phytochemistry, 24, 369–371.
- Mensor LL, Menezes FS, Leitao GG, Reis AS, Dos Santos TC, Coube CS, Leitao SG. (2001). Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res, 15, 127–130.
- Mohammad AH, Shigefumi M, Kataro M, Hiroshi K, Eisuke S, Kazunori T, Takayoshi T, Shigeru K. (1999). In vitro and in vivo activities of SCH56592 against Cryptococcus neoformans. J Antimicrob Chemother, 44, 827–829.
- Ngamga D, Fanso Free SNY, Fomum ZT, Martin MT, Bodo B. (1993). Millaurine and acetylmillaurine: Alkaloids from Millettia laurentii. J Nat Prod, 56, 2126–2132.
- Ngamga D, Fanso Free SNY, Fomum ZT, Martin MT, Bodo B. (1994). A new guanidine alkaloid from Millettia laurentii. J Nat Prod, 57, 1022–1024.
- Ngamga D, Fanso Free SNY, Tane P, Fomum ZT. (2007). Millaurine A, a new guanidine alkaloid from seeds of Millettia laurentii. Fitoterapia, 78, 276–277.
- Nyaa TBL, Tapondjou AL, Barboni L, Tamokou JD, Kuiate JR, Tane P, Park HJ. (2009). NMR Assignment and antimicrobial/antioxidant activities of 1β-hydroxyeuscaphic acid from the seeds of Butyrospermum parkii. Nat Prod Sci, 15, 76–82.
- Pietta P, Sionetti P, Mauri P. (1998). Antioxidant activity of selected medicinal plants. J Agric Food Chem, 46, 4487–4490.
- Palazzino G, Rasoanaivo P, Federici E, Nicoletti M, Galeffi C. (2003). Prenylated isoflavonoids from Millettia pervilleana. Phytochemistry, 63, 471–474.
- Rahman S, Akbor MM, Howlader A, Jabbar A. (2009). Antimicrobial and cytotoxic activity of alkaloids of Amlaki (Emblica officinalis). Pak J Biol Sci, 12, 1152–1155.
- Savafi L, Duran N, Savafi N, Onlem Y, Ocak S. (2005). The prevalence and resistance patterns of Pseudomans aeruginosa in intensive care units in a university hospital. Turk J Med Sci, 35, 317–322.
- Singhal A, Sharma RP, Baruah JN, Govindanand SV, Herz W. (1982). Rotenoids from roots of Millettia pachycarpa. Phytochemistry, 21, 949–951.
- Sofowora A. (1982). Medicinal plants and traditional medicine in Africa: Traditional medicine: Definitions and terminology; advantage and inconvenient. John Wiley and Sons Limited, New York, pp. 9–12, pp.142–146.
- Tamokou JD, Kuiate JR, Tene M, Nwemeguela KTJ, Tane P. (2011). The antimicrobial activities of extract and compounds isolated from Brillantaisia lamium. Iran J Med sci, 24–31.
- Tamokou JD, Tala FM, Wabo KH, Tane P. (2009). Antimicrobial activities of methanol extract and compounds from stem bark of Vismia rubescens. J Ethnopharmacol, 124, 571–575.
- Tian M, Dai H, Li X, Wang B. (2009). Chemical constituents of marine medicinal mangrove plant Sonneratia caseolaris. Chin J Oceanol Limnol, 27, 288–296.
- Uchiyama T, Furukama M, Isobe S, Makino M, Akiyama T, Koyama T, Fujimoto Y. (2003). New oleanane-type triterpene saponins from Millettia speciosa. Heterocycles, 60, 655–661.
- Vivien J, Faure JJ. (1986). Arbres des forêts denses d’Afrique Centrale. A.C.C.T. ed., Paris, pp. 352–353.
- Yankep E, Fomum ZT, Bistrat D, Dagne E, Hellewi V, Steglich W. (1998). O-Geranylated isoflavones and a 3-phenylcoumarin from Millettia griffoniana. Phytochemistry 49, 2521–2523.