Abstract
Context: Carpolobia lutea G. Don (Polygalaceae) leaf is reputable as an antidiarrheal agent among the Efik and Ibibio tribe of Akwa Ibom State, Nigeria. The crude extract is reported to show antidiarrheal and antiulcer effects in rodents.
Objective: The isolation and characterization of drug molecules from the leaf fraction with antidiarrheal bioactivity and determination of mechanism of action are reported.
Material and methods: Gradient extraction by maceration yielding n-hexane, chloroform, ethyl acetate, and ethanol fractions (770 mg/kg) were used to establish the fractions suitable for drug discovery. The antidiarrheal effect of the leaf fractions of Carpolobia lutea was evaluated using castor oil–induced diarrhea, castor oil–induced intestinal transit, and enteropooling.
Results: Results indicate that all fractions produced a significant (p < 0.01–0.001) decrease in castor oil–induced diarrhea in rats. This effect was not antagonized by isosorbide dinitrate (150 mg/kg, p.o), diphenoxylate (5 × 10−3 mg/kg p.o) and yohimbine (1 mg/kg, s.c.) except for the chloroform fraction. The ethyl acetate fraction produced 100% inhibition of intestinal transit, an effect greater than pure drug. Phytochemical analysis of the ethyl acetate fraction yielded polyphenolic compounds.
Conclusion: The leaf fractions contain two types of antidiarrheal agents, one mediating its effect through α1-presynaptic adrenoceptor while the other does not. Polyphenols isolated may in part lend credence for observed antidiarrheal activity.
Introduction
Diarrhea is a common and major public health problem among people with poor standards of hygiene, especially in developing countries, and it remains the leading cause of morbidity and mortality in all age groups, with as many as 4 million cases occurring each year (CitationFarthing, 2002). Diarrhea remains one of the major health threats to populations in the tropical poor countries. According to CitationHeinrich et al. (2005), the World Health Organization (WHO) estimates that 3–5 billion cases occur each year (1 billion in children less than 5 years of age), and that approximately 5 million deaths are due to diarrhea annually (2.5 million in children less than 5 years of age). A study by CitationMartinez et al. (1998) which looked at the form of treatment that is administered by primary caretakers of young children including mothers showed that herbal remedies are still important in the treatment of diarrhea.
Studies have validated the use of antidiarrheal medicinal plants by investigating the biological activity of extracts of such plants, which have antispasmodic effects, delay intestinal transit, suppress gut motility, stimulate water adsorption, or reduce the intraluminal fluid accumulation (CitationAlmeida et al., 1995; CitationAtta & Mouneir, 2005).
C. lutea G. Don (Polygalaceae) is a small tree distributed in West and Central tropical Africa; it is reputable and widely acknowledged among the Efik, Ibibio, Ibo, and Yoruba speaking ethnic groups in Nigeria for its antidiarrheal, antiinflammatory, antiarthritic, antiulcer, and aphrodisiac potential. C. lutea is called cattle stick (English), Ikpafum (Ibibio), Agba or Angalagala (Igbo), and Egbo Oshunshun (Yoruba) in Nigeria (CitationEtukudo, 2003; CitationMuanya, 2008). It has androgenic properties, is used to cure rheumatism, fever, analgesic, insanity, dermal infection, venereal diseases, to combat sterility and promote child birth, and as a taeniafuge and vermifuge (CitationBurkill, 1985; CitationEtukudo, 2003; CitationMuanya & Odukoya, 2008). The stem bark is dried and taken as snuff to cure migraine headache (CitationIrvine, 1961). The leaf is reported to have antiinflammatory and antiarthritis properties (CitationIwu & Anyanwu, 1982) and used in the management of evacuant fever, headache, leprosy, snakebite, venereal disease, and wound healing (CitationLewis & Elvin-Lewis, 1977; CitationAjibesin et al., 2008).
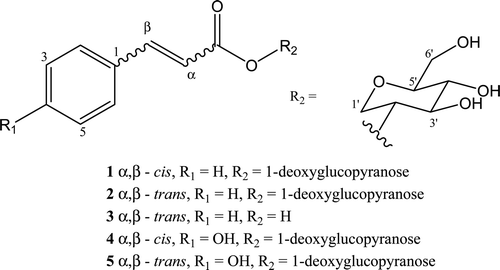
Evaluations of the antidiarrheal and antiulcerogenic potential of crude ethanol extract of C. lutea leaf was observed to have gastroprotective and antidiarrheal effects in rodents (CitationNwafor & Bassey, 2007). Preliminary gastroprotective evaluation of various fractions of the leaf extracts of C. lutea revealed that the ethyl acetate extract as promising antiulcer source (CitationNwidu & Nwafor, 2009). Although C. lutea leaf has wide patronage traditionally for the management of diarrheal and this pharmacological activity has been verified (CitationNwafor & Bassey, 2007), the mechanisms of action are yet to be scientifically elucidated. Therefore, gradient extraction using n-hexane, chloroform, ethyl acetate, and ethanol fractions were used to evaluate the diarrheal activity. HPLC fingerprint of the polar extract revealed the presence of polyphenolics with the ethyl acetate fraction preconcentrating the phenolics of the leaf extract. Polygalaceae is known to contain species with a variety polyphenolic compounds such as xanthones, flavonoids, and biphenyl derivatives, which exhibit significant biological activities (CitationCervellati et al., 2004). Two new cinnamoyl 1-deoxy glucosides (1 and 2), cinnamic acid (3), besides two new coumaroyl 1-deoxy glucosides (4 and 5), were isolated and characterized from the ethyl acetate fraction using semipreparative HPLC and structures was established using NMR experiments (CitationLatza et al., 1996; CitationHidradate et al., 2004).
Materials and methods
Preparation of plant extracts
C. lutea leaves were collected and supplied by Mr. Etefia, the traditional herbalist, attached to the Pharmacognosy Department, University of Uyo, Akwa Ibom State, Nigeria. The plant was identified and authenticated by Dr. (Mrs.) Margaret Bassey of Department of Botany of the same University. A voucher specimen (UUH 998) was deposited at the University Herbarium. The leaves were air dried, powdered with pestle and mortar. The pulverized leaves were stored at room temperature until used.
Extraction and fractionation of C. lutea
Procedure of gradient solvent extraction is described in a previous article (CitationNwidu & Nwafor, 2009). The chemical constituents present in C. lutea were analyzed according to CitationWagner et al. (1984). The chromatographic analyses were performed by thin layer chromatography (TLC; Fluka, silica gel plates on glass, 20 × 20 cm × 0.25 mm) eluted with: CHCl3/MeOH/H2O (43:37:20, v/v/v), CHCl3/MeOH/H2O (80:18:2, v/v/v), CHCl3/MeOH/H2O/Acetic acid (43:37:30:1, v/v/v/v), and CHCl3/MeOH/n-PrOH/H2O (310:380:60:250, v/v/v/).
An aliquot of 4.00 g of the ethyl acetate fraction (EAF) was dissolved in 10 ml methanol and centrifuged at 2,500 g for 15 min. This procedure was performed three times. The supernatant was filtered and submitted to gel permeation chromatography using Sephadex LH-20 (Pharmacia) in a glass column (700 × 35 mm, i.d.) using methanol 98.98%. Fractions were obtained using an automatic fraction collector set at 9 ml per minute at a flow rate of 3 ml/min. Eighteen fractions (C1-C18) were obtained after combining into different groups based on the chromatographic profile on TLC and revealed either with NP/PEG reagent or anisaldehyde/sulfuric acid solution. Fraction C7 (1.5 g) were analyzed by HPLC and after submitted to the isolation of compounds.
HPLC analysis and isolation
The EAF and fraction C7 aliquots were dissolved in 80% aqueous MeOH and filtered through a 0.50 µm Teflon syringe filter, then the filtrate (30 µl) was injected into the HPLC. HPLC analysis was conducted on an HPLC system using a Jasco (Tokyo, Japan) liquid chromatography equipped with a PU-2089 quaternary solvent pump, a MD-2010 PAD and an AS-2055 autosampler injector with a 20 μl sample loop. The analytical column was a Phenomenex Synergi Hydro RP18 (250 × 4.6 mm i.d.; 4μm) equipped with a Phenomenex security guard column (4.0 × 2.0 mm i.d.). The mobile phase composition was as follows: Water (eluent A) and methanol (eluent B) both containing 0.05% of TFA. The gradient program was linear starting with 0% B to 100% B in 60 min (Flow rate = 1.0 mL/min). EZChrom Elite Data System software (Chromatec, Idstein, Germany) was used for both the operation of detector and for data processing. All solvents used for analysis were HPLC grade and water was purified by a Milli-Q plus system (Millipore, Bedford, MA, USA) to a resistivity of 18.2 MΩcm.
The C7 fraction (1.5 g) was subjected to Solid Phase Extraction (SPE) in reverse phase cartridge (5 g/50 ml), before isolation steps, by passing H2O (100%), H2O/MeOH (80:20, v/v), H2O/MeOH (50:50, v/v), and MeOH (100%; Rodrigues et al., 2008). The four fractions were dried under a flow of nitrogen and their weight recorded on analytical balance. The yields from the H2O (100%) and H2O/MeOH (80:20, v/v) were significant, 520.5 and 837.5 mg, respectively, and the second eluate was subjected to isolation step using a Varian (Walnut Creek, CA, USA) ProStar 210/330 HPLC system equipped with a Phenomenex RP18 semipreparative column (Luna C18(2) 250 × 10.0 mm i.d.; 10 μm) and a Rheodyne (Cotati, CA, USA) 7125 sample injector with a 400 µl sample loop. The optimized condition is isocratic elution with 45% of MeOH in 40 min, a flow rate of 5 ml/min. This separation provided the isolation of compounds 1 (42.0 mg), 2 (327.0 mg), 3 (58.0 mg), and a mixture of 4 + 5 (8.0 mg).
Structure elucidation of the compounds
The structure elucidation of the isolated compounds was based on 1D and 2D-NMR experiments (1H, HOMODEC, COSY, TOCSY, HMQC, and HMBC). Spectra were recorded in DMSO-d6 on a Varian INOVA 500 and on a Bruker AC 200. Tetramethylsilane (TMS) was used as an internal proton standard. Mass Spectra was performed on Bruker Daltonics ion trap mass spectrometer; model Esquire 4000 with electrospray interface. The data obtained were compared with those found in literature (CitationLatza et al., 1996; CitationHidradate et al., 2004).
Animals
Adult albino mice and rats were used. All the animals were housed in standard cages under laboratory conditions in the University of Uyo Pharmacology Department. The animals used were fed with pellet feeds (Vital Feed and Flour Mill Limited, Edo State, Nigeria) and water ad libitum. All animals used have free access to tap water under standard conditions of 12 h dark 12 h light and temperature (21 ± 1%). The protocols were approved by the University of Uyo Institutional animal Care and Use Committee which follows the guidelines of Committee for the purpose of control and supervision of experimental on animals (CPSCEA; UUAEC No. 2004/013).
Castor oil–induced diarrhea
The rats fasted for 24 h were randomly allocated to five groups of six animals each. Group I received 20% Tween 80 (10 ml/kg); Groups II, III, IV, and V received 770 mg/kg each of n-hexane, chloroform, ethyl acetate, and ethanol of C. lutea fractions orally, respectively. Groups VI received diphenoxylate (5 mg/kg). To establish the mechanism of actions of these fractions the above procedure was repeated with pretreatment of rat with yohimbine (1 mg/kg) and diphenoxylate (5 × 10−3 mg/kg), 15 min before the administration of C. lutea fractions. After 60 min each animal was administer 2 ml of castor oil by orogastric polyethylene catheter and placed in a separate cage and observed for 4 h defecation (CitationAwouteur et al., 1978; CitationNwodo et al., 1991).
Transparent plastic dishes containing filter paper were placed beneath each cage, and the characteristic diarrheal droppings were noted. The parameters observed are as follows: Onset of diarrhea, number of wet feces, the number of semisolid feces, and total frequency of fecal outputs. The filter papers were inspected for the presence of diarrheal droppings at hourly intervals for a period of 4 h. The total number of diarrheal episodes was counted group-wise. At hour 4, its absence was considered as a protection from diarrhea. A numerical score based on stool consistency was assigned-1 (normal stool), 2 (semi-solid stool), and 3 (watery stool). Each group exhibit an evacuation index (EI) expressed according to the formula: EI = 1 × (no. stool 1) + 2 × (no. stool 2) + 3 × (no. stool 3). See . The onset is measured as the time interval in min between the administration of castor oil and the first appearance of diarrhea stool.
Table 1 A numerical score based on stool consistency from the castor oil–induced diarrheal in C. lutea fractions.
Castor oil–induced fluid accumulation
The rats fasted for 24 h, but free access to water was randomized and allocated to six groups of six rats each. Group I (control) was administered 20% Tween 80 (10 ml/kg), Groups 2-5 were administered 770 mg/kg each of n-hexane, chloroform, ethyl acetate, and ethanol of C. lutea fractions, respectively; Group 6 received only diphenoxylate (5.0 × 10−3 mg/kg body wt.) p.o.; 1 h later each rat received 2 ml of castor oil (p.o.). After 30 min, the rats were killed by cervical dislocation and exsanguinated; the small intestine was ligated both at the pyloric sphincter and at the ileocaecal junctions. The entire small intestine was dissected out, its contents were expelled into a graduated measuring cylinder, the volume and the weight of the contents was recorded according to the methods of CitationRobert et al. (1976) and CitationDicarlo et al. (1994).
Small intestinal transit
Both sexes of male and female Wistar rats fasted for 24 h and deprived of water only during the experiment were weighed and randomized into six groups of six rats each. Each animal was given orally 1 ml of charcoal meal (5% activated charcoal suspended in physiological saline), 60 min after an oral dose of drugs or vehicle. Group I was administered with 20% Tween 80 (10 ml/kg) and animals in Groups II-V received C. lutea fractions (n-hexane, chloroform, ethyl acetate, and ethanol 770 mg/kg). Group V received diphenoxylate (0.5 mg/kg) as standard drug. After 30 min, animals were killed by cervical dislocation, and the intestine was removed without stretching and placed lengthwise on dissecting board. The length of the intestine (pyloric sphincter to cecum) and the distance travelled by the charcoal as a percentage of total length were evaluated for each animal, and group means were compared and expressed as percentage inhibition (CitationLutterodt, 1989).
Statistical analysis
Data obtained were analyzed by Student’s t-test and multiple comparisons were done by ANOVA followed by Dunnet Multiple Comparison Test. A probability level of less than 5% was considered significant (p < 0.05).
Results
Phytochemistry
The HPLC fingerprint of the ethyl acetate fraction showed a homologous series of compounds with λmax = 215, 280, and 310 nm, characteristic of phenolic compounds. Chromatographic fractionation of the ethyl acetate fraction (EAF; ) afforded two new cinnamoyl 1-deoxyglucopyranosides (1 and 2) and two new p-coumaroyl 1-deoxyglucopyranosides (4 and 5), besides cinnamic acid (3; Figure 2). Structures of the isolated compounds were proposed based on NMR experiments and mass spectrometry.
Castor oil–induced diarrhea
The fractions significantly reduced the number of diarrheal episodes in this order chloroform ≥ ethyl acetate ≥ n-hexane ≥ ethanol fractions when compared with the untreated controls. At 770 mg/kg dose, chloroform fraction of C. lutea showed 90.09% and ethyl acetate fraction 74.19% reduction in the number of fecal episodes, whereas diphenoxilate (5 × 10−3 mg/kg) offered 99.28% protection. In terms of protection from diarrhea at 4 h, the 770 mg/kg dose of C. lutea leaf fraction, chloroform fraction protected 5 out of 6 animals, ethyl acetate fraction protected 4 out of 6 animals, both n-hexane and ethanol fraction protected 3 out of 6 rats each while diphenoxylate (5 × 10−3 mg/kg) protected 6 out of 6 rats from diarrhea ().
Table 2 Effects of leaf extracts of C. lutea on castor oil–induced diarrhoeal in rats.
The results of pretreatment of each fraction with α2-presynaptic antagonist, yohimbine showed a significant reduction of fecal episode which increases with ethanol (82.23%), ethyl acetate (85.11%) and n-hexane (74.45%) fractions while a significant reduction (p ≤ 0.001) of fecal episode decreases with chloroform fraction (78.00%) when compared to fractions without pretreatment with yohimbine ().
The results of pretreatment of each fraction with isosorbide dinitrate, a nitrous oxide donor, similarly showed that a significant reduction of fecal episode increases with ethanol (100%), ethyl acetate (94.34%), n-hexane (89.36%) fractions, and chloroform fraction (93.60%) when compared to the group not pretreated ().
The results of pretreatment of each fraction with anticholinergics, diphenoxylate showed a 100% reduction of fecal episode in ethanol, n-hexane, and chloroform except ethyl acetate fraction (96.47%) when compared to fraction without pretreatment.
Intestinal fluid accumulation
The plant fractions did not significantly reduce the intestinal fluid accumulation induced by castor oil (). At 770 mg/kg dose, the inhibition of intestinal fluid accumulation was in this order n-hexane > ethanol > ethyl acetate > chloroform fractions. The reduction produced by n-hexane is (13.1%) and greater than ethanol fraction (11.18%) compared with the vehicle control. The reduction in the intestinal fluid accumulation by diphenoxylate (5.0 × 10−3 mg/kg) was 33.53%.
Table 3 Effects of leaf extracts of C. lutea intestinal fluid accumulation in rats.
Small intestinal transit
The distance traveled by the charcoal marker in the treated groups showed significant difference compared with control (p < 0.001). The intestinal transit of charcoal meal was 46.78% in the control group, but at 770 mg/kg dose of ethanol, ethyl acetate, chloroform, and n-hexane fractions were 74.24, 0.0, 90.8, and 79.99%, respectively. The percent transit of marker was 29.24% in the case of 5 mg/kg dose of diphenoxylate (5.0 × 10−3 mg/kg; ).
Table 4 Effects of leaf fractions of C. lutea on normal intestinal transit in rats.
Discussion
The frequency and consistency of diarrhea induced by castor oil was inhibited significantly (p < 0.001) by the C. lutea fractions with the chloroform, ethyl acetate, n-hexane, and the ethanol fractions producing 90.09, 75.19, 64.55, and 63.11% reduction of diarrhea compared to diphenoxylate (99.28%). It appears from the study that the four fractions of the leaves of C. lutea had demonstrable antidiarrheal efficacy in one or the other experimental models, though the effects were more pronounced with the chloroform fraction followed by the ethyl acetate fraction. With respect to the castor oil–induced diarrhea model, the results revealed that the C. lutea chloroform fraction showed slightly better protection for diarrhea in the animals as compared to ethyl acetate, ethanol, and n-hexane fractions, but this was not the case with castor oil induced-induced enteropooling, where n-hexane followed by ethanol fractions produce greater inhibition. Drugs affecting motility frequency and consistency of diarrhea also affect secretion (CitationDiCarlo et al., 1994). The intraluminal fluid accumulation induced by castor oil was also blocked by the C. lutea fractions. The C. lutea fractions did not produce any significant effect on intestinal fluid accumulation. It is likely that the fractions bring out the aforementioned action either through their proabsorptive property that promotes faster fluid absorption in the intestine or through an antisecretory mechanism (CitationYadav & Tangpu, 2007). The first speculation may gain support from the fact that castor oil, which was used as a diarrhea-inducing agent in the experimental protocol, is known to induce diarrhea by increasing the volume of intestinal content by preventing water absorption. Therefore, any agent that allows or promotes water absorption in the intestine obviously would have an antidiarrheal potential (CitationKatzung, 2001). It is widely known that the castor oil is metabolized into ricinoleic acid in the gut, which in turn irritates and causes inflammation in the intestinal mucosa, resulting in release of inflammatory mediators, such as prostaglandins, histamine, and so forth (CitationLuderer et al., 1980). The prostaglandins thus released promote vasodilatation, smooth muscle contraction, and mucus secretion in the small intestines (CitationPierce et al., 1971; CitationRobert, 1973). The prostaglandins of the E series are considered to be good diarrheogenic agents in experimental animals as well as in human beings (CitationJaffe, 1979). The inhibitors of prostaglandins biosynthesis are therefore considered to delay the castor oil–induced diarrhea (CitationPierce et al., 1971).
In the small intestinal transit test, the ethyl acetate fraction produced 100% suppression of the propulsion of charcoal marker. This percentage inhibition of propulsion of charcoal marker ethyl acetate fraction is three times that of pure drugs, diphenoxylate. A decrease in the motility of gut muscles increases the stay of substances in the intestine. This allows better water absorption. It is therefore presumed that the reduction in the intestinal propulsive movement in the charcoal meal model may be due to antispasmodic properties of the ethyl acetate fraction. The polyphenols isolated from the ethyl acetate fraction could mediate this activity as plants that have quercetin, a common dietary flavonoid, in their composition can produce antidiarrheic effects mainly due to their antihistamine and antiinflammatory activities (CitationIzzo, 1994).
Recently, attention has focused on the 5-HT3 receptors because of their apparent roles in visceral pain and motility (CitationMiura et al., 1999). Acetylcholine, a neurotransmitter release by the parasympathetic nervous system plays an important physiological role to regulate the peristaltic movements of the gut (CitationBrown & Taylor, 1996) and atropine blocks all muscarinic receptor sites (CitationArunlashana & Schild, 1959).
Yohimbine, IDN, and diphenoxylate were employed in this study to elucidate the mechanism of action of C. lutea. The role of nitric oxide donors (e.g., IDN, l-arginine, S-nitro-l-gluthione, sodium nitroprusside, molsidomine, and so on) in intestinal fluid and electrolyte secretion depends on study conditions (CitationIzzo et al., 1998). In normal physiological conditions, endogenous nitric oxide is proabsorptive as a result of influence on enteric nervous system, suppression of prostaglandin formation, and opening of basolateral K+ channels. It is established that that nitric oxide synthase inhibitors (e.g. l-NG-nitro-arginine methyl ester, l-NAME; NG-monoethyl-arginine, NMMA; NG-nitro-arginine, NNA; 7-nitroindazole; s-methylisothiourea, SMT, and so on) reverses net fluid absorption to net secretion in mice, rats, guinea pigs, rabbits, and dogs (CitationAdeyemi & Akindele, 2008). In pathophysiological conditions, nitric oxide synthase is produced at higher concentrations that evoke net secretion, thus it is said to mediate the laxative action of several secreatagogues, including castor oil, phenolphthalein, bisacodyl, magnesium sulfate, bile salts, senna, and cascara in the rat (CitationIzzo et al., 1998). The fact that nitric oxide plays a role in the laxative effect of castor-induced diarrhea by inducing the release of nitric oxide (NO), which in turn mediate the generation of prostaglandin by colonic cells, evoking net fluid secretion rather than net absorption thus worsening the pathology have been reported (CitationMascolo et al., 1994). It has been concluded that castor oil–induced diarrhea in rats involves the l-arginine nitric oxide pathways based on experimental findings that isosorbide dinitrate (IDN) and isosorbide-5-monitrate (nitric oxide donors) when administered to castor oil treated rats, prevented dose dependently the inhibitory effects of l-NAME (nitric oxide synthase inhibitor; CitationAdeyemi & Akindele, 2008).
The adrenergic agonist at α2-adrenergic receptor promotes fluid and electrolyte absorption (CitationBurks, 1991), hence, yohimbine a specific α2-adrenergic receptor antagonist, will do the opposite thus promoting diarrhea. Diphenoxylate on the other hand, a muscarinic receptor antagonist, inhibits gastrointestinal motility (propulsion), reduced intestinal fluid secretion, and delay gastric emptying thus blunting diarrhea.
Ethyl acetate fraction (770 mg/kg) of C. lutea inhibited the intestinal propulsive movements (IPM) in rat by 100%. This result from ethyl acetate fraction suggest that the effect on IPM may not be mediated by α2-adrenoceptor and nitric oxide dependent mechanism because α2-adrenoceptor antagonist, yohimbine, and nitric oxide donor, isosorbide dinitrate, did not reduced extract-induced transit delay in rats. An inhibitory action on intestinal transit, as elicited by the ethyl acetate fraction in this study, delays the passage of gastrointestinal contents and allows feces to become desiccated, thus further retarding movement through the colon (CitationAkindede & Adeyemi, 2006). The effect of C. lutea in reducing intestinal propulsion is linked to delay in gastric emptying, as observed for the case with atropine and morphine (CitationIzzo et al., 1999; CitationAkindele & Adeyemi, 2006). The dose 770 mg/kg of ethyl acetate fraction inducing 100% inhibition of IPM is critical as other doses (median or lower) did not produce significant dose-dependent inhibition of gastric emptying (unpublished data).
The chloroform fraction inhibition on the diarrhea effect was however antagonized by yohimbine, indicating the role of α2-adrenoceptor in the secretion, frequency, and consistency model induction of diarrhea. However, the ethyl acetate, n-hexane and ethanol fractions were not mediated by antagonism of α2-adrenoceptor or nitric oxide donor (IDN) dependent mechanism. This precludes interference with the receptors in explaining the effectiveness of C. lutea. Nitric oxide plays an important role in intestinal fluid and electrolyte secretion in the intestine (CitationIzzo et al., 1998). Inhibitors of nitric oxide synthesis seem to block the laxative action of diarrheal agents (CitationIzzo et al., 1994). Based on the above facts, it seems reasonable to suggest that the antidiarrheal effect of C. lutea been enhanced by IDN and may not be mediated by any such mechanisms. However, the antidiarrhea effects by all the fractions were potentiated when combined with diphenoxylate producing almost 100% reductions of diarrhea.
Conclusion
This study corroborated reported presence of antidiarrheal activity in C. lutea leaves, which are used for humans suffering from diarrheal disorders. The pharmacological study reveals the presence of two types of antidiarrheal bioactive agents. One mediated through α1-adrenoceptor mechanism in chloroform fraction and the other through nonadrenergic mechanism or nonnitric oxide donor (IDN) dependent mechanism in ethyl acetate, ethanol, and n-hexane fractions but by proabsorptive property that promotes faster fluid absorption in the intestine or antisecretary mechanism and/or delayed gastric emptying by antispasmodic action.
Acknowledgements
Dr. Clinelson Martins Rodriques and Dr. Viviani da Silva (Organic Chemistry Dept., UNESP) provided technical assistance in structural isolation and characterization; Mr. Nsikang Malachy and Mrs. Siphon (Pharmacology Department, University of Uyo) assisted in antidiarrheal assay.
Declaration of interest
This study was supported by Niger Delta University Postgraduate Fellowship. Khana Local Government Council, Bori, Ogoni, Nigeria supported the predoctoral fellowship with part payment for airfare; FAPESP provided stipends for accommodation at UNESP, Araraquara.
References
- Adeyemi OO, Akindele AJ. (2008). Antidiarrhoeal activity of the ethyl acetate extract of Baphia nitida (Papilionaceae). J Ethnopharmacol, 116, 407–412.
- Ajibesin KK, Ekpo BA, Bala DN, Essien EE, Adesanya SA. (2008). Ethnobotanical survey of Akwa Ibom State of Nigeria. J Ethnopharmacol, 115, 387–408.
- Akindele AJ, Adeyemi OO. (2006). Evaluation of the antidiarrhoeal activity of Byrsocarpus coccineus. J Ethnopharmacol, 108, 20–25.
- Almeida CE, Karnikowski MG, Foleto R, Baldisserotto B. (1995). Analysis of antidiarrhoeic effect of plants used in popular medicine. Rev Saude Publica, 29, 428–433.
- Arunlakshana O, Schild HO. (1959). Some quantitative uses of drug antagonists. Br J Pharmacol Chemother, 14, 48–58.
- Atta AH, Mouneir SM. (2005). Evaluation of some medicinal plant extracts for antidiarrhoeal activity. Phytother Res, 19, 481–485.
- Awouters F, Niemegeers CJ, Lenaerts FM, Janssen PA. (1978). Delay of castor oil diarrhoea in rats: A new way to evaluate inhibitors of prostaglandin biosynthesis. J Pharm Pharmacol, 30, 41–45.
- Brown JH, Taylor P. (1996). Muscarinic receptor agonists and antagonists In: Hardman JG, Limbird LE, Molinoff PB, Ruddon RW, Gilman AG, eds. Goodman and Gilman’s Pharmacological Basis of Therapeutics 9th ed. New York: McGraw-Hill, 141–160.
- Burkill HM. (1985). The Useful Plants of West Tropical Africa (Vol. 1, 2nd ed.). Kew, UK: Royal Botanic Gardens drugs.
- Burks TF. (1991). Gastrointestinal drugs. In: Kist K, ed. Human Pharmacology: Molecular to Clinical. London, UK: Wolfe Publishing Ltd., 789–800.
- Cervellati, R., Innocenti, G., Dall’Acqua, S., Costa, S., Sartinia, E. (2004). Chemistry and Biodiversity 1, 415–417.
- DiCarlo GD, Mascolo N, Izzo AA, Capasso F, Autore G. (1994). Effects of quercetin on gastrointestinal tract in rats and mice. Phytother Res, 8, 42–45.
- Etukudo I. (2003). Ethnobotany: Conventional and traditional uses of plants. Nigeria: The Verdict Press, p. 111.
- Farthing MJG. (2002). Novel targets for the control of secretary diarrhoeal. Gut, 50, 15–18.
- Heinrich M, Heneka B, Ankli A, Rimpler H, Sticher O, Kostiza T. (2005). Spasmolytic and antidiarrhoeal properties of the Yucatec Mayan medicinal plant Casimiroa tetrameria. J Pharm Pharmacol, 57, 1081–1085.
- Hiradate S, Morita S, Sugie H, Fujii Y, Harada J. (2004). Phytotoxic cis-cinnamoyl glucosides from Spiraea thunbergii. Phytochemistry, 65, 731–739.
- Irvine FR. (1961). Woody Plants of Ghana. London, UK: Oxford University Press, pp. 1–868.
- Iwu MM, Anyanwu BN. (1982). Phytotherapeutic profile of Nigerian herbs. I: Anti-inflammatory and anti-arthritic agents. J Ethnopharmacol, 6, 263–274.
- Izzo AA. (1994). Effects of quercetin on gastrointestinal tract: Further studies. Phytother Res, 8, 179–185.
- Izzo AA, Mascolo N, Capasso F. (1998). Nitric oxide as a modulator of intestinal water and electrolyte transport. Dig Dis Sci, 43, 1605–1620.
- Izzo AA, Mascolo N, Capasso R, Germanò MP, De Pasquale R, Capasso F. (1999). Inhibitory effect of cannabinoid agonists on gastric emptying in the rat. Naunyn Schmiedebergs Arch Pharmacol, 360, 221–223.
- Jaffe BM. (1979). Prostaglandins and serotonin: Nonpeptide diarrheogenic hormones. World J Surg, 3, 565–578.
- Katzung BG, Chatterjee K. (2001). Vasodilators and treatment of angina pectoris-basic pharmacology of drugs used to treat angina. In: Katzung BG, ed. Basic Clinical Pharmacology. New York: McGraw Hill Company Inc. 183–189.
- Latza S, Ganser D, Berger RG. (1996). Identification accumulation of 1-d-glucopyranose in developing strawberry fruit (Fragaria ananassa Duch. Cv. Kent). J. Agric and Food Chem, 44, 1367–1370.
- Lewis WH, Elvin-Lewis MP. (1977). Medical Botany. New York: John Wiley and Sons, pp.1–51.
- Luderer JR, Dermers IM, Hayes AH Jr. (1980). Advances in Prostaglandin and Thromboxane Research. New York: Raven Press, 1633–1638.
- Lutterodt GD. (1989). Inhibition of gastrointestinal release of acetylcholine by quercetin as a possible mode of action of Psidium guajava leaf extracts in the treatment of acute diarrhoeal disease. J Ethnopharmacol, 25, 235–247.
- Martinez H, Ryan GW, Guiscafre H, Gutierrez G. (1998). An intercultural comparison of home case management of acute diarrhea in Mexico: Implications for program planners. Arch Med Res, 29, 351–360.
- Mascolo N, Angel AI, Autore G, Barbato F, Capasso F. (1994). Nitricoxide and castor oil-induced diarrhoea. J. Pharmacol. Exp. Ther. 263:291–295
- Miura M, Lawson DC, Clary EM, Mangel AW, Pappas TN. (1999). Central modulation of rectal distension-induced blood pressure changes by alosetron, a 5-HT3 receptor antagonist. Dig Dis Sci, 44, 20–24.
- Muanya CA. (2008). Natural health-how local plants help boost libido, by researchers, The Guardian, Nigerian Newspaper Ltd. p 24.
- Muanya CA, Odukoya OA (2008). Lipid peroxidation as index of activity in aphrodisiac herbs. J. Plant Sci, 3(1), 92–98.
- Nwafor PA, Bassey AI. (2007). Evaluation of anti-diarrhoeal and anti-ulcerogenic potential of ethanol extract of Carpolobia lutea leaves in rodents. j Ethnopharmacol, 111, 619–624.
- Nwidu LL, Nwafor PA. (2009). Gastroprotective effects of leaf extracts of Carpolobia lutea (Polygalaceae) G. Don. in rats. African J. Biotech, 8(12), 15–19.
- Nwodo OF, Alumanah EO. (1991). Studies on Abrus precatorius seeds. II: Antidiarrhoeal activity. J Ethnopharmacol, 31, 395–398.
- Pierce NF, Carpenter CC Jr, Elliott HL, Greenough WB 3rd. (1971). Effects of prostaglandins, theophylline, and cholera exotoxin upon transmucosal water and electrolyte movement in the canine jejunum. Gastroenterology, 60, 22–32.
- Robert A. (1973). Prostaglandins and gastric secretion. Res Prostaglandins, 2, 1–4.
- Robert A, Nezamis JE, Lancaster C, Hanchar AJ, Klepper MS. (1976). Enteropooling assay: A test for diarrhea produced by prostaglandins. Prostaglandins, 11, 809–828.
- Rodrigues CM, Rinaldo D, dos Santos LC, Montoro P, Piacente S, Pizza C, Hiruma-Lima CA, Brito AR, Vilegas W. (2007). Metabolic fingerprinting using direct flow injection electrospray ionization tandem mass spectrometry for the characterization of proanthocyanidins from the barks of Hancornia speciosa. Rapid Commun Mass Spectrom, 21, 1907–1914.
- Yadav AK, Tangpu V. (2007). Antidiarrheal activity of Lithocarpus dealbata and Urena lobata extracts: Therapeutic implications. Pharm Biol, 45, 223–229.
- Wagner H, Bladt S, Zgainsky EM. (1984). Plant Drug Analysis. Berlin: Springer Berlin Heidelberg, p. 320 (1984).