Abstract
Context: Irradiation is the process of exposing food such as herbal plant to ionizing radiation to destroy microorganisms. Zataria multiflora Boiss (Lamiaceae), known as Avishan-e-Shirazi in Persian, is a thyme-like plant that grows naturally in central and southern parts of Iran and is used in traditional folk medicine.
Objective: In this study, the effects of γ-radiation on chemical composition and antioxidant properties of Z. multiflora were investigated.
Materials and methods: The plants were first irradiated with Co60 source (0, 10, and 25 kGy) and then subjected to Clevenger extraction to obtain essential oils. The composition of the oil was analyzed by a gas chromatography and compared with samples pretreated under different conditions. In parallel, the hydroalcoholic extract was prepared and used for measuring flavonoid content. Thereafter, the free-radical scavenging and antioxidant properties of essential oils and hydroalcoholic extract were examined.
Results: Despite the minor change in the individual oil constituents, the total percentage of the main components remained unaffected before and after irradiation (~95%). In addition, the total flavonoid content of hydroalcoholic extract was also unchanged due to irradiation (~32 mg QE/g extract). The high radical scavenging activity of the oil (~67%) and hydroalcoholic extract (~71%), in addition, the antioxidant properties of the oil (~91%) and hydroalcoholic extract (~95%), were unaffected after irradiation.
Discussion and conclusions: These findings may suggest the sustainability of Z. multiflora extract properties pretreated with γ-radiation. With a view to its antioxidant applications, resistance of Z. multiflora and its properties against radiation effects are promising findings.
Introduction
Irradiation of dried foods, particularly herbs and spices, has a great application potential and has already been implemented in many countries (CitationLuckman, 2002). Many reports indicated that herbal plant and spices are susceptible to insect and disease attacks (CitationAbou-Arab & Donia, 2001; CitationKatuin-Raem et al., 2001), contamination by pesticides, heavy metals, and aflatoxins through different ways such as environment in developing countries, pollution in irrigation water, atmosphere, soil as well as sterilization methods and storage conditions (CitationAbou-Arab et al., 1999; CitationEvenhuis et al., 1995).
Irradiation treatment of food is becoming an increasingly accepted processing option for countries in the Asia-Pacific region wishing to meet growing sanitary and phytosanitary requirements in international trade (CitationLuckman, 2002). The amount of spices irradiated commercially has been estimated in 2002 to reach about 100,000 tons worldwide. The advantages of irradiation of different food and food products are well established. The first application of irradiation is to reduce the number of pathogenic and spoilage microorganism (CitationFarkas, 2011). The antimicrobial effect of γ-irradiation has been established by routine examination of food products before and after treatments. γ-Irradiation, by reducing the load of microorganisms, can increase the shelf life of the products, which is important in food preservation technology. In Iran, various products including dried spices and herbs are routinely irradiated. The conventional dose of γ-irradiation applied is 10 kGy, but radiation doses up to 30 kGy have been authorized for decontamination of dried food and spices (CitationMahindru, 2005; CitationFarkas, 1977). Our routine analysis indicated high microbial load in Iranian Zataria multiflora Boiss (Lamiaceae) plant (data not shown). Therefore, as an herbal plant, its decontamination by a safe method can be considered as an important goal in herbal drug processing.
Z. multiflora, known as Avishan-e-Shirazi in Persian, is a thyme-like plant that grows naturally in central and southern parts of Iran. In Iran, Z. multiflora is used in traditional folk remedies for its antiseptic, analgesic, and carminative (antiflatulence and intestine-soothing) properties. It is extensively used as flavor ingredients in a wide variety of food especially in yoghurt flavoring. The essential oil was found to contain thymol and carvacrol as the major components, which are antimicrobial and antifungal agents (CitationSharififar et al., 2007; CitationMahboubi & Bidgoli, 2010; CitationSaei-Dehkordi et al., 2010; CitationGandomi et al., 2009). Aqueous and alcoholic extracts of Z. multiflora have been therapeutically used for relieving nociceptive pain (CitationHosseinzadeh et al., 2000; CitationRamezani et al., 2004) and recurrent aphthous stomatitis (CitationJafari et al., 2003), and for preventing growth of oral streptococci (CitationOwlia et al., 2004), Plasmodium falciparum (CitationZiegler et al., 2004), and Trichomonas vaginalis (CitationAbdollahy et al., 2004), as well as used as an insect repellent (CitationSaleem et al., 2004).
The usefulness of γ-irradiation is well known, provided that the chemical composition and biochemical properties of food and food products are retained. Despite the routine use of γ-irradiation, our knowledge about the influence of radiation on the chemical composition and pharmacological properties of spices is not well documented. The aim of this study was to investigate the chemical composition of Z. multiflora extracts pretreated with γ-irradiation. Moreover, the effect of γ-irradiation on antioxidant properties of the oils and hydroalcoholic preparations has been investigated.
Materials and methods
Chemicals
Aluminum trichloride (AlCl3), chloroform, ethanol, dimethylsulfoxide, ascorbic acid, and methanol were purchased from Merck, Frankfurt, Germany. Quercetin, 2,2-diphenylpicrylhydrazyl (DPPH), trolox, β-carotene, linoleic acid, Tween 40, and butylated hyroxytoluene (BHT) were obtained from Sigma -Aldrich, St. Louis, MO.
Plant materials and radiation treatments
Fresh Z. multiflora was collected in May 2010 from the Shiraz city, Iran. Dr. Younes Asri (Botanist) authenticated the plant materials from herbarium of Iranian botanical garden (TARI) (Voucher Number: 41754). The whole aerial parts of the plants were divided into three batches (50 g) and packed in heat-sealed polyethylene pouches, and they were then passed to a Co60 source for irradiation at two different doses (10 and 25 kGy) using a high-dose rate research irradiator (Co60 Gammacell 220; Canada) calibrated with Fricke standard dosimeter (Co60 Gammacell 220 [Atomic Energy of Canada Limited Radiochemical Company], Canada), which is installed in Radiation application research school of atomic energy organization of Iran. The dose was controlled by the exposure time of each container to the source. The temperature and dose rate for all the samples were 22–23°C and 0.37 Gy/sec, respectively. The dose range within the samples was ±20% of the actual dose. The control and irradiated samples were stored in plastic containers at room temperature (28–30°C) under a similar condition.
Oil extraction and analysis
Oil extraction from the aerial parts of nonirradiated and irradiated Z. multiflora was carried out using a Clevenger-type apparatus. The extraction was carried out for 2 h, and the oils were stored in dark glass bottles in a freezer until further use. The oil extraction yield from both the nonirradiated and irradiated Z. multiflora seeds was approximately 4% (w/w). Gas chromatography (GC) analysis was performed using a GC (9-A Shimadzu, Kyoto, Japan) equipped with a flame ionization detector. Quantitation was carried out on Euro Chrom 2000 software (KNAUER Company, Berlin, Germany) by the area normalization method. The analysis was carried out using a DB-5 fused-silica column (30 m × 0.25 mm, film thickness 0.25 µm) and a temperature program of 40–250°C at a rate of 4°C/min, injector temperature of 250°C, detector temperature of 265°C, and the carrier gas was helium (99.99%). The GC/MS unit consisted of a Varian-3400 GC coupled to a Saturn II ion trap detector. The column of GC/MS was the same as of the GC under the same conditions that the above analysis was carried out. The constituents were identified by comparing their mass spectra with those in the computer library and with the authentic standards.
Preparation of hydroalcoholic extract
A known amount (30 g) of the powder prepared from Z. multiflora before and after irradiation was mixed with 50-mL distilled water and 50-mL methanol at 70–80°C and maintained at 60°C for 24 h. The hydroalcoholic extract was filtered through a Whatman filter #4 (pore size, 20–25 µm). The filtrate was then freeze-dried for further use. The extraction yield for the hydroalcohol extracts derived from nonirradiated and irradiated Z. multiflora seeds was ~9% (w/w).
Estimation of total flavonoids
Total flavonoid content of the hydroalcoholic extract was determined using the Dowd method, which is adapted by CitationArvouet-Grand et al. (1994). Briefly, 5 mL of extract solution (1 mg/ mL) was mixed with an equal volume of 2% AlCl3 prepared in methanol. The absorbance was recorded at 415 nm using Shimadzu UV-3100 spectrophotometer after 10 min against a blank sample. The blank sample was prepared by mixing 5 mL of the extract with 5 mL of methanol alone. Total flavonoids were determined using a standard curve prepared with quercetin (0–100 mg/L) as the standard. The mean of three readings was used to calculate the flavonoid content, which was finally expressed as mg quercetin equivalents (QE)/g of hydroalcoholic extract.
Radical-scavenging capacity (DPPH assay) of the oils
The hydrogen atom or electron donation abilities of the extracts and pure compounds were measured from the bleaching of the purple-colored methanol solution of 2,2-DPPH. This spectrophotometric assay uses the stable radical DPPH as reagent (CitationBurits & Bucar, 2000; CitationCuendet et al., 1997). Among the different concentrations of essential oil preparations, 20% v/v (in methanol) was found suitable for DPPH test. Fifty microliters of the essential oils in methanol was added to 5 mL of DPPH solution (0.004% DPPH in methanol). Trolox (1 mM), a stable antioxidant, was used as a synthetic reference. After 30 min of incubation period at room temperature, the absorbance was read against the blank at 517 nm. The inhibitory effects of the extracts in percent (I%) was calculated by the following formula:
where, Ablank is the absorbance of the control reagent (containing all reagents except the test compound), and Asample is the absorbance of the test compound. All the assays were carried out in triplicate.
Radical-scavenging capacity of hydroalcoholic extract
The radical scavenging activity of the Z. multiflora hydroalcoholic extract was measured spectrophotometrically using DPPH radical (CitationBlois, 1958). In this assay, 2 mL of the hydroalcoholic extract (50 µg/mL) was added to equal volume of DPPH solution (125 μM in methanol). The solution was then mixed and incubated at 37°C in dark for 30 min. The decrease in absorbance of DPPH was recorded at 517 nm. A parallel experiment was performed in which Z. multiflora extract was replaced with vitamin C (5 µg/mL) and considered as a positive control. The inhibition percentage was calculated by comparing the absorbance of the blank and the samples as discussed.
β-Carotene-linoleic acid assay of the oils
The antioxidant activity of essential oils was determined using the β-carotene bleaching test (CitationTaga et al., 1984). Approximately, 10 mg of β-carotene (type I synthetic) was dissolved in 10 mL of chloroform, and then, 0.2 mL of this solution was added to a boiling flask containing 20-mg linoleic acid and 200-mg Tween 40. Chloroform was removed using a rotary evaporator at 40°C for 10 min. Then, 50 mL of distilled water saturated with oxygen was added slowly with vigorous agitation to form an emulsion. The emulsion (5 mL) was added to a tube containing 0.2 mL of essential oil solution prepared according to CitationChoi et al. (2000). The absorbance was immediately measured at 470 nm against a blank consisting of an emulsion without β-carotene. The tubes were placed in a water bath at 50°C and the oxidation of the emulsion was monitored spectrophotometrically by measuring absorbance at 470 nm over a 60-min period. Samples containing 0.2 mL of ethanol instead of essential oils were also monitored and used as control. Butylated hydroxytoluene (BHT; 1 mM in ethanol), a stable antioxidant, was used as reference. The antioxidant activity was expressed as inhibition percentage with reference to the control sample after 60 min of incubation, using the following equation: AA = 100(DRC − DRS)/DRC, where
AA = antioxidant activity,
DRC = degradation rate of control = [ln(a/b)/60],
DRS = degradation rate in presence of sample = [ln(a/b)/60],
a = absorbance at time 0,
b = absorbance at 60 min.
β-Carotene-linoleic acid assay of hydroalcoholic extract
In this assay, antioxidant capacity was determined by measuring the inhibition of the volatile organic compounds and the conjugated diene hydroperoxides arising from linoleic acid oxidation (CitationDapkevicius et al., 1998). A stock solution of β-carotene–linoleic acid mixture was prepared as follows: 5-mg β-carotene was dissolved in 10 mL of chloroform (HPLC grade), and then, 25-µL linoleic acid together with 200-mg Tween 40 were added. Chloroform was completely evaporated using a vacuum evaporator before adding 100-mL distilled water saturated with oxygen. After dissolving the residue, an aliquot (5 mL) of this mixture was dispensed into a test tube. Then, 350-µL of the Z. multiflora extract, prepared at 2 g/L concentrations, was added, and the emulsion system was incubated for 2 h in a water bath at 50°C. Assay containing BHT (2 g/L) was also carried out and considered as positive control. After the incubation period, the absorbance was recorded at 470 nm. Antioxidative capacity of the hydroalcoholic extract was compared with those of BHT and the blank.
Statistical analysis
Data are presented as means ± standard error. The results were subjected to one-way analysis of variance followed by Tukey’s Honestly Significant Differences using SPSS13.0 software. The significance was considered as p < 0.05.
Results and discussion
Effect of γ-irradiation on chemical composition and antioxidant properties of Z. multiflora essential oils
GC/GC-MS analysis of essential oil extracted from aerial parts of Z. multiflora cultivated in Shiraz city, Iran, resulted in identification of 12 known compounds (). The major compounds were thymol (61.8%), carvacrol (10.5%), p-cymene (7.5%), and γ-terpinene (4.4%). Similarly, other studies also indicated thymol and carvacrol as the main constituents of this plant (CitationAli et al., 2000; CitationShaffiee & Javidnia, 1997; CitationBasti et al., 2007; CitationSharififar et al., 2007; CitationMahboubi & Bidgoli, 2010). Characterization of the chemical composition of essential oils in Z. multiflora from different parts of Iran also indicated that thymol, a phenolic compound of oxygenated monoterpens, was the most abundant component in GC/MS ranging from 27.05% to 64.87% with the high antioxidant activities (CitationSaei-Dehkordi et al., 2010).
Table 1. Chemical composition of essential oils from Zataria multiflora pretreated with γ-irradiation.
Comparison of the essential oil fractions in the Z. multiflora before and after γ-irradiation (10 and 25 kGy) analyzed by GC/MS showed minor changes in the total percentage of oil chemical constituent (). These changes were mainly recorded in thymol (−16.5%), carvacrol (−4.2%), p-cymene (+10%), and γ-terpinene (+4.9%) irradiated at 25 kGy. Irradiation at 10 kGy caused small changes in the level of some essential oils, particularly thymol (−12.5%), carvacrol (−3.9%), p-cymene (+8.7%), and γ-terpinene (+4.2%). These data were further supported by showing equal oil yields of approximately 4% (w/w) in irradiated and control seeds.
The antioxidative properties of the Z. multiflora essential oils before and after γ-irradiation (10 and 25 kGy) analyzed by DPPH and β-carotene bleaching tests are presented in . When compared with a standard antioxidant agent, that is, trolox (15%), it was found that essential oils extracted from Z. multiflora have strong radical scavenging activity (67%). Addition of the plant oils to the reaction mixture containing β-carotene and linoleic acid, brought about ~91% inhibition in formation of peroxidation products that was maintained even after irradiation (). The DPPH assay data revealed that γ-irradiation at lower dose (10 kGy), which is routinely used for food preservation, and also sterile dose (25 kGy) cannot influence the DPPH radical scavenging capacity of the oils (p > 0.05).
Table 2. Free radical scavenging and antioxidant activities (%) of Zataria multiflora essential oils.
In regard to these data, we can demonstrate that the unaffected radical scavenging and antioxidant properties of the oils can be attributed to the unchanged total oil components due to γ-irradiation obtained by GC/MS analysis. Despite changes in the level of some individual oil constituents due to irradiation, the total percentage of the main components, that is, thymol, carvacrol, p-cymene, and γ-terpinene remained unaffected before (95.82%) and after irradiation at 10 and 25 kGy (95.69% and 95.47%, respectively). Nevertheless, irradiation caused decrease in thymol and carvacrol levels and increase in γ-terpinene and p-cymene percentages. Studies indicated that all of these constituents possessed antioxidant activities, which compensate the individual oil decreasing (CitationMechergui et al., 2010; CitationCelik et al., 2010). This was confirmed by experiments using γ-terpinene as an inhibitory factor in the peroxidation process of linoleic acid, which facilitated cross-reactions between HOO˚ and LOO˚ radicals leading to rapid chain termination of the peroxidation pathway (CitationFoti & Ingold, 2003). One study showed that the phenolic -OH groups present in oil of thymol, which act as hydrogen donors to the peroxy radicals produced during the first step of lipid oxidation, is probably responsible for retardation of hydroxyl peroxide formation (CitationFarag et al., 1989). CitationTeissedre and Waterhouse (2000) also reported a good correlation (r = 0.75) between the total phenol content of essential oils containing thymol, carvacrol, and p-cymene and human low-density lipoprotein oxidation in vitro (CitationTeissedre & Waterhouse, 2000). Our previous studies on caraway seeds also indicated that γ-irradiation could not influence the components and also antioxidant properties of the seeds extracts in vivo and in vitro systems (CitationFatemi et al., 2010a,Citationb & Citation2011). Also, complete decontamination of peppermint after low-dose γ-irradiation (0.5, 1.0, and 2.66 kGy) did not lead to significant loss in essential oil components (CitationMachhour et al., 2011).
Effect of γ-irradiation on total flavonoid content and antioxidant properties of the Z. multiflora hydroalcoholic extracts
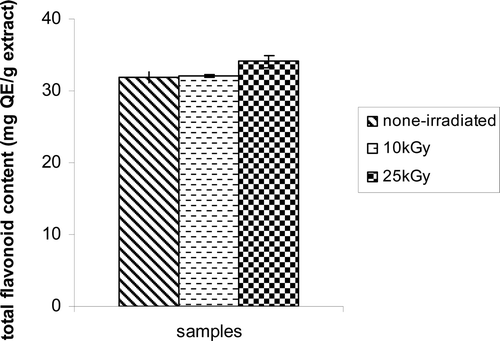
The total flavonoid content of Z. multiflora was found to be ~32 mg of quercetin (QE)/g hydroalcoholic extract, which was significantly unaffected in samples preexposed to 10 and 25 kGy radiation (p > 0.05) (). However, the extraction yields of hydroalcoholic extracts after γ-irradiation remained at the same level (~9% w/w) in nonirradiated sample. The antioxidant properties of hydroalcoholic extracts derived from Z. multiflora assessed by the DPPH and β-carotene bleaching test indicates strong antioxidant ability of the extract. In comparison to the standard antioxidant agent, that is, vitamin C (~48%), it was found that Z. multiflora hydroalcoholic extract possessed stronger radical scavenging activity (~71%) (). In β-carotene bleaching test, it was also found that Z. multiflora hydroalcoholic extract caused ~95% inhibition in formation of peroxidation products (). The antilipid peroxidation activities of hydroalcoholic extracts from other natural sources have also been reported (CitationTepe et al., 2005). One study also indicated that sub-fractions of the methanol extract of Z. multiflora were able to reduce the stable free radical 2,2-DPPH with the high inhibition values of linoleic oxidation (CitationSharififar et al., 2007). The results presented in shows that γ-irradiation could not change the radical scavenging activity of the hydroalcoholic extracts (p > 0.05). As well, the extracts from γ-irradiated seeds failed to alter the lipid peroxidation reaction (β-carotene bleaching test) (). These results are in agreement with the report of CitationFarag and el-Khawas (1998) showing the lack of influence of γ-irradiation on antioxidant property of caraway extracts. The antioxidant properties of sage, thyme, and oregano in chloroform and methanol extracts as well as in their mixture were also unaffected after irradiation at 10 kGy (CitationBrandstetter et al., 2009). The result of one study indicated that γ-irradiation 0.5–10 kGy does not have adverse changes in biological activity of Schizandra chinensis Baillon (Schisandraceae) extract (CitationLee et al., 2011).
Table 3. Free radical scavenging and antioxidant activities (%) of Zataria multiflora hydroalcoholic extracts.
The lack of changing in antioxidant and DPPH scavenging activity due to irradiation were associated with the amount of unaffected flavonoid content in the irradiated Z. multiflora preparations (). Our results together with reports from other laboratories suggest that the total flavonoid content of plant play a major role in antioxidant and radical scavenging activity of the hydroalcoholic extract (CitationJimoh et al., 2007; CitationJeong et al., 2007). It is likely that flavonoids with 3-OH group have a major contribution to the antioxidant activity of the natural products (CitationJeong et al., 2007). A correlation between the total flavonoid content and antioxidant activity was also reported in methanol extracts of Paullinia pinnata L. (Sapindaceae) leaves (CitationJimoh et al., 2007). One study also indicated that γ-radiation dose of up to 10 kGy was found to be sufficient for complete microbial decontamination without affecting the bioactive properties of herbal formulations, including antioxidant potential, which was high in rasayan, shatpatryadi, scrub, rose, and guggul. The antioxidant property of these herbals could be attributed to components such as phenolics, flavonoids, and color pigments (CitationKumar et al., 2010). Another study also showed that γ-irradiation at 5 kGy could be a potential method to decontaminate the microbial load of Polygoni multiflori Radix without significant changes in its total phenols and antioxidant properties (CitationChiang et al., 2011). The 30 kGy dose applied to dry sage and oregano for sanitization did not significantly affect the capacity to inhibit the DPPH radical or the reducing power, nor did it affect the total phenolic content of the methanol and aqueous extract (CitationPérez et al., 2011).
Conclusions
These findings suggest sustainability of Z. multiflora extract properties on exposure to γ-radiation. With a view to its antioxidant applications, resistance of Z. multiflora and its properties against radiation effects are promising findings. The evidences presented in this article show that neither the chemical composition nor the in vitro antioxidant properties of the Z. multiflora extracts are affected by γ-irradiation. Nevertheless, it appears that there are positive effects on the in vitro antioxidant properties due to the conventional method of γ-irradiation used in food preservation.
Declaration of interest
The authors declared no conflict of interest.
References
- Abdollahy F, Ziaei H, Shabankhani B, Azadbakht M. (2004). Effect of essential oils of Artemisia aucheri Boiss. Zataria multiflora Boiss, and Myrtus communis L. on Trichomonas vaginalis. Iran J Pharm Res, 2(Suppl 2), 35–35.
- Abou-Arab AAK, Abou Donia MA. (2001). Pesticide residues in some Egyptian spices and medicinal plants as affected by processing. Food Chem, 72, 439–445.
- Abou-Arab AAK, Soliman Kawther M, El Tantawy ME, Ismail Badeaa R, Khayria N. (1999). Quantity estimation of some contaminants in commonly used medicinal plants in the Egyptian market. Food Chem, 67, 357–363.
- Ali MS, Saleem M, Ali Z, Ahmad VU. (2000). Chemistry of Zataria multiflora (Lamiaceae). Phytochemistry, 55, 933–936.
- Arvouet-Grand A, Vennat B, Pourrat A, Legret P. (1994). [Standardization of propolis extract and identification of principal constituents]. J Pharm Belg, 49, 462–468.
- Basti AA, Misaghi A, Khaschabi D. (2007). Growth response and modelling of the effects of Zataria multiflora Boiss. essential oil, pH and temperature on Salmonella typhimurium and Staphylococcus aureus. Food Sci Technol, 40, 973–981.
- Blois MS. (1958). Antioxidant determination by use of a stable free radical. Nature, 181, 1199–1200.
- Brandstetter S, Berthold C, Isnardy B, Solar S, Elmadfa I. (2009). Impact of gamma-irradiation on the antioxidative properties of sage, thyme, and oregano. Food Chem Toxicol, 47, 2230–2235.
- Burits M, Bucar F. (2000). Antioxidant activity of Nigella sativa essential oil. Phytother Res, 14, 323–328.
- Celik A, Nur Herken E, Arslan I, Zafer Ozel M, Mercan N. (2010). Screening of the constituents, antimicrobial and antioxidant activity of endemic Origanum hypericifolium O. Schwartz & P.H. Davis. Nat Prod Res, 24, 1568–1577.
- Chiang YC, Huang GJ, Ho YL, Hsieh PC, Chung HP, Chou FI, Chang YS. (2011). Influence of gamma irradiation on microbial load and antioxidative characteristics of Polygoni multiflori Radix. Process Biochem, 46, 777–782.
- Choi HS, Song HS, Ukeda H, Sawamura M. (2000). Radical-scavenging activities of citrus essential oils and their components: detection using 1,1-diphenyl-2-picrylhydrazyl. J Agric Food Chem, 48, 4156–4161.
- Cuendet M, Hostettmann K., Potterat O. (1997). Iridoid glucosides with free radical scavenging properties from Fagraea blumei. Helv Chim Acta, 80, 1144–1152.
- Dapkevicius A, Venskutonis R, Van-Beek TA, Linssen PH. (1998). Antioxidant activity of extracts obtained by different isolation procedures from some aromatic herbs grown in Lithuania. J Sci Food Agric, 77, 140–146.
- Evenhuis A, Verdam B, Gerlagh M, Goossen-van de Geijn HM. (1995). Studies on major diseases of caraway (Carum carvi) in the Netherlands. Ind Crop Prod, 4, 53–61.
- Farag RS, Badei AZMA, Hewedi FM, El-Baroty GSA. (1989). Antioxidant activity of some spice essential oils on linoleic acid oxidation in aqueous media. J Am Oil Chem Soc, 66, 792–799.
- Farag RS, el-Khawas KH. (1998). Influence of gamma-irradiation and microwaves on the antioxidant property of some essential oils. Int J Food Sci Nutr, 49, 109–115.
- Farkas J. (1977). Radiation processing of dry food ingredients - a review. Radiat Phys Chem, 25, 271–280.
- Farkas J. (2011). Radiation decontamination of spices, herbs, condiments, and other dried food ingredients. In: Molins, R.A. (Ed.). Food Irradiation: Principles and Applications. Wiley-Interscience, New York, 291–312.
- Fatemi F, Allameh A, Khalafi H, Rajaee R, Rezaei MB. ((2011). Biochemical properties of γ-irradiated caraway essential oils. J Food Biochem, 35, 650–662.
- Fatemi F, Allameh A, Khalafi H, Ashrafihelan J. (2010a). Hepatoprotective effects of gamma-irradiated caraway essential oils in experimental sepsis. Appl Radiat Isot, 68, 280–285.
- Fatemi F, Allameh A, Khalafi H, Rezaei MB, Seyhoon M. (2010b). [The effect of essential oils and hydroalcoholic extract of caraway seed on oxidative stress parameters in rats suffering from acute lung inflammation before and after γ-irradiation]. I J Med Aroma Plant, 25, 441–455.
- Foti MC, Ingold KU. (2003). Mechanism of inhibition of lipid peroxidation by gamma-terpinene, an unusual and potentially useful hydrocarbon antioxidant. J Agric Food Chem, 51, 2758–2765.
- Gandomi H, Misaghi A, Basti AA, Bokaei S, Khosravi A, Abbasifar A, Javan AJ. (2009). Effect of Zataria multiflora Boiss. essential oil on growth and aflatoxin formation by Aspergillus flavus in culture media and cheese. Food Chem Toxicol, 47, 2397–2400.
- Hosseinzadeh H, Ramezani M, Salmani G. (2000). Antinociceptive, anti-inflammatory and acute toxicity effects of Zataria multiflora Boiss extracts in mice and rats. J Ethnopharmacol, 73, 379–385.
- Jafari S, Amanlou M, Borhan-Mohabi K, Farsam H. (2003). Comparative study of Zataria multiflora and Anthemis nobelis extracts with Myrthus communis preparation in the treatment of recurrent aphthous stomatitis. Daru, 11, 1–5.
- Jeong JM, Choi CH, Kang SK, Lee IH, Lee JY, Jung H. (2007). Antioxidant and chemosensitizing effects of flavonoids with hydroxy and/or methoxy groups and structure-activity relationship. J Pharm Pharm Sci, 10, 537–546.
- Jimoh FO, Sofidiya MO, Afolayan AJ. (2007). Antioxidant properties of the methanol extracts from the leaves of Paullinia pinnata. J Med Food, 10, 707–711.
- Katuin-Raem B, Novak B, Raem D. (2001). Microbiological decontamination of botanical raw materials and corresponding pharmaceutical products by irradiation. Radiat Phys Chem, 62, 261–275.
- Kumar S, Gautam S, Powar S, Sharma A. (2010). Microbial decontamination of medicinally important herbals using gamma radiation and their biochemical characterization. Food Chem, 119, 328–335.
- Lee SS, Lee EM, An BC, Kim TH, Lee KS, Cho JY, Yoo SH, Bae JS, Chung BY. (2011). Effects of irradiation on decolourisation and biological activity in Schizandra chinensis extracts. Food Chem, 125, 214–220.
- Luckman GJ. (2002). Food irradiation: regulatory aspects in the Asia and Pacific region. Radiat Phys Chem, 63, 285–288.
- Machhour H, Hadrami I., El,Imziln B, Mouhib M, Mahrouz M (2011). Microbial decontamination by low dose gamma irradiation and its impact on the physico-chemical quality of peppermint (Mentha piperita). Radiat Phys Chem, 80, 604–607.
- Mahboubi M, Bidgoli FG. (2010). Antistaphylococcal activity of Zataria multiflora essential oil and its synergy with vancomycin. Phytomedicine, 17, 548–550.
- Mahindru, SN. (2005). Food Preservation and Irradiation., 1st edn. Saujanya Books, New Delhi.
- Mechergui K, Coelho JA, Serra MC, Lamine SB, Boukhchina S, Khouja ML. (2010). Essential oils of Origanum vulgare L. subsp. glandulosum (Desf.) Ietswaart from Tunisia: chemical composition and antioxidant activity. J Sci Food Agric, 90, 1745–1749.
- Pérez MB, Banek SA, Croci CA. (2011). Retention of antioxidant activity in gamma irradiated Argentinian sage and oregano. Food Chem, 126, 121–126.
- Owlia P, Pirveicy H, Saderi H, Rezvani MB, Mansouri S. (2004). Evaluation of the antimicrobial effects of extract of Zataria multiflora against oral Streptococci. Iran J Pharm Res, 2, 74–75.
- Ramezani M, Hosseinzadeh H, Samizadeh S. (2004). Antinociceptive effects of Zataria multiflora Boiss fractions in mice. J Ethnopharmacol, 91, 167–170.
- Saei-Dehkordi SS, Tajik H, Moradi M, Khalighi-Sigaroodi F. (2010). Chemical composition of essential oils in Zataria multiflora Boiss. from different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem Toxicol, 48, 1562–1567.
- Saleem M, Nazli R, Afza N, Sami A, Ali MS. (2004). Biological significance of essential oil of Zataria multiflora boiss. Nat Prod Res, 18, 493–497.
- Shafiee A, Javidnia K. (1997). Composition of essential oil of Zataria multiflora. Planta Med, 63, 371–372.
- Sharififar F, Moshafi MH, Mansouri SH, Khodashenas M. (2007). In vitro evaluation of antibacterial and antioxidant activities of the essential oil and methanol extract of endemic Zataria multiflora Boiss. Food Control, 18, 800–805.
- Taga MS, Miller EE, Pratt DE. (1984). Chia seeds as a source of natural lipid antioxidant. J Am Oil Chem Soc, 61, 928–931.
- Teissedre PL, Waterhouse AL. (2000). Inhibition of oxidation of human low-density lipoproteins by phenolic substances in different essential oils varieties. j Agric Food Chem, 48, 3801–3805.
- Tepe B, Daferera D, Sokmen A, Sokmen M, Polissiou M. (2005). Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem, 90, 333–340.
- Ziegler HL, Franzyk H, Sairafianpour M, Tabatabai M, Tehrani MD, Bagherzadeh K, Hägerstrand H, Staerk D, Jaroszewski JW. (2004). Erythrocyte membrane modifying agents and the inhibition of Plasmodium falciparum growth: structure-activity relationships for betulinic acid analogues. Bioorg Med Chem, 12, 119–127.