Abstract
Context: The rhizome of Wikstroemia indica (L.) C. A. Mey (Thymelaeaceae) is widespread in China which has been widely used in China as folk medicine for the treatment of syphilis, arthritis, whooping cough, and cancer. Due to its multiactivities, its extract has an attractive potential as a promising natural agent in the pharmaceutical industries.
Objective: Aims of this study were to optimize the extraction process of the flavonoids from W. indica, and evaluate its multiple activities.
Materials and methods: An orthogonal test design was employed to optimize the extraction procedure of flavonoids from W. indica. And multichromatography and spectroscopy were used to study the chemical compounds of W. indica, while several bioactivity assays were used to evaluate the antibacterial, anti-inflammatory, and antitumor activities of W. indica.
Results: Optimal extraction conditions were determined: ethanol concentration was 60%; extraction time was 60 min; liquid–solid ratio was 16:1 and the power of ultrasonic instrument was 160 W. Four compounds: daphnoretin, chrysophanol, myricitrime and rutin were purified from W. indica, and chrysophanol was identified from this plant for the first time. The extract of W. indica displayed significant antimicrobial and anti-inflammatory activities. Daphnoretin showed a significant inhibition effect on CNE cells and HeLa cells lines at the concentrations ranging from 15.6 to 125 μg/mL, the tendency of antitumor effect was displayed in a concentration-dependent manner.
Discussion and conclusions: Extracts of W. indica could potentially be used as a promising natural agent in the pharmaceutical industries.
Introduction
The genus Wikstroemia (Thymelaeaceae) includes ∼70 species with a ubiquitous distribution in Southeastern Asia, Oceania, and Pacific islands. There are more than 40 species distributed in China Wikstroemia indica (L) C. A. Mey (W. indica) is regionally abundant in Zhejiang, Jiangxi, Fujian, Hunan, and Guangdong provinces of South China.
The rhizome of W. indica, named ‘Liao Ge Wang,’ has been widely used in China both as food and folk medicine for the treatment of syphilis, arthritis, whooping cough, and cancer (CitationKato, 1979). China Southerners used to add a little of ‘Liao Ge Wang’ to soup for healthcare when they boil broth. Previous studies demonstrated therapeutic potentialities of W. indica such as anti-inflammatory (CitationWang et al., 2005b), antiviral (CitationHu et al., 2000), antitumor (CitationLee et al., 1981; CitationHu et al., 2000), and antimalarial activities (CitationNunome et al., 2004). Chemical investigations showed there are many constituents in W. indica, including flavonoids, biflavones, coumarin, and lignans compounds (CitationKato, 1979; CitationChen et al., 2009), daphnoretin (CitationKato, 1979), bis-5,5-nortrachelogenin (CitationWang et al., 2005a), and d-primev-ersyl genkwaine (CitationGeng et al., 2006), etc.
The antibacterial activity of W. indica has been reported previously, but the constituents which have antibacterial activity were not clarified yet. As one of the characteristic compositions of W. indica, flavonoids constitutes an important nutrient component of human diet due to its reported multiple bioactivities (CitationCarlo et al., 1999). Exploration was made in the present research to determine whether there is certain correlation existing between the crude flavonoids and its antimicrobial activity. Firstly, flavonoids were extracted from W. indica with the help of orthogonal test; secondly, the antimicrobial activity of the crude flavonoids was investigated in vitro.
Moreover, the evaluation of anti-inflammatory activity of W. indica together with chemical studies on active fractions was implemented. Daphnoretin, a compound of coumarin, is considered as one of the main compounds of antitumor activity. To increase awareness of its antitumor activity, several tumor cell lines, which were not studied before, were used to evaluate the cytotoxicity of daphnoretin.
Materials and methods
Plant material
The dried rhizome of the W. indica, was purchased (25 December 2006) at Qingping market for Chinese medicinal material of Guangzhou, China. The original plant of the material was authenticated as W. indica (L.) C. A. Mey, and the voucher specimen is stored in Department of Natural Products Studies, School of Light Chemistry and Food Science, South China University of Technology.
Optimization of ultrasonic-assisted extraction of flavonoids
An orthogonal test design (CitationPeng et al., 2006) was employed to optimize the procedure of ultrasound-assisted extraction of flavonoids from W. indica. According to the results of preliminary experiments, four independent variables, with three levels, had been selected (). Nine extractions were carried out, under the experimental conditions shown in . The extraction ratio of flavonoids was quantified by measuring the absorbance of test samples at 510 nm using UV-Vis spectrophotometer (CitationZhang et al., 2008a).
Table 1. Factors and levels of the orthogonal test design.
Table 2. Orthogonal test design and the experimental ratio of extraction.
By the assistance of ultrasound, ∼50 g powder of W. indica was extracted under corresponding conditions and filtered through 0.45-μm filter paper, and was extracted for two times. Then, the filters of the two extractions were combined and concentrated using a rotary evaporator at 45°C. The obtained dry powder was dissolved by ethanol (30%) to a defined volume (1,000 mL), and kept at 4°C till analysis. Standard calibration curves were prepared using the solution of rutin (0.4 mg/mL) as standard in series of volume (1, 2, 3, 4, and 5 mL). Then added 12.5 mL ethanol (concentration was 30%) and 0.7 mL Al(NO3)3 (0.1 g/mL) to each volumetric flask; 6 min late, added 5 mL NaOH (1 mol/mL), then diluted to defined volume (25 mL) with ethanol (30%). The absorbance of the sample at 510 nm was measured by UV-Vis spectrophotometer which was used to calculate the quality of flavonoids based on the analysis of the standard curve.
Antimicrobial activity of the crude flavonoids
The crude flavonoids from W. indica extracted in the best suitable conditions (prepared in series of concentrations 0.85, 0.425, and 0.106 g/mL) was individually assayed against a panel of microorganisms, including Staphylococcus aureus, Escherichia coli, and Mucor racemosus. The agar well diffusion method (CitationTyagi & Malik, 2011) was intended for the determination of antimicrobial activities of the W. indica.
The strains of bacteria used in this study were grown in Mueller–Hinton broth at 37°C for 24 h, and fungi was grown in Potato Dextrose Broth medium at 28°C for 48 h. At the end of the incubation, the cultures were centrifuged at 4,000 r.p.m. for 15 min to pellet the cells. The pellets were suspended in sterile distilled water (∼1.5 × 106 colony-forming unit/ mL, obtained following a 0.5 McFarland turbidity standard). About 0.2 mL suspension of the tested microorganism was spread on the solid media plates (∼18 mL). Four holes were dug by punch (6 mm) and infused with solution of extract with different concentrations. The diameters of the inhibition zones were measured and expressed in millimeters. All tests were applied in triplicate.
The minimum inhibitory concentration (MIC) of W. indica was determined by the broth microdilution method (CitationPuglisi et al., 2009). MIC was defined as the lowest concentration of extract at which there was no visible growth, bacterium incubated at 37°C for 24 h, mycetes at 28°C for 48 h.
Anti-inflammatory activity of the crude extracts and its fractions
The anti-inflammatory activity of the W. indica was evaluated against nitrogen monoxidum (NO) release, based on the production of nitric oxide in mice RAW264.7 stimulated by lipopolysaccharide (LPS), according to literature (CitationZhou et al., 2009). Diluted RAW 264.7 cells (200 μl) culture at 5 × 105 cells/mL were seeded in 96-well plates and allowed cell number at 1 × 105 cells/mL. After 1-h incubation, each well was added with some volume of appropriate media containing LPS and 0.4 μl of sample concentrations, leading to the final LPS concentration of 1 μg/mL and series of sample concentrations. Meanwhile, the ranks only with LPS and only with dimethyl sulfoxide (without both LPS and sample) were set in the same plate as blank. Each sample had four parallel wells in a rank.
After 24-h incubation at 37°C in CO2 incubator, samples (100 μl) of cell culture supernatants were taken and incubated with 100 μl of Griess reagent (1:1; 1% sulfanilic amide, 0.1% N-1-naphthyl-ethylenediamine dihydrochloride in 3% phosphoric acid solution) at room temperature for 10 min in 96-well plates. Absorbance at 540 nm was read using an ELISA plate reader (Biotek, City of Winooski, VT, USA). Standard calibration curves were prepared using sodium nitrite as standard in series of concentrations (1, 5, 10, and 50 μmol/mL) of NaNO2. The NO2− concentrations in cell culture supernatants and the inhibitory rates of NO release were calculated on the standard curve.
Extraction and isolation of chemical compounds
The air dried root bark of W. indica (3.0 kg) were powdered and extracted with 95% EtOH three times under reflux. The crude extract (257.6 g) obtained by vacuum concentration was suspended in water, and partitioned with petroleum ether, ethyl acetate (EtOAc) and n-butanol, successively, to afford 5.7, 83.5, and 90.2 g of extracts respectively, and 78.2 g water-layer residue. Considering the anti-inflammatory activities and the weight of each fraction, ethyl acetate fraction was the active fraction and been chosen for the following isolation. The EtOAc fraction (83.5 g) was subjected to silica gel column eluted with petroleum ether-ethyl acetate (100:0–0:100) to afford eight fractions. Fraction 2 was followed by recrystallization to give compound 1 (12 mg). Fraction 4 was applied to silica gel column with cyclohexane-acetone as elute to provide compound 2 (8 mg). In addition, the n-butanol fraction was experimented by silica gel column eluted with chloroform–methanol (100:0–0:100) to afford 10 fractions. Fraction 6 (using chloroform–methanol 7:3 as elute) was repeatedly chromatographed on sephadex-LH 20 to lead to the isolation of compounds 3 (15 mg) and 4 (11 mg).
Cytotoxicity assay of daphnoretin
The cytotoxicity of daphnoretin was detected using 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) assay (CitationZhang et al., 2008b; CitationHu et al., 2008). Exponentially growing cells (8 × 104 cells/mL, 100 μl) were seeded in 96-well microculture plates and attached for 24 h, then the supernatant was removed. Daphnoretin (125, 62.5, 31.25, 15.625, 7.18125, 3.9, 1.95, 0.975 μg/mL, final concentrations) (100 μl) was seeded in each well for 72 h at 37°C. Blanks were treated with the same amount of DMSO as used in the corresponding experiments. Surviving cells were detected based on their ability to metabolize MTT into formazan crystals. Optical density at 570 nm was used as a measure of cell viability. Cell curvival was calculated as the fraction of cells alive relative to control for each point through the following formula:
where OD represents the optical density at 570 nm.
Results and discussion
Optimization extraction of flavonoids
Ultrasonic-assisted extraction is an extraction technique that combines ultrasonic wave and traditional solvent extraction (CitationKhajeh, 2009). In traditional solvent extractions, the samples were crushed or cut into granular, and soaked in the extraction solvent, such as water, ethanol, or other organic solvents. Ultrasonic could produce the ‘cavitation’ and the mechanical action in the extraction solvent. On the one hand, this can effectively break the cell walls and promote the salvation of the ingredient. The other could accelerate the molecular motion, and accelerate the mixture of active ingredients and solvent. Thus, ultrasonic-assisted extraction has many advantages, such as shorter time, less solvent, higher extraction rate, better products with lower cost (CitationHao et al., 2002; CitationBugallo et al., 2007). The present paper adopts an orthogonal array design to optimize of ultrasound-assisted extraction procedure of flavonoids from W. indica. The results of experiments (presented in ) indicated that the maximum extraction yield of crude flavonoids was 1.23%.
Orthogonal analysis of the results of L9(34) was showed in . The extreme difference (K) was applied to analyze the data, and the results indicated the influence (R) of extraction factors on the extraction yields decreases in the order: C>D>B>A (CitationPeng et al., 2006). Namely, liquid–solid ratio > power of ultrasonic instrument > ethanol concentration > extraction time. And the most suitable conditions for extraction would be combination C3 D3 B2A3 (extraction time 60 min, ethanol concentration 60%, the ratio of liquid–solid 16:1 and the power of ultrasonic instrument 160 W) according to the values of K. In these conditions, the ratio of the extraction was 1.23%.
Table 3. Analysis of L9(34) test results.
Antimicrobial activity of flavonoids
The therapeutic potentialities of W. indica, such as anti-inflammatory (CitationWang et al., 2005a), antiviral (CitationHu et al., 2000), antitumor (CitationLee et al., 1981; CitationHu et al., 2000) and antimalarial activities (CitationNunome et al., 2004), have already been investigated. However, there isn’t any report about the antimicrobial activities of the flavonoids from W. indica.
In this research, the antimicrobial activities of W. indica were qualitatively and quantitatively assessed for the first time by the presence or absence of growth inhibition areas, diameters of inhibition areas, and MIC. Results () obtained from agar well diffusion method indicate that the W. indica showed significant antimicrobial activity against S. aureus and E. coli, however, no evident activity to M. racemosus. The following measurements of MIC showed the MIC against S. aureus and E. coli were 53 and 106.25 mg/mL, accordingly.
Table 4. The inhibition effect of the crude flavonoids from W. indica on three microorganism (the diameters of the inhibition zones, mm).
Anti-inflammatory activity of W. indica
NO plays an important role in the inflammatory process, and an inhibitor of NO release may be considered as a therapeutic agent in the inflammatory diseases (CitationLee et al., 1999) Results of the anti-inflammatory test () indicated that the total extraction, petroleum ether fraction and ethyl acetate fraction had different inhibitory activity. Both n-butanol and water fractions showed no inhibitory activity (IC50 >100 μg/mL). Considering the recovery of each fraction of total extraction (), ethyl acetate fraction should be the activity fraction.
Table 5. The inhibitory effect of total extraction and its different fractions against the production of NO in RAW 264.7 stimulated by LPS.
In 2005, the anti-inflammatory activities of W. indica were reported by CitationWang et al. (2005a,Citationb), and eight compounds were isolated from the active fraction. These reports were well in agreement with the result of our experiment that ethyl acetate fraction of the root of W. indica had significant inhibitory effect on the production of nitric oxide in RAW264.7 cells stimulated by LPS.
Isolation and identification of chemical composition
In order to study the chemical constituents of W. indica, multichromatographic separation techniques, such as silica column, Sephadex LH-20, octadecyl silane, were used to isolated chemical constituents from W. indica, by which four compounds were archived. The chemical structures of these compounds were determined by spectroscopic analysis (MS, 1H-NMR, 13C-NMR), and identified as daphnoretin (CitationGeng et al., 2006), chrysophanol (CitationYang et al., 2007), myricitrime (CitationLi et al., 2008) and rutin (CitationLiu & Liu, 2009). Among these compounds, chrysophanol was isolated from this plant for the first time. The chemical structures of the isolated compounds were presented in . Spectroscopic data of these compounds were listed below: Daphnoretin (compound 1) was obtained as slight yellow needle crystal, mp 250–252°C. ESI-MS (negative ion mode) m/z 351 [M-H]−. 1H-NMR (DMSO-d6, 400 MHz) δ: 10.27 (1H, s), 8.04 (1H, d, J = 9.6 Hz), 7.87 (1H, s), 7.71 (1H, d, J = 8.4 Hz), 7.21 (1H,s), 7.18 (1H, d, J = 2.4 Hz), 7.11 (1H, dd, J = 2.4, 8.4 Hz), 6.87 (1H, s), 6.38 (1H, d, J = 9.6 Hz), 3.82 (3H, s). 13C-NMR (DMSO-d6, 100 MHz) δ: 160.2 (C-2′), 159.9 (C-2), 157.2 (C-7′), 155.2 (C-9′), 150.6 (C-7), 147.7 (C-9), 145.9 (C-6), 144.3 (C-4′), 136.0 (C-3), 131.1 (C-4), 130.1 (C-5′), 114.6 (C-10′), 114.1 (C-3′), 113.7 (C-6′), 110.4 (C-10), 109.7 (C-5), 104.3 (C-8′), 103.0 (C-8), 56.3 (−OCH3).
Figure 1. Chemical structures of the compounds isolated from W. indica. Daphnoretin and chrysophanol were both isolated from the ethyl acetate (EtOAc) fraction by using silica gel column and recrystallization techniques. Rutin and myricitrime were got from the n-butanol fraction, silica gel column, and sephadex-LH 20 chromatograph were used.
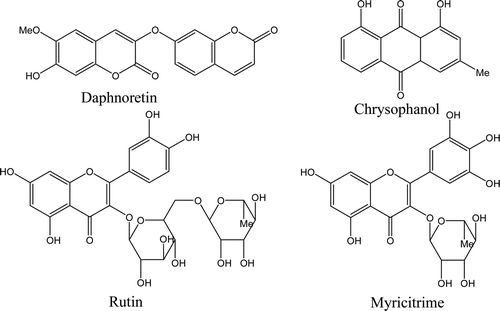
Chrysophanol (compound 2) was obtained as orange crystal flake, mp 197–198°C. ESI-MS (negative ion mode) m/z 253 [M-H]−. 1H-NMR (DMSO-d6, 400 MHz) δ: 11.97 (1H, s), 11.87 (1H, s), 7.81 (1H, t, J = 8.0 Hz), 7.71 (1H, d, J = 8.0 Hz), 7.55 (1H, s), 7.38 (1H, d, J = 8.0 Hz), 7.22 (1H, s), 2.44 (3H, s). 13C-NMR (DMSO-d6, 100 MHz) δ: 191.9 (C-9), 181.7 (C-10), 161.8 (C-1), 161.6 (C-8), 149.4 (C-3), 137.6 (C-6), 133.6 (C-10a), 133.3 (C-4a), 124.6 (C-5), 124.3 (C-2), 120.8 (C-4), 119.6 (C-7), 116.1 (C-8a), 114.0 (C-9a), 21.9 (-CH3).
Myricitrime (compound 3) was obtained as orange needle crystal, mp 187–189°C. ESI-MS (negative ion mode) m/z 463 [M-H]−. 1H-NMR (DMSO-d6, 400 MHz) δ: 6.94 (1H, s), 6.35 (1H, d, J = 2.0 Hz), 6.19 (1H, d, J = 2.0 Hz), 5.31 (1H, d, J = 1.2 Hz), 4.56 (1H, brs), 4.21 (1H, brs), 3.78 (1H, dd, J = 3.4, 9.4 Hz), 3.50 (1H, m), 3.33 (1H, m), 0.95 (3H, d, J = 6.0 Hz). 13C-NMR (DMSO-d6, 100 MHz) δ: 178.1 (C-4), 164.2 (C-7), 161.6 (C-5), 157.8 (C-9), 156.9 (C-2), 145.2(C-3′,5′), 136.3(C-4′), 134.7(C-3′), 120.3(C-1′), 108.0(C-2′, 6′), 104.3(C-10), 102.0(C-1″), 98.2(C-6), 93.1(C-8), 71.7(C-4″), 70.5(C-3″), 70.4(C-2″), 70.3(C-5″), 16.2(C-6″).
Rutin (compound 4) was obtained as yellow powder. ESI-MS (negative ion mode) m/z 609 [M-H]−. 1H-NMR (DMSO-d6, 400 MHz) δ: 7.66 (1H, d, J = 2.0 Hz), 7.62 (1H, dd, J = 2.0, 8.4 Hz), 6.86 (1H, d, J = 8.4 Hz), 6.38 (1H, d, J = 2.0 Hz), 6.19 (1H, d, J = 2.0 Hz), 5.09 (1H, d, J = 7.6 Hz), 4.51 (1H, d, J = 1.2 Hz), 3.63 (1H, dd, J = 1.6, 3.2 Hz), 1.11 (3H, d, J = 6.0 Hz). 13C-NMR (DMSO-d6, 100 MHz) δ: 177.8 (C-4), 164.4 (C-7), 161.3 (C-5), 157.7 (C-9), 156.9 (C-2), 148.2 (C-4′), 144.2 (C-3′), 134.0 (C-3), 122.0 (C-6′), 121.5 (C-1′), 116.1 (C-5′), 114.4 (C-2′), 104.0 (C-10), 103.2 (C-1″), 100.8 (C-1″′), 98.4 (C-6), 93.3 (C-8), 76.6 (C-3″), 75.6 (C-5″), 74.1 (C-2″), 72.3 (C-4″′), 70.7 (C-3″′), 70.5 (C-2″′), 69.8 (C-4″), 68.1 (C-5″′), 67.0 (C-6″), 16.3 (C-6″′).
Cytotoxic activity of daphnoretin
During the chemical studies, we found that W. indica contains an abundance of daphnoretin (a bis-coumarin), which was reported possessing extensive bioactivity. In this study, the antitumor activity of daphnoretin was investigated in vitro by testing the inhibition effect on proliferation of four kinds of human cancer cell lines, HeLa, A549, CNE, and HEp-2. The CNE, HeLa, and A549 cells lines were used for the first time to test the cytotoxicity of daphnoretin. The results are shown in , indicating its ability to inhibit the proliferation of cancer cells. In particular, it had significant inhibition to CNE cells and HeLa cells at the concentrations ranging from 15.6 to 125 μg/mL, the tendency of antitumor effect was displayed in a concentration-dependent manner. The proliferation of A549 was significantly suppressed at the concentration of 31.25 μg/mL, with the inhibition ratio of 32.46%. To HEp-2 cells, daphnoretin shows significant inhibition at the highest concentration (125 μg/mL), however, no evident activity (inhibition <10%) while the concentration ≤62.5 μg/mL.
Figure 2. The in vitro inhibition ratio of HeLa, A549, CNE, and HEp-2 cells by the daphnoretin from W. indica at different concentrations. Cells were treated with different concentrations of daphnoretin for 72 h. Viability was quantitated by MTT assay. Results are mean ± SEM (n = 3). The asterisms are indicator of statistical differences obtained separately at different concentration points compared to controls shown in figure as *p < 0.05, **p < 0.01, ***p < 0.001.
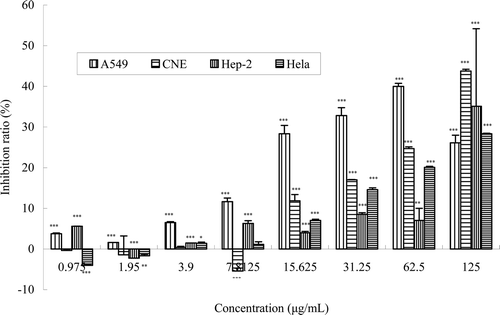
At low concentrations, the negative inhibition ratio did not mean that daphnoretin had a promotion effect on the proliferation of the HEp-2, CNE, and HeLa cells, because the absolute value of inhibition ratio was <10% at the concentration of daphnoretin lower than 10 μg/mL.
Previous studies have authenticated the good anticancer activity of daphnoretin, such as inhibiting the tyrosine-specific protein kinase activity of human epidermal growth factor receptors (CitationMohamed et al., 1998), inhibiting P-388 lymphocytic leukemia in vitro experiment (CitationHanda et al., 1983), and strong suppressive effects on the expression of the hepatitis B surface antigen in human hepatoma Hep 3B cells (CitationChen et al., 1996), etc. In this research, the suppressive effects of daphnoretin on the proliferation of four human cancer cells lines were evaluated. We found that daphnoretin had a good inhibition activity on the CNE and HEp-2 cells and reported for the first time.
Conclusions
The optimal conditions for ultrasound-assisted extraction of flavonoids from W. indica were: ethanol concentration 60%, extraction time 60 min, the ratio of liquid–solid 16:1 and the power of ultrasonic instrument 160 W, in this conditions, the ratio of the extraction was 1.23%. The crude flavonoids extracted from W. indica showed good antimicrobial activities and ethanol extract of W. indica displayed significant anti-inflammatory activities. The chemical studies isolated and identified four compounds, daphnoretin, chrysophanol, myricitrime, and rutin, from W. indica, of which chrysophanol was isolated from this plant for the first time. The results of antitumor activity assay indicated that daphnoretin had good potent for clinical usage, particularly for nasopharyngeal carcinoma and laryngeal carcinoma.
Declaration of interest
This project was supported by National Key Technology R&D Program (2006BAD27B04) and China Postdoctoral Science Foundation Funded Project (20080430826, 200801250).
References
- Bugallo RA, Segade SR, Gómez EF. (2007). Comparison of slurry sampling and microwave-assisted digestion for calcium, magnesium, iron, copper and zinc determination in fish tissue samples by flame atomic absorption spectrometry. Talanta, 72, 60–65.
- Di Carlo G, Mascolo N, Izzo AA, Capasso F. (1999). Flavonoids: old and new aspects of a class of natural therapeutic drugs. Life Sci, 65, 337–353.
- Chen HC, Chou CK, Kuo YH, Yeh SF. (1996). Identification of a protein kinase C (PKC) activator, daphnoretin, that suppresses hepatitis B virus gene expression in human hepatoma cells. Biochem Pharmacol, 52, 1025–1032.
- Chen Y, Fu WW, Sun LX, Wang Q, Qi W, Yu H. (2009). A new coumarin from Wikstroemia indica (L.) C. A. Mey. Chin Chem Lett, 20, 592–594.
- Geng LD, Zhang C, Xiao YQ. (2006). [Studies on the chemical constituents in stem rind of Wikstroemia indica]. Zhongguo Zhong Yao Za Zhi, 31, 817–819.
- Handa SS, Kinghorn AD, Cordell GA, Farnsworth NR. (1983). Plant anticancer agents. XXVI. Constituents of Peddiea fischeri. J Nat Prod, 46, 248–250.
- Hao JY, Han W, Huang S, Xue BY, Deng X. (2002). Microwave assisted extraction of artemisinin from Artemisia annua L. Sep Purif Technol, 28, 191–196.
- Hu K, Kobayashi H, Dong A, Iwasaki S, Yao X. (2000). Antifungal, antimitotic and anti-HIV-1 agents from the roots of Wikstroemia indica. Planta Med, 66, 564–567.
- Hu KW, Huang CC, Hwu JR, Su WC, Shieh DB, Yeh CS. (2008). A new photothermal therapeutic agent: core-free nanostructured Au x Ag1-x dendrites. Chemistry, 14, 2956–2964.
- Kato A, Hashimoto Y, Kidokoro M. (1979). (+)-Nortrachelogenin, a new pharmacologically active lignan from Wikstroemia indica. J Nat Prod, 42, 159–162.
- Khajeh M. (2009). Optimization of microwave-assisted extraction procedure for zinc and copper determination in food samples by Box-Behnken design. J Food Compos Anal, 22, 343–346.
- Lee HJ, Kim NY, Jang MK, Son HJ, Kim KM, Sohn DH, Lee SH, Ryu JH. (1999). A sesquiterpene, dehydrocostus lactone, inhibits the expression of inducible nitric oxide synthase and TNF-alpha in LPS-activated macrophages. Planta Med, 65, 104–108.
- Lee KH, Tagahara K, Suzuki H, Wu RY, Haruna M, Hall IH, Huang HC, Ito K, Iida T, Lai JS. (1981). Antitumor agents. 49 tricin, kaempferol-3-o-beta-d-glucopyranoside and (+)-nortrachelogenin, antileukemic principles from Wikstroemia indica. J Nat Prod, 44, 530–535.
- Li D, Liu MS, Li ZL, Kang SL, Hua HM. (2008). [Studies on chemical constituents of Heliciopsis lobata II]. Zhongguo Zhong Yao Za Zhi, 33, 409–411.
- Liu JJ, Liu XK. (2009). Chemical constituents from edible part of Pistacia chinensis. China Tradit Herbal Drugs, 40, 186–189.
- Mohamed AK, Nagwa ES, Thomas SW. (1998). Inhibition of oncogene product enzyme activity as an approach to cancer chemoprevention. Tyrosine-specific protein kinase inhibition by daphnoretin from Thymelaea hirsuta root. Phytother Res, 12, 282–284.
- Nunome S, Ishiyama A, Kobayashi M, Otoguro K, Kiyohara H, Yamada H, Omura S. (2004). In vitro antimalarial activity of biflavonoids from Wikstroemia indica. Planta Med, 70, 76–78.
- Peng J, Dong F, Xu Q, Xu Y, Qi Y, Han X, Xu L, Fan G, Liu K. (2006). Orthogonal test design for optimization of supercritical fluid extraction of daphnoretin, 7-methoxy-daphnoretin and 1,5-diphenyl-1-pentanone from Stellera chamaejasme L. and subsequent isolation by high-speed counter-current chromatography. J Chromatogr a, 1135, 151–157.
- Puglisi S, Speciale A, Acquaviva R, Ferlito G, Ragusa S, De Pasquale R, Iauk L. (2009). Antibacterial activity of Helleborus bocconei Ten. subsp. siculus root extracts. J Ethnopharmacol, 125, 175–177.
- Tyagi AK, Malik A. (2011). Antimicrobial potential and chemical composition of Eucalyptus globulus oil in liquid and vapour phase against food spoilage microorganisms. Food Chem, 126, 228–235.
- Wang LY, Unehara N, Kitanaka S. (2005). Lignans from the roots of Wikstroemia indica and their DPPH radical scavenging and nitric oxide inhibitory activities. Chem Pharm Bull, 53, 1348–1351.
- Wang LY, Unehara T, Kitanaka S. (2005). Anti-inflammatory activity of new guaiane type sesquiterpene from Wikstroemia indica. Chem Pharm Bull, 53, 137–139.
- Stone WL, Yang H, Qui M. (2006). Assays for nitric oxide expression. Methods Mol Biol, 315, 245–256.
- Yang SM, Liu XK, Qing C, Wu DG, Zhu DY. (2007). Chemical constituents from the roots of Homonoia riparia. Yao Xue Xue Bao, 42, 292–296.
- Zhang F, Zhou F, Wang GH, Yang Y, Guo ZK. (2008). [Determination of total flavones from euonymus alatus sieb by microwave-assisted extraction and absorption spectroscopy]. Guang Pu Xue Yu Guang Pu Fen Xi, 28, 1633–1636.
- Zhang Q, Zhao XH, Wang ZJ. (2008). Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem Toxicol, 46, 2042–2053.
- Zhou GX, Lu CL, Wang HS, Yao XS. (2009). An acetyl flavonol from Nervilia fordii (Hance) Schltr. J Asian Nat Prod Res, 11, 498–502.