Abstract
Context: Jacaranda decurrens subsp. symmetrifoliolata Farias & Proença (Bignoniaceae) is a species widely used for their medicinal properties. At least to our known, no study has been conducted concerning its toxicological profile after gestational and lactational exposure.
Objective: The present study was carried out to evaluate the effects of J. decurrens on development of the reproductive system in male rats.
Materials and methods: Pregnant rats were treated daily (gavage) with 250 or 500 mg/kg/day of aqueous extract of J. decurrens or vehicle, from day 12 of pregnancy to day 21 of lactation.
Results and Discussion: Both doses of J. decurrens significantly anticipated (p < 0.05) the age of testicular descent to the scrotum, a parameter indicative of puberty initiation. Furthermore, at puberty, there was a significant reduction (p < 0.05) in testicular and epididymis weights in the offspring exposed to the higher dose of extract, without effect on sperm production and the histology of reproductive organs. On the other hand, at adulthood, the reproductive parameters analyzed did not differ among groups.
Conclusions: J. decurrens, in this experimental model, interfered with the initial development of the reproductive system, but without lasting effects on sperm production in adulthood.
Introduction
Phytotherapy is becoming an alternative for the treatment of various diseases (CitationCoulter & Willis,2004)Citation. However, few plants selected for use have been scientifically studied to assess their quality, safety, and efficacy (CitationCalixto, 2005).
One of the factors that favor an increase in the use of plants is the fact that their adverse effects are much less marked when compared to synthetic drugs, which makes these substances very attractive to pregnant women. However, clinical studies have revealed adverse effects of medicinal plants (CitationIkegami et al., 2003; CitationSowemimo et al., 2007) and several species have been associated with significant reproductive changes (CitationCapasso et al., 2000; CitationErnst & Pittler, 2002), such as reduced fertility, miscarriages, and uterine stimulation, among others (CitationFarnsworth et al., 1975; CitationAlmeida & Lemonica, 2000; CitationMazaro et al., 2002; CitationGupta et al., 2006).
Jacaranda decurrens subsp. symmetrifoliolata Farias & Proença (Bignoniaceae), popularly known as “carobinha-do-campo,” “carobinha” or “caroba”, is an endemic species of the savanna-like formation found in the Brazilian states of Goias, Mato Grosso, Mato Grosso do Sul, Minas Gerais and São Paulo (CitationMauro et al., 2007). According to folk medicine, tea prepared from its root is used as a blood cleanser, for wound healing in the uterus and ovary, prostate inflammation, and allergies (CitationSangalli, 2000; CitationNunes et al., 2003).
Pharmacological evaluation revealed that different species of Jacaranda possess antitumor, cytotoxic, anthelmintic, antibacterial and anti-hypertensive properties (CitationOgura et al., 1977; CitationWeniger et al., 2001; CitationNicasio & Meckes, 2005; CitationZatta et al., 2009). Furthermore, Jacaranone was demonstrated to be the compound responsible for the cytotoxic and antitumor activity of these species (CitationOgura et al., 1977).
Despite the wide use of this plant in folk medicine, few studies have examined the toxic effects of the species J. decurrens. CitationZatta et al. (2009) concluded that the crude ethanol extract of leaves of J. decurrens showed no acute toxicity in rats and mice. However, no study was conducted to evaluate the possible effects of this plant if employed during pregnancy and lactation. Thus, this investigation aimed to evaluate the effects of the aqueous extract of J. decurrens on the development of the reproductive system in male rats exposed during pregnancy and lactation.
Materials and methods
Plant material
J. decurrens subsp. symmetrifoliolata roots were collected in the Horto de Plantas Medicinais of the Federal University of Grande Dourados (UFGD) between April and May 2008. A voucher specimen was identified by Dr. Rosana Farias Singer and deposited (register: W.G. Garcia 14.008) in the herbarium of the Department of Botany at the Biology Institute of the State University of Campinas (UNICAMP).
Preparation of the extracts
The roots of J. decurrens were washed with water dried and prepared by adding 500 ml of distilled water to 500 g of plant material at 95°C and leaving to macerate for 24 h. After this period, the sample was dried in a desiccator, resulting in the final plant extract.
Animals and obtainment of pregnant females
Adult male (90 days old, weighing 300g, n = 7) and female (60 days old, weighing 200 g, n = 25) Wistar rats were maintained in polypropylene cages (43 × 30 × 15 cm) with bedding of wood shavings, two animals/cage, under controlled temperature (23°C), with a constant 12 h light-dark cycle and free access to food and water. The rats were allowed to mate during the dark period of the cycle, by placing two females in the box with each male. Gestational day 0 (GD 0) was determined by the presence of sperm in vaginal smears of females in estrus. The pregnant or lactating rats were weighed on alternating days to permit calculation of the extract volume to be administered and to investigate clinical signs of maternal toxicity. Consumption of water and chow by mothers during the experimental period was registered. After birth, the number of pups per litter was reduced to eight, in order to maintain, preferentially, male offspring. Experimental procedures were in accord with the Ethical Principles in Animal Research adopted by the Brazilian College of Animal Experimentation and approved by the Committee for Ethics in Animal Experimentation at the Centro Universitário da Grande Dourados (UNIGRAN) (Protocol number: 332/08).
Experimental design
Pregnant rats were treated orally (gavage) with 250 or 500 mg/kg/day of aqueous extract of J. decurrens, from gestational day (GD) 12 until postnatal day (PND) 21, which corresponds to the critical period of sexual differentiation in rats (CitationSommer et al., 1996; CitationMcIntyre et al., 2003). The dose regimens were based on previous studies (CitationMazaro et al., 2002; CitationYakubu et al., 2007). Control rats received only distilled water (vehicle).
Reproductive development of male offspring
At birth and at 22 days of age, 10 male rats per group, one or two per litter were weighed. In order to confirm the age of puberty initiation, these same animals were examined for testicular descent by scrotal palpation, starting from PND 15. Preputial separation also was investigated, starting from PND 33, by manual retraction of the prepuce.
Body weight and weight of reproductive organs
At 60 (puberty) and 90 (adult) days old, rats from different experimental groups (10 animals/group, one or two/litter), were anesthetized, weighed and killed by decapitation. The left testis and epididymis, vas deferens, ventral prostate and seminal vesicle (without the coagulating gland and full of secretion) were removed and their weights (absolute and relative to body weights) were determined. Testis and epididymis were used for sperm counts.
Daily sperm production per testis, sperm counts, and transit time in the epididymis
Homogenization-resistant testicular spermatids (Stage 19 of spermiogenesis) and sperm in the caput/corpus and cauda epididymis were enumerated as described previously by CitationRobb et al. (1978), with adaptations adopted by CitationFernandes et al. (2007). Briefly, each left testis, decapsulated and weighed soon after collection, was homogenized in 5 mL of NaCl 0.9% containing Triton X 100 0.5%, followed by sonication for 30 s. After a 10-fold dilution a sample was transferred to Newbauer chambers (4 fields per animal), preceding a count of mature spermatids. To calculate daily sperm production (DSP) the number of spermatids at stage 19 was divided by 6.1, which is the number of days of the seminiferous cycle in which these spermatids are present in the seminiferous epithelium. In the same manner, caput/corpus and cauda epididymidis portions were cut into small fragments with scissors and homogenized, and sperm counted as described for the testis. The sperm transit time through the epididymis was determined by dividing the number of sperm in each portion by the DSP.
Sperm morphology
For evaluation of sperm morphology, the interior of the left vas deferens of mature rats was washed, by the aid of a syringe and needle, with 1.5 ml of saline solution, after which were prepared histological slides. Two hundred spermatozoa (heads only or intact sperm) per animal were evaluated for head and/or flagellar defects by phase-contrast microscopy (×200, total magnification) in wet preparations (CitationFiller, 1993).
Histopathological evaluation
The right testis and epididymis in five animals per group were removed and fixed in Alfac fixing solution (80% ethanol, formaldehyde and glacial acetic acid, 8.5:1.0:0.5, v/v) for 24 h. The pieces were embedded in paraffin wax and sectioned at 7 µm. The sections were stained with hematoxylin and eosin (H&E) and observed by light microscopy for general histopathological examination.
Statistical analysis
For comparison of results among the three experimental groups, statistical tests for analysis of variance were utilized—ANOVA, with the “a posteriori” Tukey-Kramer test. Differences were considered significant when p < 0.05. The statistical analyses were performed by GraphPad InStat (version 3.02).
Results
No changes were observed in the health status or behavior of pregnant or nursing rats treated with 250 or 500 mg/kg of extract of J. decurrens. Similarly, the consumption of food and water were not altered by treatment (data not shown).
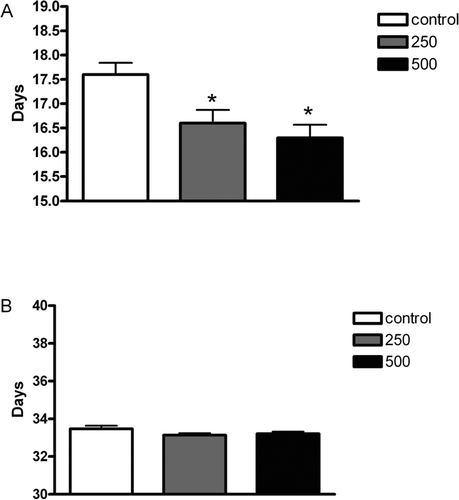
J. decurrens exposure throughout pregnancy and lactation did not affect the body weight of male offspring at birth and at weaning (Day 0: Control = 5.96 ± 0.10 g; 250 mg/kg = 6.13 ± 0.04 g; 500 mg/kg = 5.80 ± 0.10 g; Day 22: Control = 44.93 ± 0.67 g; 250 mg/kg = 44.12 ± 0.60 g; 500 mg/kg = 44.68 ± 1.81 g; n = 10). On the other hand, both doses of J. decurrens brought forward the age of testicular descent to the scrotum in male rats. However, these rats did not present differences in the age of preputial separation in relation to the control group ().
Figure 1. Sexual development landmarkers of the male offspring rats exposed to 250 or 500 mg/kg/day of J. decurrens in utero and during lactation (see text). Values expressed as mean ± SEM of 10 animals group. *p < 0.05 (ANOVA—Tukey—Kramer test) compared with control group.
The exposure to a higher dose of J. decurrens provoked a statistically significant reduction in the absolute and relative weights of testis and epididymis in animals at 60 days of age (puberty) (). However, in adult life (at 90 days of age), no difference was observed in this parameter among experimental groups (). Furthermore, the animals exposed to the higher dose of J. decurrens presented a reduction in the relative weight of vas deferens at 60 days of age, while in the adult animals a reduction was seen in the absolute weight of this organ after exposure to both doses.
Table 1. Final body weight, absolute and relative organs weights of male rats at 60 days exposed to 250 or 500 mg/kg/day of J. decurrens in utero and during lactation.
Table 2. Final body weight, absolute and relative organs weights of male rats at 90 days exposed to 250 or 500 mg/kg/day of J. decurrens in utero and during lactation.
After sperm counts in the testis, no change was observed in the daily sperm production at 60 and 90 days of age in the animals exposed to J. decurrens. Similarly, the sperm number and the sperm transit time through the epididymis were not affected by treatment ( and ).
Table 3. Sperm parameters of male rats at 60 days exposed to 250 or 500 mg/kg/day of J. decurrens in utero and during lactation.
Table 4. Sperm parameters of male rats at 90 days exposed to 250 or 500 mg/kg/day of J. decurrens in utero and during lactation.
Morphological analysis of sperm extracted from the vas deferens of 90-day-old rats showed that the percentages of normal (Control = 89.21%; 250 mg/kg = 89.33%; 500 mg/kg = 87.40%; n = 10) and abnormal forms were similar among groups. In the same way, the analysis of testis and epididymis in the light microscope did not reveal any morphological changes related to the treatment (data not shown).
Discussion
Adverse developmental outcomes in either sex can result from exposure to toxic agents in utero, through contact with exposed dams, or through contaminated milk (U.S. Environmental Protection Agency [U.S. EPA], 1996). Thus, the aim of the present work was to evaluate the effects on reproductive development in male rats whose mothers were exposed to J. decurrens during pregnancy and lactation.
To evaluate the action of a substance on a progenitor, mortality is a clear sign of toxicity, however, other variables may be indicative of more subtle adverse effects, such as loss of body mass during the treatment and clinical signs of toxicity (diarrhea, piloerection, changes in behavior, and so on; CitationMuller, 2007). Females exposed to the aqueous extract of J. decurrens did not exhibit clinical signals of toxicity, indicating an absence of maternal toxicity at the doses tested.
Some of the variables recommended for the evaluation of chemical endocrine substance interference in sexual development are testicular descent and biomarkers of the onset of puberty, which, in male rats, is analyzed by the average time (days) for preputial separation. Compounds that destabilize the hormonal balance can interfere in these processes, accelerating or delaying them (U.S. EPA, 1996). In the present study, treatment with J. decurrens (250 or 500 mg/kg) in the perinatal period did not affect the body weight of male offspring at birth and at 22 days of age. However, both doses of J. decurrens caused the age of testicular descent in the offspring to be brought forward, indicating interference of the treatment in the hormonal balance during critical stages of pregnancy. By contrast, these offspring did not present differences in the age of preputial separation, another important marker of the initiation of puberty.
Changes in the wet weight of androgen-dependent reproductive organs, such as the testis, epididymis, seminal vesicle and prostate are used as parameters to indicate changes in the levels of sex hormones (CitationZenick et al., 1994). In the case of the testis, the absolute weight is used in preference to relative weight since in normal adult male the weight of the testes and body weight are independent variables (CitationAmann, 1982; CitationZenick & Clegg, 1989). Exposure to the higher dose of J. decurrens throughout pregnancy and lactation caused a reduction in the absolute and relative weights of testis and epididymis in pubertal animals. However, over the course of sexual development the weights of these organs became normalized, since in adult life no difference was detected in this parameter in relation to the control group. Furthermore, the weight of the vas deferens was lower in pubertal animals exposed to the higher dose and in adult animals exposed to both doses of J. decurrens.
In the last few years, studies have indicated a decline in sperm quality (and especially in the sperm count) in a variety of species including the human population. Possible causes for this fact include the estrogenic and/or antiandrogenic activity of various environmental pollutants (CitationSharpe & Skakkebaek, 1993; CitationJensen et al., 1995; CitationSafe, 1995; CitationToppari et al., 1996; CitationCooper & Kavlock, 1997; CitationIrvine, 1997). When investigating the effects of chemicals on the male reproductive system the number and quality of sperm are important parameters (CitationNeubert, 1997). A toxic agent can affect the maturation, function and survival of sperm by a direct action on the sperm or by altering epididymal function (CitationZenick et al., 1994).
It has been demonstrated that several medicinal plants can interfere in sperm production and/or in sperm number in the epididymis, compromising the fertility of exposed animals (CitationFarnsworth et al., 1975; CitationMazaro et al., 2000; CitationGupta et al., 2007). For example, CitationYakubu et al. (2007) showed that the aqueous extract of Chromolaena odoratum, at doses of 250 and 500 mg/kg, possesses antiandrogenic activity, since it reduced the sperm count in male rats exposed orally for 14 days. By contrast, in the present study there was no change in the sperm count in testis and epididymis of pubertal and adult animals exposed to J. decurrens during pregnancy and lactation. Although a change was observed in the age of testicular descent and in the weights of testis and epididymis of pubertal animals, we found no evidence that the aqueous extract of J. decurrens acted as an endocrine disrupter during reproductive development, since the weights of reproductive organs and the sperm count were found to be normal in adult animals. However, fertility studies should be performed in exposed animals, through natural mating, to assess the sperm quality.
Examination of sperm morphology is used in the detection of possible spermatotoxic effects (U.S. EPA, 1986). In the present work, exposure to the extract of J. decurrens did not alter the sperm morphology in adult animals. Similarly, histopathological analysis did not reveal changes in testicular or epididymal structure in pubertal and adult animals exposed to the extract throughout pregnancy and lactation.
It may be concluded that the aqueous extract of J. decurrens, in this experimental model, interfered with the onset of reproductive development in male offspring, without compromising the sperm production in adult life. Considering that human reproductive parameters are more susceptible to damage from toxic agents than those of the test species, additional studies are needed to assess the safety of this plant in pregnant women.
Acknowledgments
This work was supported by grants from FUNDECT (Foundation to Support the Development of Education, Science and Technology of the State of Mato Grosso do Sul).
Declaration of interest
The authors report no conflicts of interest.
References
- Almeida FC, Lemonica IP. (2000). The toxic effects of Coleus barbatus B. on the different periods of pregnancy in rats. J Ethnopharmacol, 73, 53–60.
- Amann RP. (1982). Use of animal models for detecting specific alterations in reproduction. Fundam Appl Toxicol, 2, 13–26.
- Calixto JB. (2005). Twenty-five years of research on medicinal plants in Latin America: A personal view. J Ethnopharmacol, 100, 131–134.
- Capasso R, Izzo AA, Pinto L, Bifulco T, Vitobello C, Mascolo N. (2000). Phytotherapy and quality of herbal medicines. Fitoterapia, 71, 58–65.
- Cooper RL, Kavlock RJ. (1997). Endocrine disruptors and reproductive development: A weight-of-evidence overview. J Endocrinol, 152, 159–166.
- Coulter ID, Willis EM. (2004). The rise and rise of complementary and alternative medicine: A sociological perspective. Med J Aust, 180, 587–589.
- Ernst E, Pittler MH. (2002). Risks associated with herbal medicinal products. Wien Med Wochenschr, 152, 183–189.
- Farnsworth NR, Bingel AS, Cordell GA, Crane FA, Fong HH. (1975). Potential value of plants as sources of new antifertility agents. Indian. J Pharm Sci, 64, 535–598.
- Fernandes GS, Arena AC, Fernandez CD, Mercadante A, Barbisan LF, Kempinas WG. (2007). Reproductive effects in male rats exposed to diuron. Reprod Toxicol, 23, 106–112.
- Filler R. (1993). Methods for evaluation of rat epididymal sperm morphology. In: Chapin RE, Heindel JD, eds. Male Reproductive Toxicology. Academic Press Inc, San Diego, CA, 334–343.
- Gupta RS, Kachhawa JB, Chaudhary R. (2006). Antispermatogenic, antiandrogenic activities of Albizia lebbeck (L.) Benth bark extract in male albino rats. Phytomedicine, 13, 277–283.
- Gupta RS, Kachhawa JB, Sharma A. (2007). Effect of methanolic extract of Dendrophthoe falcata stem on reproductive function of male albino rats. J Herb Pharmacother, 7, 1–13.
- Ikegami F, Fujii Y, Ishihara K, Satoh T. (2003). Toxicological aspects of Kampo medicines in clinical use. Chem Biol Interact, 145, 235–250.
- Irvine DS. (1997). Declining sperm quality: A review of facts and hypotheses. Baillieres Clin Obstet Gynaecol, 11, 655–671.
- Jensen TK, Toppari J, Keiding N, Skakkebaek NE. (1995). Do environmental estrogens contribute to the decline in male reproductive health? Clin Chem, 41, 1896–1901.
- Mauro C, Pereira AMS, Silva CP, Missima J, Ohnuki T, Rinaldi RB. (2007). Anatomical study of species from savanna-like formation, Anemopaegma arvense (Vell.) Stellf. ex de Souza (catuaba), Zeyheria montana Mart. (bolsa-de-pastor) and Jacaranda decurrens Chamisso (caroba)—Bignoniaceae. Rev Bras Farmacogn, 17, 262–265.
- Mazaro R, Di Stasi LC, Vieira Filho AS, Kempinas WG. (2000). Decrease in sperm number after treatment of rats with Austroplenckia populnea. Contraception, 62, 45–50.
- Mazaro R, Di Stasi L, Kempinas WG. (2002). Effects of the hydromethanolic extract of Austroplenckia populnea (Celastraceae) on reproductive parameters of male rats. Contraception, 66, 205–209.
- McIntyre BS, Vancutsem PM, Treinen KA, Morrissey RE. (2003). Effects of perinatal loratadine exposure on male rat reproductive organ development. Reprod Toxicol, 17, 691–697.
- Muller JC. (2007). Toxicidade reprodutiva da Morinda citrifolia Linn. Dissertação (Mestrado—Área de Concentração em Farmacologia)—Universidade Federal do Paraná. 88.
- Neubert D. (1997). Vulnerability of the endocrine system to xenobiotic influence. Regul Toxicol Pharmacol, 26, 9–29.
- Nicasio P, Meckes M. (2005). Hypotensive effect of the hydroalcoholic extract from Jacaranda mimosaefolia leaves in rats. J Ethnopharmacol, 97, 301–304.
- Nunes GP, Silva MF, Resende UM, Siqueira JM. (2003). Plantas medicinais comercializadas pelos raizeiros no Centro de Campo Grande-MS. Rev Bras Farmacogn, 13(2), 83–92.
- Ogura M, Cordell GA, Farnsworth R. (1977). Potential anticancer agents. IV. Constituents of Jacaranda caucana Pittier (Bignoniaceae). Lloydia, 40, 157–168.
- Robb GW, Amann RP, Killian GJ. (1978). Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J Reprod Fertil, 54, 103–107.
- Safe SH. (1995). Environmental and dietary estrogens and human health: Is there a problem? Environ Health Perspect, 103, 346–351.
- Sangalli A. (2000). Levantamento e caracterização de plantas nativas com propriedades medicinais em fragmentos florestais e de Cerrado de Dourados-MS, numa visão etnobotânica. Monografia (Curso de Ciências Biológicas)—Universidade Federal do Mato Grosso do Sul, Dourados. 70.
- Sharpe RM, Skakkebaek NE. (1993). Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? Lancet, 341, 1392–1395.
- Sommer RJ, Ippolito DL, Peterson RE. (1996). In utero and lactational exposure of the male Holtzman rat to 2,3,7,8-tetrachlorodibenzo-p-dioxin: Decreased epididymal and ejaculated sperm numbers without alterations in sperm transit rate. Toxicol Appl Pharmacol, 140, 146–153.
- Sowemimo AA, Fakoya FA, Awopetu I, Omobuwajo OR, Adesanya SA. (2007). Toxicity and mutagenic activity of some selected Nigerian plants. J Ethnopharmacol, 113, 427–432.
- Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ Jr, Jégou B, Jensen TK, Jouannet P, Keiding N, Leffers H, McLachlan JA, Meyer O, Müller J, Rajpert-De Meyts E, Scheike T, Sharpe R, Sumpter J, Skakkebaek NE. (1996). Male reproductive health and environmental xenoestrogens. Environ Health Perspect, 104 Suppl 4, 741–803.
- U.S. Environmental Protection Agency. (1986). Guidelines for estimating exposures. Federal Register, 51(185), 34042–34054.
- U.S. Environmental Protection Agency. (1996). Guidelines for reproductive toxicity risk assessment. EPA/630/R-96/009, Washington, DC.
- Weniger B, Robledo S, Arango GJ, Deharo E, Aragón R, Muñoz V, Callapa J, Lobstein A, Anton R. (2001). Antiprotozoal activities of Colombian plants. J Ethnopharmacol, 78, 193–200.
- Yakubu MT, Akanji MA, Oladiji AT. (2007). Evaluation of antiandrogenic potentials of aqueous extract of Chromolaena odoratum (L.) K. R. leaves in male rats. Andrologia, 39, 235–243.
- Zatta DT, Pimenta FC, Tres Venzol LMF, Fiuza TS, Bara MTF, Cunha LC, Pucci LL, Garrote CFD, Oliveira FNM, Paula JR. (2009). Estudo da Atividade Antibacteriana contra cepas de Pseudomonas aeruginosa e da Toxicidade Aguda das folhas da Jacaranda decurrens. Lat Am J Pharm, 28, 485–489.
- Zenick H, Clegg ED. (1989). Assessment of male reproductive toxicity: A risk assessment approach. In: Hayes W, ed. Principles and Methods of Toxicology. New York: Raven, 275–309.
- Zenick H, Clegg ED, Perreault SD, Klinefelter GR, Gray LE. (1994). Assessment of male reproductive toxicity: A risk assessment approach. In: Hayes W, ed. Principles and Methods of Toxicology. New York: Raven, 937–988.