Abstract
Context: Ocular diseases are currently an important problem in modern societies. Patients suffer from various ophthalmologic ailments namely, conjunctivitis, dry eye, dacryocystitis or degenerative diseases. Therefore, there is a need to introduce new treatment methods, including medicinal plants usage. Aloe vera [Aloe barbadensis Miller (Liliaceae)] possesses wound-healing properties and shows immunomodulatory, anti-inflammatory or antioxidant activities.
Materials and methods: NR uptake, MTT, DPPH• reduction, Griess reaction, ELISA and rhodamine-phalloidin staining were used to test toxicity, antiproliferative activity, reactive oxygen species (ROS) reduction, nitric oxide (NO) and cytokine level, and distribution of F-actin in cells, respectively.
Aim: The present study analyzes the effect of Aloe vera extracts obtained with different solvents on in vitro culture of human 10.014 pRSV-T corneal cells.
Results: We found no toxicity of ethanol, ethyl acetate and heptane extracts of Aloe vera on human corneal cells. No ROS reducing activity by heptane extract and trace action by ethanol (only at high concentration 125 µg/ml) extract of Aloe vera was observed. Only ethyl acetate extract expressed distinct free radical scavenging effect. Plant extracts decreased NO production by human corneal cells as compared to untreated controls. The cytokine (IL-1β, IL-6, TNF-α and IL-10) production decreased after the addition of Aloe vera extracts to the culture media.
Discussion and conclusions: Aloe vera contains multiple pharmacologically active substances which are capable of modulating cellular phenotypes and functions. Aloe vera ethanol and ethyl acetate extracts may be used in eye drops to treat inflammations and other ailments of external parts of the eye such as the cornea.
Introduction
The use of medicinal plants preparations is based not only on the experience of generations but also on scientific studies. Herbal medicines are currently used in a variety of commercial products owing to their therapeutic or cosmetic properties. Among them, the perennial succulent Aloe vera [Aloe barbadensis Miller (Liliaceae)] is recognized as an effective natural remedy (CitationChoi & Chung, 2003). Aloe vera is used both internally and externally on humans in a form of alternative medicine as well as in the home in first aid. It has been shown that Aloe vera expresses wound- and burn-healing effects and shows immunomodulatory, anti-inflammatory, antiparasitic, UV protective, antioxidant, antiviral, antitumor and antidiabetic activities (CitationLee et al., 2000; CitationChoi & Chung, 2003; CitationSarkar et al., 2005; CitationYoo et al., 2008; CitationKigondu et al., 2009; CitationOzsoy et al., 2009). CitationTelefo et al. (1998, Citation2002, Citation2004) also demonstrated the effect of Aloe vera on the reproductive system in rats where the plant extract regulated the steroidogenesis and hormone levels, menstrual cycle, ovarian and uterine weight and was used to treat dysmenorrhea or infertility. These medicinal properties are mainly mediated through biologically active components in Aloe vera and its extracts such as anthraquinones, flavonoids, phenolic acids, antioxidant vitamins, enzymes and other non-nutrient compounds of the plant (CitationChoi & Chung, 2003; CitationHu et al., 2003; CitationYoo et al., 2008). Pharmacological action of Aloe vera extracts is closely related to cytokine network maintenance or radical level stabilization among others in eye tissue. Medicinal activity of Aloe vera compounds against eye disorders should be considered within two groups of actions: antioxidant [reactive oxygen species (ROS) scavenging and production of nitric oxide (NO)] and immunomodulatory (influence on mainly proinflammatory or anti-inflammatory cytokine level). The trust to alternative medicine usage in ophthalmology is generally based on relatively low side effects and well tolerated by the patients when herbal preparations are used in a form of, e.g. eye drops. However, while using herbal medicine not only clinically significant ocular advantages but also side effects can occur (CitationFraunfelder, 2004). Besides advantageous effects, Aloe vera is also considered to have adverse effects on some organs (CitationChoi & Chung, 2003; CitationCan et al., 2004; CitationRabe et al., 2005). Therefore, it is necessary to recognize these adverse events to reduce possible harmful influences of Aloe vera components on cornea or conjunctiva structure.
The aim of the present study was to analyze the effects of the usage of Aloe vera extracts obtained with different solvents on in vitro culture of human 10.014 pRSV-T corneal cells.
Materials and methods
Plant material
A voucher specimen of Aloe vera is deposited at the Department of Pharmaceutical Botany, Medical University, Lublin, Poland. The strict plant material was obtained in summer 2010 from botanical garden of Maria Curie-Skłodowska University in Lublin, Poland. The plant was identified by Professor T. Krzaczek (Medical University in Lublin). Voucher number of the plant is No. 1112.
Extracts preparation
Three fresh plant portions of 15 g each were pulverized in an electric mill (Zelmer, Rzeszów, Poland). Each was placed in a 250-ml round-bottomed flask, and to each 70 ml of 1.1% heptane, 2.1% of ethyl acetate and 3.96% of ethanol was added, respectively. The flasks were transferred into an ultrasonic bath (Bandelin, Sonorex, Germany) and a threefold exhaustive extraction at 45°C was carried out, each time using the same volume of fresh solvent. Filtered solutions of each solvent were combined (210 ml in volume) and the solvent was distilled in a rotary vacuum evaporator IKA RV 05-ST 1 (IKA-Werke, Staufen, Germany). The dry residue obtained was used in biological tests. Samples of the dry residue were analyzed in gas chromatography–mass spectrometry to check for the presence of solvents (a Varian 3400 gas chromatograph in combination with a Finnigan MAT ITS 40 ion trap detector was used), capillary chromatography column Restek Rtx-5 (0.18 mm i.d. × 20 m, 0.25 μm film; Restek Corp. Bellefonte, PA). The tests were negative: no solvents used were found in the analyzed material. After the solvents were removed, the part of dried mass was dissolved in DMSO, obtaining stock solution (100 mg/ml). Next, the working solutions (8–125 µg/ml) were prepared by extracts dissolving in culture medium.
Cell culture
Human normal corneal cell line 10.014 pRSV-T (ATCC No. CRL-11515) was used. The cells were cultured as monolayers in 25-cm2 culture flasks (Nunc, Roskilde, Denmark) coated with PureCol™ ultrapure collagen (INAMED Biomaterials, Fremont, CA) at 3.1 mg/ml concentration. Cell line was maintained in defined K-SFM (keratinocyte-serum free medium) (Gibco) supplemented with 75 µg/ml endothelial cell growth factor (Sigma), 0.05 mg/ml bovine pituitary extract (Gibco), 500 ng/ml hydrocortisone (Sigma) and 0.0005 mg/ml bovine insulin (Gibco) and antibiotics (100 U/ml penicillin, 100 µg/ml streptomycin) (Sigma, St Louis, MO) at 37°C in a humidified atmosphere with 5% CO2.
Incubation of cells with Aloe vera extracts
For the purpose of the current experiments, the total number of cells was estimated by counting using a Thoma’s haemocytometer. A dose of 100 µl of cell suspension (1 × 105 cells/ml) was added to wells of 96-well flat-bottomed microtiter plates (MTT and NR methods). After 24 h of incubation, the medium was discarded and a new one was added, with appropriate concentrations of extracts. As controls, also incubated for 24 h, cells cultured in 100 µl of medium were used, the total cell number equivalent to those in the sample wells. A blank control consisted only of culture medium.
The incubation was performed for further 24 h, with cytotoxicity and antiproliferative activity of extracts estimated with spectrophotometric methods (MTT and NR assays).
Neutral Red (NR) uptake assay
NR cytotoxicity assay is based on the uptake and lysosomal accumulation of the supravital dye, Neutral Red. Dead or damaged cells do not take up the dye (CitationGanbold et al., 2010).
Cells were grown in 96-well multiplates in 100 µl of culture medium (K-SFM) with supplements and Aloe vera extracts at three doses (25, 75 and 125 µg/ml) and standards. Subsequently, the medium was discarded and 0.4% NR (Sigma) solution medium was added to each well. The plate was incubated for 3 h at 37°C in a humidified 5% CO2/95% air incubator. After incubation, the dye-containing medium was removed, cells fixed with 1% CaCl2 in 4% paraformaldehyde, and thereafter the incorporated dye was solubilized using 1% acetic acetate in 50% ethanol solution (100 µl). The plates were gently shaken for 20 min at room temperature and the extracted dye absorbance was measured spectrophotometrically at 540 nm using a microplate reader (Emax; Molecular Devices Corp., Menlo Park, CA).
MTT assay
Cells sensitivity to Aloe vera extracts was determined in a standard spectrophotometric 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay according to CitationMosmann (1983). MTT test is based on conversion of yellow tetrazolium salt by viable cells to purple crystals of formazan. The reaction is catalyzed by mitochondrial succinyl dehydrogenase.
Cells grown in 96-well multiplates in 100 µl of culture medium were incubated for 3 h with MTT solution (5 mg/ml, 25 µl/well) (Sigma). The yellow tetrazolium salt was metabolized by viable cells to formazan purple crystals. The reaction was catalyzed by mitochondrial succinyl dehydrogenase. The crystals were solubilized overnight in a 10% sodium dodecyl sulfate in 0.01 M HCl mixture. The product was quantified spectrophotometrically by absorbance measurement at 570 nm wavelength using an Emax microplate reader (Molecular Devices Corp.).
DPPH• free radical scavenging test
Free radical scavenging activity of extracts was measured by the 1,1-diphenyl-2-picrylhydrazyl (DPPH•) assay. This method is based on the ability of antioxidants to reduce the stable dark violet radical DPPH• (Sigma) to the yellow colored diphenyl-picrylhydrazine. Briefly, 100 μl of DPPH• solution (0.2 mg/ml in ethanol) was added to 100 μl of extract concentrations (25–125 μg/ml) and standards. Trolox (Sigma) at increasing concentrations (1–50 μg/ml) was used as a standard for the free radical scavenging activity. After 20 min of incubation at room temperature, the absorbance of the solution was measured at 515 nm; the lower the absorbance, the higher the free radical scavenging activity of the extracts. The activity of each extract was determined by comparing its absorbance with that of a control solution (reagents without extract) and standard.
The capability to scavenge DPPH• radical was calculated by the following formula:
DPPH• scavenging effect (%) = [(Xcontrol− Xextract/Xcontrol) × 100]
where Xcontrol is the absorbance of the control and Xextract is the absorbance in the presence of extracts (CitationPaduch et al., 2008).
NO measurement
Nitrate, a stable end product of NO, was determined in culture supernatants by a spectrophotometric method based on the Griess reaction. The level of nitrite reflects NO production (CitationMuzitano et al., 2011).
Briefly, corneal cells were incubated for 24 h with two concentrations of Aloe vera: 8 and 20 µg/ml and then culture supernatants were collected. Next, 100 µl of supernatant was plated in 96-well flat-bottomed plates in triplicate and incubated with 100 µl of Griess reagent (1% sulfanilamide/0.1% N-(1-naphthyl)ethylenediamine dihydrochloride) (Sigma) in 3% H3PO4 (POCH Gliwice, Poland) at room temperature for 10 min. The optical density was measured at 570 nm using a microplate reader (Emax; Molecular Devices Corp.). A standard curve was achieved using 0.5–25 µM sodium nitrite (NaNO2) for calibration.
ELISA
The levels of human IL-1β, IL-6, TNF-α and IL-10 were tested immunoenzymatically (ELISA) in culture supernatants using commercially available kits (Diaclone) according to the manufacturer’s instruction. The optical density at 450 nm with the correction wavelength of 570 nm of each ELISA sample was determined using a microplate reader. The IL-1β, IL-6, TNF-α and IL-10 concentrations were calculated on the basis of a standard curve: 7 pg/ml (IL-1β), 2 pg/ml (IL-6), 8 pg/ml (TNF-α) and 5 pg/ml (IL-10) were the detection limits.
Labeling of cytoskeleton F-actin
Phalloidin staining is the useful tool for investigating the distribution of F-actin in cells (CitationChang et al., 2010).
Cells were incubated in 4-well Lab-Tek chamber slides (Nunc) in 1 ml of culture medium with plant extracts. After incubation, cells were rinsed with K-SFM medium and exposed to paraformaldehyde (10%, v/v) solution for 20 min, rinsed three times in phosphate-buffered saline (PBS), exposed to Triton X-100 (0.2%, v/v) (Sigma) solution for 5 min and rinsed three times with PBS. A volume of 0.5 ml PBS containing tetramethyl-rhodamine-isothiocyanate-phalloidin (TRITC-phalloidin, 1 µg/ml) (Sigma) was added to each well and incubated in the dark at 37°C/5% CO2 for 30 min. Cell observation was conducted under a fluorescence microscope (Olympus, BX51; Olympus). Quantitative analysis of fluorescent images was performed by an AnalySIS imaging software system.
Statistical analysis
Results are presented as means ± SD from three experiments. Data were analyzed using one-way analysis of variance with Dunnett post hoc test. Differences of p ≤ 0.01 were considered significant.
Results
Analysis of toxicity (NR uptake) and antiproliferative (MTT test) activity of extracts
The viability analysis of 10.014 pRSV-T human corneal cells cultured at a density of 1 × 105 cells/ml was performed using NR uptake or MTT assays after 24-h incubation with Aloe vera extracts.
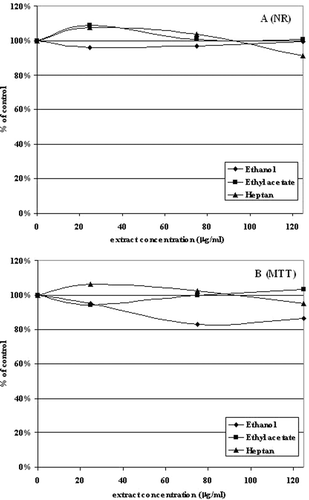
NR test showed no toxicity of ethanol, ethyl acetate and heptane extracts of Aloe vera. In all the cases, the viability of cells was higher than 90%, even at the highest extract concentration (125 µg/ml) (). Similarly, when succinyl dehydrogenase activity was tested (MTT assay), the application of plant extracts did not reduce viability and proliferative activity of corneal cells to level lower than 85% ().
Figure 1. The effect of 24-h treatment of 10.014 pRSV-T with ethanol, ethyl acetate and heptane extracts of Aloe vera in the Neutral Red assay (NR) (A) and MTT assay (B). The results are presented as a percentage of the controls, arbitrarily set to 100%. The figure shows an average of three independent experiments.
Free radical scavenging activity (DPPH tests)
ROS reducing action of Aloe vera extracts is presented in . The study was performed on the basis of stable radical (DPPH•) reduction test. The effect of radical scavenging was extract and concentration dependent. We found no ROS reducing activity by heptane and trace action by ethanol (only at high concentration 125 µg/ml) extracts of Aloe vera. Only ethyl acetate extract of this plant expressed distinct free radical scavenging effect. At the maximum dose that was used (125 µg/ml), the reduction was 10.74% higher than the control value. This reduction level was statistically significant. It was the equivalent of 3.87 µM of Trolox, a synthetic vitamin E, the activity of which was used as a ROS reduction standard (CitationLibrowski & Moniczewski, 2010).
Table 1. DPPH free radicals scavenging activity (%).
NO release
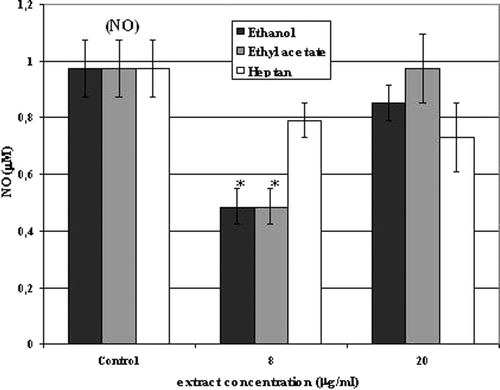
The control NO level was 0.973 µM. Two concentrations of Aloe vera extracts were used: 8 and 20 µg/ml. The presence of plant extracts decreased NO production by human corneal cells as compared to untreated controls. Moreover, in the case of ethanol and ethyl acetate extracts, the application of lower concentration of preparations (8 µg/ml) reduced NO level more than it was observed after usage of a higher concentration of extracts (20 µg/ml). The reduction was more than half (50.05%) (IC50 = 7.99 µg/ml) as compared to controls. In the case of heptane extract, the reduction of NO was comparable for both concentrations used and the level of the radical was on the average 22% lower than the control value ().
Figure 2. Nitric oxide (NO) secretion in culture of 10.014 pRSV-T human normal corneal cells during 24-h incubation with ethanol, ethyl acetate and heptane extracts of Aloe vera. Two extracts concentrations were used: 8 and 20 µg/ml. An analysis was performed using the Griess method. Columns and bars show the mean ± standard deviation (n = 3). *p ≤ 0.01− a culture of corneal cells treated with plant extracts compared to a non-treated culture control.
ELISA tests
Proinflammatory cytokine (IL-1β, IL-6 and TNF-α) levels
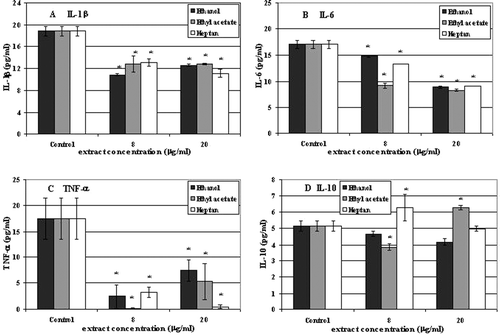
IL-1β
The control level of IL-1β released by 10.014 pRSV-T human corneal cell line was 18.81 pg/ml. Addition of Aloe vera extracts (8 and 20 µg/ml) to the cell culture medium led to the decrease of the cytokine production after 24 h of incubation ().
Figure 3. IL-1β (A), IL-6 (B), TNF-α (C) and IL-10 (D) secretion in culture of 10.014 pRSV-T human normal corneal cells during 24-h incubation with ethanol, ethyl acetate and heptane extracts of Aloe vera. Two extracts concentrations were used: 8 and 20 µg/ml. ELISA test. *p ≤ 0.01− a culture of corneal cells treated with plant extracts compared to a non-treated control culture.
IL-6
Control, non-treated, 10.014 pRSV-T cells released the amount of 17.06 pg/ml of IL-6. Similarly to IL-1β, plant extracts also decreased IL-6 production after 24 h of incubation ().
TNF-α
Non-treated corneal cells released 17.46 pg/ml of TNF-α which was accepted as a control value. The cytokine production decreased after Aloe vera extracts (8 and 20 µg/ml) were added to the culture media and incubated for 24 h ().
Anti-inflammatory cytokine IL-10 level
The control level of IL-10 released by 10.014 pRSV-T human corneal cells was 5.14 pg/ml. Incubation of cells for 24 h with Aloe vera preparations led to increase of cytokine amount to the level of 6.28 pg/ml (1.13 pg/ml above control) when 8 µg/ml of heptane and 20 µg/ml of ethyl acetate extracts were used. Ethanol and remaining ethyl acetate and heptane extract concentrations decreased IL-10 production by the tested cells ().
Organization of cytoskeletal F-actin filaments
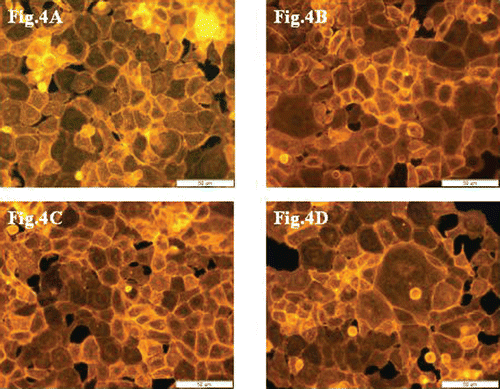
F-actin filaments were analyzed using TRITC-phalloidin fluorescent staining method after 24-h incubation of 10.014 pRSV-T cells with 125 µg/ml of Aloe vera extracts.
Control cells and samples after incubation with extracts are presented in , , and 4D. After incubation of cells with ethanol, ethyl acetate and heptane extracts of the plant, there were no cytoskeleton changes when compared to controls.
Figure 4. Cytoskeleton organization and reciprocal direct interactions of 10.014 pRSV-T human normal corneal cells. The control sample (A), after incubation with extracts at 125 µg/ml concentration: ethanol extract (B), ethyl acetate extract (C) and heptane extract (D). TRITC-phalloidin fluorescent staining. Magnification 400×. Bar 50 µm.
Discussion
Herbal medicines are currently considered to possess many active compounds which express medicinal and health benefits (CitationPaduch et al., 2007). However, there are also herbs and herbal components with potentially hazardous activity. Therefore, standardization of herbal medicines should be undertaken to efficiently reduce or eliminate noxious, adverse reactions. One of the methods to analyze toxicity of herbs is in vitro tests on cell cultures or primary cells isolated from whole organism (CitationPiersma, 2004). These tests enable to answer the question about potential usefulness of plant extracts as a remedy or complementary medicine for patients’ treatment.
In the present study, toxicity and antiproliferative activity of Aloe vera extracts on human normal 10.014 pRSV-T corneal cells was tested. Moreover, the study covers ROS scavenging, NO release and also pro- and anti-inflammatory cytokine production after corneal cells incubation with ethanol, ethyl acetate and heptane extracts.
When using higher extract concentrations (25–125 µg/ml) in toxicity and ROS scavenging tests we wanted to show that even at relatively high extract concentrations they exerted no cytotoxic effects but expressed radical reducing features. Therefore, the aim of this part of the study was to show the effectiveness and safety of Aloe vera extracts even at high concentrations used. On the other hand, low extract concentrations (8 and 20 µg/ml) were used only when specific factors produced and secreted by cells were analyzed. Therefore, when slight amounts and delicate changes in production of specific factors were analyzed, we had to be sure that under any circumstance we did not influence the amount of the cells, their viability or metabolism. Low extract concentrations enabled then to demonstrate that slight amounts of plant substances may significantly regulate NO production and show immunomodulatory activity.
Corneal epithelium is the most external layer of eye surface and therefore it is especially exposed to various harmful factors (CitationWylęgała et al., 2009). Among typical environmental agents influencing corneal lineage uniformity, there are also plant components which could be potentially used in eye drops. To analyze morphology, functionality and viability of corneal layer to chemical stress, we used established 10.014 pRSV-T cell line to test Aloe vera extract activity. This evaluation was performed to assess the potential usefulness of Aloe components to be applied in eye drops in the future. Our experiments revealed that Aloe extracts are non-toxic even at high concentrations (see ) and can be safely used in eye disorder therapies or prophylaxis. Aloe vera contains multiple pharmacologically active substances which are capable of modulating cellular metabolism, activation of some enzymes and express antibacterial, antifungal and anti-inflammatory activities (CitationWilliams et al., 1996). Generally, what was observed in folk medicine and also in experimental studies, Aloe vera extracts do not influence on cells viability. This observation was confirmed by CitationGreen and colleagues (1996) who analyzed healing of corneal epithelial lesions in rabbits. They revealed no toxicity without redness or chemosis after Aloe vera gel application to the animal eyes. We confirmed these results (MTT and NR assays) showing that Aloe vera extracts have no cytotoxic effect and no simultaneous influences on cellular cytoskeleton on in vitro–cultured human corneal cells. Moreover, therapeutic activity may be associated with substance mixture coming from ethyl acetate extraction of Aloe vera which demonstrated concentration-dependent ROS scavenging action. It is accepted that antioxidant activity of Aloe vera is related to the presence of phenolic compounds in its extracts (CitationOzsoy et al., 2009). This effect is also regarded the most beneficial therapeutic activity of this plant. However, CitationLindsey et al. (2003) concluded in their study that promising antioxidant properties of Aloe vera are limited by simultaneous insufficient prevention of lipid peroxidation. However, ROS reducing effect is very important considering the fact that Aloe extracts also reduced NO production. This result is complementary and in agreement with CitationChen et al. (2005) who showed inhibitory activity of Aloe vera polysaccharides on the release of NO in cultured human keratinocytes. NO is not only an important physiological signaling molecule but also an effector radical in biological processes. However, it may also exert pathological effects depending upon its local concentration and kind of tissue it influence on (CitationSarkar et al., 2005). The reduced NO production in corneal cell culture in the presence of Aloe vera extracts, as was shown in our study (see ), may be a result of constitutive and inducible NO synthase inhibition and possible cyclooxygenase (COX) activity modulation. COXs are key enzymes in the prostaglandin biosynthesis that is implicated in inflammatory processes (CitationLindsey et al., 2002). Therefore, a feedback consisting of a limitation in inflammatory conditions by Aloe vera extracts, via proinflammatory cytokine expression inhibition, and prostaglandin production, which, in consequence, could be connected with decreased NO level, exists. However, this thesis needs further study. Decreased NO level may, in consequence, lead to formation of very active toxic intermediates (RNOS). They are capable of nitrosylation of selected amino acid residues of proteins, which may disturb protein structure, and, in consequence, may lead to cell death (CitationKim et al., 2001). However, proper level of NO enables to maintain homeostasis of eye surface and may serve as a health preventive factor against, e.g. chronic inflammations. Therefore, considering dual role of NO both in healthcare or toxicity depending on its local concentration, it has to have suitably matched quantities of Aloe vera active substances in, e.g. eye drops to achieve progressive curative effect during successive units of therapy. Administration of herb-derived substances or plant extracts to specific parts of the organism like eyeball influences local homeostasis among others on cytokine network (CitationChen et al., 2010). The changes in pro- and anti-inflammatory cytokine levels are most important. The first group consists of IL-1β, IL-6 or TNF-α, whereas the second one consists of IL-10. In our study, we showed that Aloe vera extracts, generally, decreased proinflammatory cytokine level while anti-inflammatory IL-10 was concentration and kind of extract dependent (see ). It has been already shown that Aloe vera contains many biologically active compounds which create anti-inflammatory or immunomodulatory effects. The active plant agents act alone or in concert and consist mainly of glycoproteins, anthraquinones, polysaccharides or low-molecular-mass species (CitationChoi & Chung, 2003). Literature data indicate that anti-inflammatory or immunomodulatory effects might also be connected with Aloe vera sterols. It was shown that they may inhibit phospholipase A2 enzyme which is responsible for arachidonic acid liberation, the substrate for prostaglandin production. Therefore, the anti-inflammatory action of the contents of the Aloe vera extracts may be related to the inhibition of prostaglandin and leukotriene synthesis. Furthermore, anti-inflammatory effect of Aloe vera could be related to COX inhibition and in consequence, what was shown in our study, a decrease in the level of proinflammatory cytokines (CitationVázquez et al., 1996). Moreover, this plant can inhibit inflammatory process not only by decreasing cytokine level but also by reduction of leukocyte adhesion in the place of wound or injury (CitationDuansak et al., 2003). Our results also confirmed the close relationship between IL-1β and NO levels. It is known that IL-1β is an important mediator of secretory processes acting at least in part through the generation of NO (CitationEutamene et al., 1995; CitationIzzo et al., 1999). Therefore, as we confirmed in our experiments, lowered IL-1β level after Aloe vera extract administration to the corneal cell culture was also followed by limited NO level. Summing up, we showed that immunomodulatory or inflammation-reducing effect based on limitation of proinflammatory cytokine levels by Aloe vera extracts may also occur in the eye tissue.
Conclusions
Aloe vera ethanol and ethyl acetate extracts may be used in eye drops. In a limited range of concentrations, they are not toxic to corneal cells, show immunomodulatory activity and might have an antiinflammatory effect. Therefore, Aloe vera provides a scientific basis for the use of this plant in the treatment of inflammations and other ailments of external parts of the eye such as the cornea.
Acknowledgment
We would like to express our thanks to Professor T. Krzaczek (Medical University in Lublin) for Aloe vera identification.
Declaration of interest
The authors declare that they have no conflict of interest.
References
- Can A, Akev N, Ozsoy N, Bolkent S, Arda BP, Yanardaq R, Okyar A. (2004). Effect of Aloe vera leaf gel and pulp extracts on the liver in type-II diabetic rat models. Biol Pharm Bull, 27, 694–698.
- Chang HR, Huang HP, Kao YL, Chen SL, Wu SW, Hung TW, Lian JD, Wang CJ. (2010). The suppressive effect of Rho kinase inhibitor, Y-27632, on oncogenic Ras/RhoA induced invasion/migration of human bladder cancer TSGH cells. Chem Biol Interact, 183, 172–180.
- Chen M, Hu DN, Pan Z, Lu CW, Xue CY, Aass I. (2010). Curcumin protects against hyperosmoticity-induced IL-1beta elevation in human corneal epithelial cell via MAPK pathways. Exp Eye Res, 90, 437–443.
- Chen XD, Huang LY, Wu BY, Jiang Q, Wang ZC, Lin XH. (2005). Effect of Aloe vera polysaccharide on the release of cytokines and nitric oxide in cultured human keratinocytes. Chin Crit Care Med, 17, 296–298.
- Choi S, Chung MH. (2003). A review on the relationship between Aloe vera components and their biologic effects. Semin Integr Med, 1, 53–62.
- Duansak D, Somboonwong J, Patumraj S. (2003). Effects of Aloe vera on leukocyte adhesion and TNF-alpha and IL-6 levels in burn wounded rats. Clin Hemorheol Microcirc, 29, 239–246.
- Eutamene H, Theodorou V, Fioramonti J, Bueno L. (1995). Implication of NK1 and NK2 receptors in rat colonic hypersecretion induced by interleukin 1 beta: role of nitric oxide. Gastroenterology, 109, 483–489.
- Fraunfelder FW. (2004). Ocular side effects from herbal medicines and nutritional supplements. Am J Ophthalmol, 138, 639–647.
- Ganbold M, Barker J, Ma R, Jones L, Carew M. (2010). Cytotoxicity and bioavailability studies on a decoction of Oldenlandia diffusa and its fractions separated by HPLC. J Ethnopharmacol, 131, 396–403.
- Green K, Tsai J, Luxenberg MN. (1996). Effect of Aloe vera on corneal epithelial wound healing. J Toxicol Cutaneous Ocul Toxicol, 15, 301–304.
- Hu Y, Xu J, Hu Q. (2003). Evaluation of antioxidant potential of aloe vera (Aloe barbadensis miller) extracts. J Agric Food Chem, 51, 7788–7791.
- Izzo AA, Sautebin L, Borrelli F, Longo R, Capasso F. (1999). The role of nitric oxide in aloe-induced diarrhoea in the rat. Eur J Pharmacol, 368, 43–48.
- Kigondu EV, Rukunga GM, Keriko JM, Tonui WK, Gathirwa JW, Kirira PG, Irungu B, Ingonga JM, Ndiege IO. (2009). Anti-parasitic activity and cytotoxicity of selected medicinal plants from Kenya. J Ethnopharmacol, 123, 504–509.
- Kim PK, Zamora R, Petrosko P, Billiar TR. (2001). The regulatory role of nitric oxide in apoptosis. Int Immunopharmacol, 1, 1421–1441.
- Lee KY, Weintraub ST, Yu BP. (2000). Isolation and identification of a phenolic antioxidant from Aloe barbadensis. Free Radic Biol Med, 28, 261–265.
- Librowski T, Moniczewski A. (2010). Strong antioxidant activity of carane derivatives. Pharmacol Rep, 62, 178–184.
- Lindsey KL, Jäger AK, Viljoen AM. (2002). Cyclooxygenase inhibitory activity of Aloe species. S Afr J Botany, 68, 47–50.
- Lindsey KL, Viljoen AM, Jäger AK. (2003). Screening of Aloe species for antioxidant activity. S Afr J Bot, 69, 599–602.
- Mosmann T. (1983). Rapid colorimetric assay for cellular growth and cytotoxicity. J Immunol Methods, 65, 55–63.
- Muzitano MF, Bergonzi MC, De Melo GO, Lage CL, Bilia AR, Vincieri FF, Rossi-Bergmann B, Costa SS. (2011). Influence of cultivation conditions, season of collection and extraction method on the content of antileishmanial flavonoids from Kalanchoe pinnata. J Ethnopharmacol, 133, 132–137.
- Ozsoy N, Candoken E, Akev N. (2009). Implications for degenerative disorders: antioxidative activity, total phenols, flavonoids, ascorbic acid, beta-carotene and beta-tocopherol in Aloe vera. Oxid Med Cell Longev, 2, 99–106.
- Paduch R, Matysik G, Wójciak-Kosior M, Kandefer-Szerszeń M, Skalska-Kamińska A, Nowak-Kryska M, Niedziela P. (2008). Lamium album extracts express free radical scavenging and cytotoxic activities. Polish J Environ Stud, 17, 569–580.
- Paduch R, Matysik G, Wójciak-Kosior M, Niedziela P. (2007). The biological activity of medicinal plant extract. Houston, TX: Stadium Press LLC.
- Piersma AH. (2004). Validation of alternative methods for developmental toxicity testing. Toxicol Lett, 149, 147–153.
- Rabe C, Musch A, Schirmacher P, Kruis W, Hoffmann R. (2005). Acute hepatitis induced by an Aloe vera prepration: A case report. World J Gastroenterol, 11, 303–304.
- Sarkar D, Dutta A, Das M, Sarkar K, Mandal C, Chatterjee M. (2005). Effect of Aloe vera on nitric oxide production by macrophages during inflammation. Indian J Pharmacol, 37, 371–375.
- Telefo PB, Moundipa PF, Tchana AN, Tchouanguep Dzickotze C, Mbiapo FT. (1998). Effects of an aqueous extract of Aloe buettneri, Justicia insularis, Hibiscus macranthus, Dicliptera verticillata on some physiological and biochemical parameters of reproduction in immature female rats. J Ethnopharmacol, 63, 193–200.
- Telefo PB, Moundipa PF, Tchouanguep FM. (2002). Oestrogenicity and effect on hepatic metabolism of the aqueous extract of the leaf mixture of Aloe buettneri, Dicliptera verticillata, Hibiscus macaranthus and Justicia insularis. Fitoterapia, 73, 472–478.
- Telefo PB, Moundipa PF, Tchouanguep FM. (2004). Inductive effect of the leaf mixture extract of Aloe buettneri, Justicia insularis, Dicliptera verticillata and Hibiscus macranthus on in vitro production of estradiol. J Ethnopharmacol, 91, 225–230.
- Vázquez B, Avila G, Segura D, Escalante B. (1996). Antiinflammatory activity of extracts from Aloe vera gel. J Ethnopharmacol, 55, 69–75.
- Williams MS, Burk M, Loprinzi CL, Hill M, Schomberg PJ, Nearhood K, O’Fallon JR, Laurie JA, Shanahan TG, Moore RL, Urias RE, Kuske RR, Engel RE, Eggleston WD. (1996). Phase III double-blind evaluation of an aloe vera gel as a prophylactic agent for radiation-induced skin toxicity. Int J Radiat Oncol Biol Phys, 36, 345–349.
- Wylegala E, Dobrowolski D, Nowinska A, Tarnawska D. (2009). Anterior segment optical coherence tomography in eye injuries. Graefes Arch Clin Exp Ophthalmol, 247, 451–455.
- Yoo EA, Kim SD, Lee WM, Park HJ, Kim SK, Cho JY, Min W, Rhee MH. (2008). Evaluation of antioxidant, antinociceptive, and anti-inflammatory activities of ethanol extracts from Aloe saponaria Haw. Phytother Res, 22, 1389–1395.