Abstract
Context: Dietary botanicals are often consumed together with allopathic medicines, which may give rise to pharmacokinetic interactions. In vitro intestinal models are useful to identify botanical-drug interactions, but they may exhibit different expressions of transporters or enzymes.
Objective: To compare the effects of selected dietary botanical extracts on cimetidine transport across two in vitro intestinal models.
Materials and Methods: Bi-directional transport of cimetidine was measured across Caco-2 cell monolayers and excised porcine jejunum tissue in the absence (control) as well as the presence of verapamil (positive control) and selected plant extracts.
Results: Sclerocarya birrea Hochst. (Anacardiaceae) (marula) and Psidium guajava L. (Myrtaceae) (guava) crude extracts significantly decreased cimetidine efflux in both in vitro models resulting in increased absorptive transport of the drug. On the other hand, Dovyalis caffra Sim. (Flacourtiaceae) (Kei-apple), Prunus persica (L.) Batsch (Rosaceae) (peach), Aspalathus linearis (Burm. f.) R. Dahlgren (Fabaceae) (rooibos tea), Daucus carota L. (Apiaceae) (carrot), Prunus domestica A. Sav. (Rosaceae) (plum), Beta vulgaris L. (Chenopodiaceae) (beetroot) and Fragaria x ananassa (Weston) Duchesne ex Rozier. (Rosaceae) (strawberry) crude extracts exhibited different effects on cimetidine transport between the two models.
Discussion: Caco-2 cells were more sensitive to changes in cimetidine transport by the plant extracts and therefore may overestimate the effects of co-administered plant extracts on drug transport compared to the excised pig tissue model, which is congruent with findings from previous studies.
Conclusions: The excised porcine jejunum model seemed to provide a more realistic estimation of botanical-drug pharmacokinetic interactions than the Caco-2 cell model.
Introduction
Plant materials in the form of fruits, vegetables, herbs and beverages contain various phytoconstituents and are often taken in conjunction with allopathic medicines (CitationIngersoll, 2005). Pharmacokinetic and/or pharmacodynamic interactions may occur between phytoconstituents from these plants and co-administered drugs (CitationHarris et al., 2003). Pharmacokinetic interactions between dietary botanicals and conventional drugs may involve interferences with drug bioavailability and disposition by means of altered absorption, metabolism, distribution and/or elimination (CitationTarirai et al., 2010). Although not all pharmacokinetic interactions encountered by means of in vitro assays are necessarily clinically significant in the in vivo situation, some of them should be considered potentially harmful (CitationFarkas & Greenblatt, 2008; CitationTarirai et al., 2010).
It has been reported that several phytoconstituents could improve the permeability of drugs in the absorptive direction by inhibition of secretory transporters such as p-glycoprotein (p-gp), several multidrug resistance proteins (MRPs) or breast cancer resistance protein (BCRP) (CitationLegen et al., 2005; CitationColalto, 2010). An important herb-drug pharmacokinetic interaction mechanism that has been extensively studied in vitro (Caco-2 cells), in vivo (rats) and clinically is mediated through inhibition or induction of p-gp (CitationSun et al., 2004, CitationVarma et al., 2006).
Different intestinal epithelia models such as Caco-2 cells, rat and human jejunum, colon of rabbit, monkey, dog and pig jejunum have been investigated for studying the effectiveness of various non-botanical permeation enhancers and/or evaluating the permeability of drugs (CitationRubas et al., 1993; CitationHsiu et al., 2002; CitationLaffont et al., 2003; CitationLegen et al., 2005; Tang et al., 2005). In the intestine, drug efflux transporters like p-gp are localized in the apical domain of the epithelial cells and actively pump compounds from within the cell back into the intestinal lumen (CitationMandava et al., 2010). These drug efflux transporters play an important role in the absorption, distribution and elimination of many clinically important therapeutic substances (CitationDoppenschmitt et al., 1999; CitationKatragadda et al., 2005). Understanding the regulation of drug transporters is paramount to designing strategies for improving the therapeutic efficacy of their substrate drugs (CitationSukhai & Piquette-Miller, 2000; CitationVarma et al., 2006).
Cimetidine, a relatively poorly permeable drug, has been shown to be a substrate of efflux proteins like p-gp, multidrug resistance proteins (MRPs), breast cancer resistance protein (BCRP) and organic cation transporter (OCT) (CitationTaur & Rodriguez-Proteau, 2008). It has also been shown that the secretion (or efflux) of cimetidine across intestinal epithelia was reduced by the co-application of efflux transport modulators (CitationCollett et al., 1999). Thus, cimetidine is one of the drugs recommended for use as a model drug to study bioequivalence and permeability enhancement to indicate the effects of co-applied compounds on drug efflux in vitro (CitationJantratid et al., 2006; CitationTakagi et al., 2006). Cimetidine permeability data from cell cultures appear to be variable and lower than those from human perfusion techniques (CitationJantratid et al., 2006). Cimetidine and atenolol are both hydrophilic and are transported via the paracellular route through the intestinal membrane, which is less available in cell cultures (high TEER values) than in intestinal tissue (low TEER values) (CitationJantratid et al., 2006).
Conventional drugs like verapamil have shown potential to inhibit drug efflux proteins like p-gp thus improving the bioavailability of substrate drugs. However, high toxicities, side effects and the dangers associated with clinically active inhibitors limit their use for reversing drug efflux. Limitations associated with clinically active inhibitors have channeled research efforts on drug absorption enhancement via efflux inhibition towards natural product extracts as potential sources of new generation efflux inhibiting excipients (CitationVarma et al., 2003).
Plant materials and herbal products that have been found to exhibit strong modulatory effects on efflux transport proteins across the intestinal epithelium include Citrus paradisi Macfad. (Rutaceae) (grapefruit juice), Ginkgo biloba L. (Ginkgoaceae) (ginkgo), Curcuma longa L. (Zingiberaceae) (turmeric) and Hypericum perforatum L. (Clusiaceae) (St. John’s Wort) (CitationColalto, 2010). CitationLegen et al. (2005) reported that the concentration of potential permeation enhancers that is needed to improve the permeability of polar drugs across the native intestine is usually very high (>10 mM), while the permeation enhancers may promote absorption of drugs across Caco-2 cells at lower concentrations. The Caco-2 cell monolayer model has therefore been found to exagerate the effects of absorption enhancers such as surfactants on drug transport across the intestinal epithelium.
Although a plethora of information is available in the scientific literature on the modulatory effects of various phytoconstituents on drug transport proteins, many commonly consumed plant materials still remain under-investigated in terms of their ability to cause pharmacokinetic interactions. Caco-2 cells have been used extensively as an in vitro intestinal epithelium model for drug transport screening experiments (CitationElsby et al., 2008). A few published studies that used excised pig jejunum tissue as an in vitro model are available (CitationHsiu et al., 2002; CitationLaffont et al., 2003; CitationTang et al., 2004; CitationLegen et al., 2005), but the effects of plant extracts on cimetidine transport has not been investigated in this model. Therefore, it was the purpose of this study to compare the effects of extracts from selected dietary fruits, vegetables and a herbal tea beverage on the permeability of cimetidine across Caco-2 cell monolayers and excised porcine jejunum tissue.
Methods
Materials
The ripe fruit of Prunus persica (L.) Batsch (Rosaceae) (peach), Prunus domestica A. Sav. (Rosaceae) (plum), and Fragaria x ananassa (Weston) Duchesne ex Rozier. (Rosaceae) (strawberry), mature tubers of the vegetables Daucus carota L. (Apiaceae) (carrot), and Beta vulgaris L. (Chenopodiaceae) (beetroot) as well as aerial parts of the herb Aspalathus linearis (Burm. f.) R. Dahlgren (Fabaceae) (rooibos tea) were purchased from a local supplier in South Africa. The fruit of Dovyalis caffra Sim. (Flacourtiaceae) (Kei-apple), Sclerocarya birrea Hochst. (Anacardiaceae) (marula) and Psidium guajava L. (Myrtaceae) (guava) were collected from the wild in Southern Africa between January and May 2006. The plants were identified by a botanist (Prof AM Viljoen) and voucher specimens (Tarirai2006 #1-9) of the plants were deposited in the Natural Products Laboratory of the Department of Pharmaceutical Sciences at Tshwane University of Technology (TUT), South Africa. Cimetidine was a donation from Sandoz, South Africa and verapamil was purchased from Sigma-Aldrich, Germany. All other ingredients were of analytical grade and were used as received.
Preparation and chemical fingerprinting of crude extracts
The fruit pulp (200 g) of S. birrea (marula), P. guajava (guava), D. caffra (Kei-apple), P. persica (peach), P. domestica (plum), and F. ananassa (strawberry) was each liquidized with 200 mL distilled water with a food blender, then frozen and lyophilized (Model LP3 Jouan freeze-drier, Germany). The dried aerial parts (200 g) of the herbal beverage A. linearis (rooibos tea) were grounded with a mortar and pestle. The tubers (200 g) of D. carota (yellow carrot) and B. vulgaris (beetroot) were shredded and ultrasonicated (Sonorex Digital 10P, Germany) for 24 h at 25°C in 500 mL distilled water and filtered into a volumetric flask. The remaining plant material was ultrasonicated with 300 mL distilled water at 30°C and stirred for a further 12 h. The two extract portions were combined (800 mL), frozen and lyophilized (Model LP3 Jouan freeze-drier, Germany). The resultant freeze-dried powders of the different plant extracts were micronized (250 µm) and stored in labeled moisture-free air-tight glass vials until used.
The chromatographic profile of the crude extracts from all the plant materials was captured by recording Liquid Chromatography-Mass Spectrometry (LC-MS) chromatograms (Waters Alliance 2690 HPLC; a 996 photodiode array detector; Waters BEH C18 column 1.7 μm, 2.1 × 4850 mm; Waters API Quattro Micro MS detector). At least 20 mg samples of dry crude extract powder were resuspended in 1 mL of 50% acetonitrile and 0.1% formic acid in water. The MS detector was operated in electron impact mode with capillary voltage at 3.5 kV, cone voltage at 35 (positive switching− ES+), RFI of 40, source temperature at 100°C, desolvation temperature at 350°C, desolvation gas at 350 L/h and cone gas at 50 L/h. The samples were introduced to the MS Waters Acquity UPLC at a rate of 270 µL/min and the injection volume was 2 µL.
In vitro drug permeability studies
Culturing and seeding of Caco-2 cells
Cryopreserved cells from the human colonic adenocarcinoma origin namely Caco-2 (HTB-37) were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum, 1% non-essential amino acids (NEAA), and 1% pen/strep/fungizone solution (10,000 U penicillin/mL, 10,000 µg streptomycin/mL and 25 µg fungizone/mL) sourced from Invitrogen (Auckland, New Zealand). The cells were incubated in a sterile humidified atmosphere at 37°C with 5% carbon dioxide and 95% air in a Forma series II incubator (Thermo Electron Corporation, USA). The medium was changed every second day. A cell suspension was obtained by means of trypsinization of the cells using trypsin/EDTA in growth flasks (25 cm2). The Caco-2 cells were counted on an Olympus IX71 microscope (Olympus Optical Co., Japan) with a haemocytometer and diluted to a concentration of 6.20 × 104 cells/mL, then seeded onto Transwell® 6-well membrane filters (Corning Costar Corporation, Acton, MA) with 0.4 µm pore polycarbonate membrane inserts and surface area of 4.71 cm2. Drug permeation studies were conducted 18–21 days after seeding. The confluence of the monolayers was checked by measurement of the transepithelial electrical resistance (TEER) with a Millicell meter equipped with chopstick electrodes (Millipore Corporations, Johannesburg, South Africa) based on a method previously described by CitationArtursson et al. (2001).
Porcine jejunum tissue preparation for the Sweetana-Grass diffusion chamber
Porcine jejunum tissue was obtained from a local slaughterhouse (R & R Abattoirs, Pretoria North, South Africa). Immediately after slaughter of the pigs (aged between 15 and 17 weeks), a 40 cm piece of the proximal jejunum extending from the ascending loop of the duodenum was excised. The excised jejunum segment was submerged in freshly prepared ice-cold Krebs-Ringer bicarbonate buffer (pH 7.4) (Sigma-Aldrich, South Africa) in a cooler box during transport. In the laboratory, the porcine jejunum segment was pulled over a glass test tube. The serosal layer with overlaying longitudinal and circular muscles was stripped off using blunt dissection with the aid of tweezers (CitationLegen et al., 2005). The jejunum segment was then cut along the mesenteric border with the aid of dissection scissors and the resultant jejunum sheet was washed off from the glass test tube onto a sheet of filter paper in a clean glass dish to maintain smoothness of the surface texture. Each jejunum sheet was cut into smaller segments of tissue, which were then mounted onto the diffusion chamber inserts of 1.13 cm2 effective surface area. Each of the six inserts were then slotted into and clamped between two diffusion chamber half cells linked to the heating block of a Sweetana-Grass diffusion chamber system. The transepithelial electrical resistance (TEER) was measured with a Millicell meter equipped with chopstick electrodes (Millipore Corporations).
Preparation of test solutions
An amount of 125 mg of cimetidine was dissolved in 5 mL of pre-prepared 5% v/v methanol in distilled water solution by stirring it at 25°C for 30 min. The cimetidine solution was then made up to 25 mL with the appropriate transport medium. An amount of 125 mg of each crude plant extract was dissolved in and made up to a final volume of 25 ml with transport medium. The transport medium for the Caco-2 cell monolayers consisted of Hank’s balanced salt solution (HBSS) supplemented with 15 mM glucose and 10 mM HEPES (4-hydroxylethylpiperazin-N-2-ethansulfonacid) [ratio 39:1] and that for excised porcine jejunum tissue consisted of Krebs-Ringer bicarbonate buffer. For the transport experiments, the cimetidine solution was mixed with each crude plant extract solution at a 1:1 ratio by volume. The mixture was stirred for approximately 5 min before the pH was adjusted to 7.4 using 1.0 M NaOH or 1.0 M HCl solutions as necessary. The final concentration of the components in each test solution was therefore 2.5 mg/mL (or 9.907 mM) cimetidine and 2.5 mg/mL plant extract.
A control group consisting of cimetidine alone was prepared by mixing 12.5 mL of the cimetidine stock solution with 12.5 mL of transport medium. The mixture was stirred for approximately 5 min before the pH was adjusted to 7.4 using 1.0 M NaOH or 1.0 M HCl solutions as necessary. A positive control was prepared by dissolving approximately 125 mg of verapamil in 2.5 mL of distilled water and stirring at 25°C for 30 min, which was then made up to 25 mL with transport medium. A volume of 12.5 mL of the cimetidine stock solution was mixed with 12.5 mL of the verapamil solution. The mixture was stirred for approximately 5 min before the pH was adjusted to 7.4 using 1.0 M NaOH or 1.0 M HCl solutions as necessary. The final concentration of the components in the mixture for the positive control group was therefore 2.5 mg/ml (or 9.907 mM) cimetidine and 2.5 mg/mL (or 5.499 mM) verapamil.
Transport across Caco-2 cell monolayers
The growth medium was removed from the chambers in the 6-well Transwell® plates with confluent Caco-2 cell monolayers on the membrane filters and replaced with 2.5 mL transport medium in each compartment. The cells were equilibrated in this medium at 37°C for 15 min before replacement of the medium in the donor compartment with a 2.5 mL of the test solution. Samples of 200 µL were drawn from each receptor compartment at 0, 20, 40 and 60 min and immediately replenished by an equal volume of fresh and warm transport medium (CitationDudley & Brown, 1996; CitationLegen et al., 2005). Both apical-to-basolateral (A-B) and basolateral-to-apical (B-A) transport of cimetidine was measured and reported as the mean of two inserts for each experiment (CitationHayeshi et al., 2006).
Transport across excised porcine jejunum tissue
The mounted porcine jejunum tissue pieces in the Sweetana-Grass diffusion chamber apparatus were incubated with 7 mL transport medium in each chamber at 37°C for 20 min before commencement of the transport study to allow the tissue to equilibrate to this environment. A mixture of 5% carbon dioxide and 95% oxygen was bubbled continuously through the chambers to stir the contents and to more closely simulate intestinal conditions by preventing stagnant layer formation. The medium in each donor chamber was then replaced by 7 mL aliquots of the test solution. Samples of 200 µL were withdrawn from the receptor chamber at 0, 20, 40 and 60 min and immediately replenished by an equal volume of fresh and warm transport medium (CitationDudley & Brown, 1996; CitationLegen et al., 2005). Both A-B and B-A directional transport of cimetidine was measured and reported as the mean of two inserts (CitationHayeshi et al., 2006).
Analysis of cimetidine concentration in samples
Cimetidine samples were analyzed using a validated method on HP1100 series high performance liquid chromatography (HPLC) equipped with a pump, auto sampler, UV detector and Chemstation Rev. A.08.03 data acquisition and analysis software (Hewllet-Packard, Palo Alto, CA, USA). A stock solution of cimetidine standard (dried substance: 99.50%, loss on drying: 0.04%) was prepared by dissolving 200 mg of cimetidine in a small volume of 40% v/v methanol solution, which was made to 100 mL to obtain a stock solution of 2.0 mg/mL. A reference solution of cimetidine was prepared by diluting 5 mL of the stock solution to 10 mL with 40% methanol to obtain a 1.0 mg/mL reference solution. The chromatographic analysis was performed on a Synergi Max-RP-column, 150 × 4.6 mm, 4 µm (Phenomenex, Torrance, CA) using an acetonitrile/water solvent with 0.005 M Na-heptane sulfonate, adjusted to pH 3.5 with orthophosphoric acid (H3PO4), 20:80 as the mobile phase in isocratic elution mode at 37°C, flow rate of 1.0 mL/min and injection volume of 5 µL. The detector was set at 220 nm, 0.13 AUFS and the retention time was ± 4.5 min. The cimetidine detection was linear over the concentration range 0.9–895 µg/mL, R2 = 0.9992 and slope = 20.054 within the 0–120% range of reference solution. The method yielded a mean recovery (accuracy) of 99.4% and a precision of 1.5% over the above-stated range. System performance proved well with relative standard deviation (RSD) values of 0.44% for peak area and 0.225% for retention time. Cimetidine was stable over a period of 12 h. The method was able to tolerate small changes in the chromatographic conditions and performed well under normal use. It was concluded that the method was suitable to analyze cimetidine in the transport samples.
Analysis of in vitro cimetidine transport data
Apparent permeability coefficient
Apparent permeability coefficient (Papp, x 10−6 cm/s) values in each direction, which represent the diffusion rate normalized for the effective cell monolayer or excised porcine jejunum tissue segment surface area and drug concentration in the donor chamber were calculated using equation 1 (CitationHansen & Nilsen, 2009):
Where dQ/dt µg/s represents the increase in the amount of drug in the acceptor compartment over time, A (cm2) is the effective surface area of the Caco-2 cell monolayer or the excised porcine jejunum tissue segment exposed to transport medium and Co µg/mL is the initial drug concentration in the donor chamber.
The efflux ratio (RE) was calculated to determine any asymmetry in the transport of cimetidine under the influence of each of the extracts using the following equation (CitationHansen & Nilsen, 2009):
Where (B-A) and (A-B) indicate the direction of cimetidine transport for which Papp was calculated. Plant extracts containing p-gp inhibiting phytoconstituents would exhibit net absorptive transport in the apical-to-basolateral direction resulting in an efflux ratio of 1 or lower.
Flux
Cimetidine flux (J, µg/cm2/min) in each direction and net flux (JNet, µg/cm2/min) were calculated using the following equations (CitationHansen & Nilsen, 2009):
Where V (mL) is the receiver chamber volume, dC (µg/mL) is the specific receiver chamber cimetidine concentration at a specific time t min, dt (min) is the overall specific transport time and A (cm2) is the effective surface area of the Caco-2 cell monolayer or the porcine jejunum tissue segment exposed to transport medium. For a p-gp inhibitor, JB-A was expected to be less than JA-B.
Where (B-A) and (A-B) indicate the direction of cimetidine transport for which flux was calculated. A negative JNet value indicates net absorptive cimetidine transport which means the test compound inhibited p-gp mediated cimetidine efflux.
Statistical analysis of permeability data was performed with Microsoft Office Excel 2007 using a one-way analysis of variance (ANOVA, p < 0.05) to indicate significant statistical differences in the effects of various extracts on cimetidine transport compared to the controls. Inhibition and/or induction plots for selected permeability parameters of various test samples were done in Microsoft Office Excel 2007.
Results
Chemical fingerprinting of plant extracts
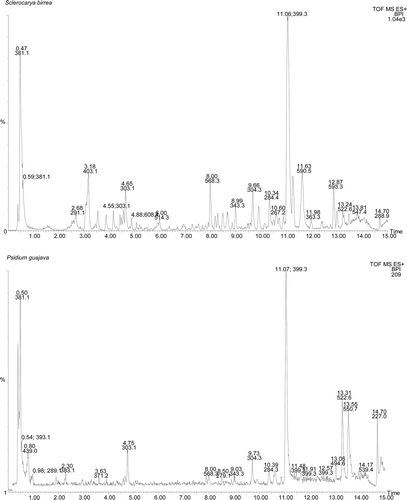
The LC-MS chromatograms of the crude extracts are shown in indicating the chemical fingerprinting data obtained for the plant materials investigated in this study. Although it is evident from the LC-MS chromatograms that the chemical composition of the crude plant extracts varied considerably, it was also noted that there were some common molecular ions and/or fragments with the same molecular masses that existed in some of the different plant materials.
As indicated on the LC-MS chromatograms, a peak at 11.06 min representing a molecular ion of 399.3 mass/charge (m/z) ratio was present in the chromatograms of all the crude plant extracts. A peak corresponding to a fragment of 381.1 m/z was only absent from the chromatograms of A. linearis and F. ananassa crude extracts, while it was present on all the other chromatograms. The results suggested that the p-gp related efflux modulatory effects of some of the plant materials could most probably not be due to the presence of these common molecules because some of the plant extracts containing them did not show any effect on drug efflux.
On the other hand, S. birrea and P. guajava crude extracts had one or more common phytoconstituents with molecular weights of 303.1–304.3, 341.1–343.3 m/z, which potentially correspond to quercetin dihydrate (C15H10O7.2H2O, MW = 338.3 g/mol) or derivatives of quercetin (C15H10O7, MW = 302.3 g/mol). Other molecular ions that were inferred from the LC-MS chromatograms of S. birrea and P. guajava extracts exhibiting p-gp inhibitory activity potentially corresponded to molecules such as β-caryophyllene, isocaryophyllene, α-humulene or their isomers with a molecular weight of 204.3 m/z.
Effect of crude plant extracts on cimetidine transport
For the Caco-2 cell monolayers only wells with TEER values above 230 Ω.cm2 were used and for the excised pig intestinal tissues only chambers with TEER values above 100 Ω.cm2 were used, which were regarded as acceptable (CitationJantratid et al. 2006; Neirinckx et al. 2010).
The Papp and RE values for cimetidine transport across Caco-2 cell monolayers and pig jejunum tissue are shown in . Papp values in each direction represent the diffusion rate normalized for the effective cell monolayer or excised porcine jejunum tissue segment surface area and drug concentration in the donor chamber while RE values indicate asymmetry in the transport of cimetidine under the influence of each of the extracts (CitationHansen & Nilsen, 2009).
Table 1. Apparent permeability (Papp) and efflux ratio (RE) values for cimetidine transport in the presence of crude plant extracts over a 1 h period at pH 7.4
The deductions that can be made from the Papp and RE values for cimetidine transport in the presence of the different crude plant extracts in terms of inhibition or induction in the respective in vitro models are summarized in .
Table 2. Summary of the effects of each crude plant extract on cimetidine efflux as determined by two in vitro models.
The values of J and JNet are presented in based on the transport across Caco-2 cell monolayers and in based on the transport across excised pig jejunum tissue. A negative JNet value is interpretive of net absorptive cimetidine transport indicating that the test compound inhibited p-gp mediated cimetidine efflux.
Figure 2. Flux values for cimetidine (9.9 mM) in the presence of different crude plant extracts across Caco-2 cell monolayers over a 1 h period at pH 7.4. The values are the mean ± SD of two inserts and N = 4.
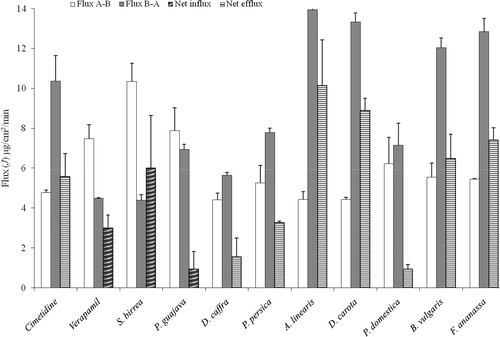
Figure 3. Flux values for cimetidine (9.9 mM) in the presence of different crude plant extracts across excised pig jejunum tissue over a 1 h period at pH 7.4. The values are the mean ± SD of two inserts and N = 4.
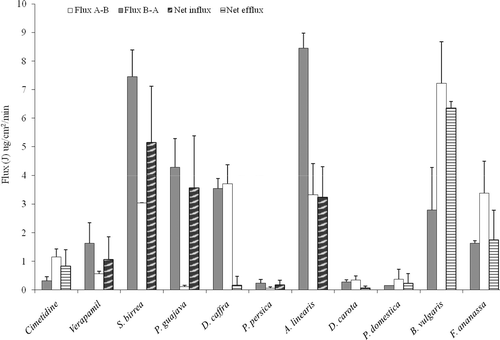
In accordance with the Papp and RE values, verapamil, S. birrea and P. guajava crude extracts showed negative net flux values, which are clearly indicated as net influx of cimetidine in the absorptive direction on and across both Caco-2 cell monolayers and excised porcine jejunum tissue. This clearly shows that the inhibition of the cimetidine efflux by the crude extracts of these two fruits increased transport of the drug in the absorptive direction.
The crude extracts of P. persica and A. linearis showed positive net flux values, which are indicated as net efflux of cimetidine in the secretory direction on across Caco-2 cell monolayers, but they showed negative net flux values indicated as net influx of cimetidine on across the excised pig jejunum.
Discussion
Chemical fingerprinting of plant extracts
From the LC-MS chromatographs, it is clear that the different plant materials exhibited complex chemical compositions with some peaks indicating presence of similar compounds with the same molecular fragments shown by similar mass/charge (m/z) ratios, but they also showed vast differences in terms of their phytochemical contents. It was observed from the LC-MS chemical fingerprints that the extracts which exhibited p-gp inhibitory activity namely S. birrea and P. guajava crude extracts had one or more common phytoconstituents that could potentially be related to the flavonoid quercetin dihydrate, derivatives of quercetin, α-humulene and β-caryophyllene (CitationGuti’érrez et al., 2008; CitationViljoen et al., 2008). Since dietary flavonoids have been shown before to exhibit modulatory effects on p-gp related cimetidine efflux, it is possible that these phytochemicals contributed to the changes in cimetidine transport caused by these plant materials (CitationHsiu et al., 2002; CitationTaur & Rodriguez-Proteau, 2008). However, it is important to note that the crude extracts of S. birrea and P. guajava were investigated and the effect on cimetidine efflux may be due to the combination of phytoconstituents rather than a single phytochemical.
Effect of crude plant extracts on cimetidine transport
In general, the Papp values for cimetidine transport across Caco-2 cell monolayers were much higher compared to those obtained for cimetidine transport across excised pig jejunum tissue. This can possibly be explained by the leakier characteristics of the Caco-2 cell culture epithelium monolayer compared to the excised porcine intestinal tissue that has been formed in vivo fully connected to nerve cells and blood vessels. This is in line with results obtained from a study where a linear permeability relationship was obtained between Caco-2 cells and animal intestinal tissues, but the Caco-2 cell monolayers were twice as permeable as rabbit and five times as permeable as monkey colon epithelial tissue (CitationRubas et al., 1993).
Cimetidine efflux across both the in vitro intestinal epithelial models was confirmed as indicated by an average Papp (B-A) >> average Papp (A-B) values for the negative control group (cimetidine alone) with a net transport in the secretory direction. Verapamil was shown to be an appropriate positive control for inhibition of the efflux of compounds across Caco-2 cell monolayers by CitationElsby et al. (2008) and in this study it consistently improved the transport of cimetidine in the absorptive direction. These results from the negative and positive control groups demonstrated that both in vitro models used in this study were suitable to measure the potential effects of plant extracts on cimetidine efflux. The Caco-2 cell model showed a higher RE value for cimetidine alone (negative control) than the excised porcine intestinal tissue.
Based on the Papp and RE values for the experimental groups, it was evident that the crude extract of S. birrea significantly (p < 0.05) inhibited cimetidine efflux in both in vitro models compared to the negative control group. In fact, the improved absorptive transport of cimetidine caused by S. birrea crude extract was similar to that caused by verapamil with RE values of 0.58 for S. birrea extract vs. 0.52 for verapamil across Caco-2 cell monolayers and 0.43 vs. 0.47 across excised pig jejunum tissue. P. guajava crude extract also consistently increased the transport of cimetidine in the absorptive direction across both in vitro intestinal models by decreasing its efflux in the secretory direction, which was significantly (p < 0.05) different from the negative control group. The effect of P. guajava crude extract on cimetidine transport was more pronounced in the Caco-2 model than in the pig jejunum tissue, but it was consistently less than that obtained for S. birrea crude extract in both models.
The crude extracts of A. linearis and D. carota significantly (p < 0.05) increased the efflux of cimetidine compared to the negative control group across the Caco-2 cell monolayers, but this effect was not observed in the excised pig jejunum tissue. Similarly, different effects were observed for the crude extracts of D. caffra, P. persica, P. domestica, B. vulgaris and F. ananassa in the two in vitro models where they exhibited non-significant or non-conclusive effects on cimetidine transport. The findings on A. linearis, D. caffra, P. persica, D. carota, P. domestica, B. vulgaris, and F. ananassa showed a lack of correlation between the two intestinal epithelial models. Contradictory findings between different studies have been reported for the effect of grapefruit juice on the bioavailability of the p-gp substrate talinolol where increased bioavailability was reported in vitro (Caco-2 cell monolayers) in one study and decreased bioavailability of talinolol was found in vivo in humans in another study (CitationSpahn-Langguth & Langguth, 2001; CitationSchwarz et al., 2005). These discrepancies may be explained by potential differences in expression of active transporters between models because cimetidine transport may also be affected by other transporters than p-gp as described below.
Although the transport of cimetidine has been shown to be modulated by compounds that affect p-gp efflux (CitationTaur & Rodriguez-Proteau, 2008), its efflux may also involve other active transporters such as OCT, MRP2 and BCRP. The effects of the plant extracts on cimetidine efflux therefore involved p-gp related transport, but are not restricted to this efflux transporter only. Furthermore, since the in vitro inhibitory effects of S. birrea and P. guajava crude extracts on cimetidine efflux were similar to that of verapamil in the positive control group, the two extracts may also produce effects similar to that of verapamil in the in vivo situation. For example, it was shown that verapamil increased the bioavailability of the p-gp substrate irinotecan significantly in vivo in rats (CitationBansal et al., 2009). It is also known that the juice of C. paradisi (grapefruit) increases the bioavailability of cyclosporine due to p-gp efflux inhibition and also because of cytochrome P450 enzyme inhibition (CitationHsiu et al., 2002; CitationSica, 2006). This type of pharmacokinetic interaction is especially important when drugs that are substrates for p-gp efflux and have narrow therapeutic indices are taken in conjunction with modulators of p-gp. However, further studies are required on the enzyme inhibition effects of the extracts or juice of S. birrea and P. guajava and in vivo pre-clinical (animals) and clinical trials will have to be conducted in order to be conclusive in terms of their effects on the bioavailability of co-administered drugs.
The RE values obtained for the experimental groups are mostly higher in the Caco-2 cell model than in the excised porcine tissue model (). The different RE values indicate that the effects of the plant extracts on cimetidine transport were more pronounced in the Caco-2 cells than in the porcine jejunum tissue. These results are similar to a previous study where the Caco-2 cells were found to be more expressive of the effects of absorption enhancers on drug transport as compared to the porcine intestinal tissue model (CitationLegen et al., 2005). The Caco-2 cell monolayers may therefore overestimate or exaggerate the effects of co-administered plant extracts on the transport of drugs across the intestinal epithelium (CitationFarkas & Greenblatt, 2008). Furthermore, the results from this study indicated that differences exist between the types of effects of plant extracts on cimetidine transport in the Caco-2 cells and excised porcine jejunum tissue. The inter-model variability highlights the fact that a single in vitro model may not be suitable to accurately and adequately predict the effects of co-administered compounds on drug transport.
Conclusions
The results of the in vitro transport study indicate that the crude extracts of S. birrea and P. guajava significantly affect cimetidine transport by inhibiting its efflux in two in vitro models, Caco-2 cell monolayers and excised porcine jejunum tissue. This may indicate potentially unwanted pharmacokinetic interactions such as increased drug bioavailability that may be experienced in the in vivo situation when these fruits are taken simultaneously with drugs that are substrates for efflux transporters such as p-gp. An increase in bioavailability may be potentially harmful in the case of drugs with narrow therapeutic indices. On the other hand, controlled herb-drug interactions via efflux inhibition potentially presents an opportunity for enhancing the permeability of orally administered drugs with poor bioavailabilities.
The Caco-2 cells were in general more sensitive to the effects of the plant extracts than the excised porcine jejunum tissue. This may lead to an overestimated effect of co-administered plant extracts on drug transport. Based on this, the excised porcine jejunum model seemed to give a more realistic estimate of botanical-drug pharmacokinetic interactions than the Caco-2 cell model. In addition, differences were observed in the effects of some of the plant extracts between the two in vitro models highlighting potential inter-model variability in terms of botanical-drug pharmacokinetic interactions possibly due to differences in expression of active transporters.
Acknowledgements
The authors want to acknowledge Mr. M. Kleinschmidt and the staff of R & R abattoir for generously supplying pig jejunum tissue. Financial support by Tshwane University of Technology and the National Research Foundation is acknowledged.
Declaration of interest
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Artursson P, Palm K, Luthman K. (2001). Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv Drug Deliv Rev, 46, 27–43.
- Bansal T, Mishra G, Jaggi M, Khar RK, Talegaonkar S. (2009). Effect of P-glycoprotein inhibitor, verapamil, on oral bioavailability and pharmacokinetics of irinotecan in rats. Eur j Pharm Sci, 36, 580–590.
- Colalto C. (2010). Herbal interactions on absorption of drugs: Mechanisms of action and clinical risk assessment. Pharmacol Res, 62, 207–227.
- Collett A, Higgs NB, Sims E, Rowland M, Warhurst G. (1999). Modulation of the permeability of H2 receptor antagonists cimetidine and ranitidine by P-glycoprotein in rat intestine and the human colonic cell line Caco-2. J Pharmacol Exp Ther, 288, 171–178.
- Döppenschmitt S, Spahn-Langguth H, Regårdh CG, Langguth P. (1999). Role of P-glycoprotein-mediated secretion in absorptive drug permeability: An approach using passive membrane permeability and affinity to P-glycoprotein. J Pharm Sci, 88, 1067–1072.
- Dudley AJ, Brown CD. (1996). Mediation of cimetidine secretion by P-glycoprotein and a novel H(+)-coupled mechanism in cultured renal epithelial monolayers of LLC-PK1 cells. Br J Pharmacol, 117, 1139–1144.
- Elsby R, Surry DD, Smith VN, Gray AJ. (2008). Validation and application of Caco-2 assays for the in vitro evaluation of development candidate drugs as substrates or inhibitors of P-glycoprotein to support regulatory submissions. Xenobiotica, 38, 1140–1164.
- Farkas D, Greenblatt DJ. (2008). Influence of fruit juices on drug disposition: Discrepancies between in vitro and clinical studies. Expert Opin Drug Metab Toxicol, 4, 381–393.
- Guti’érrez RMP, Mitchell S, Solis RV. (2008). Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J Ethnophamacol, 117, 1–27.
- Hansen TS, Nilsen OG. (2009). Echinacea purpurea and P-glycoprotein drug transport in Caco-2 cells. Phytother Res, 23, 86–91.
- Harris RZ, Jang GR, Tsunoda S. (2003). Dietary effects on drug metabolism and transport. Clin Pharmacokinet, 42, 1071–1088.
- Hayeshi R, Masimirembwa C, Mukanganyama S, Ungell AL. (2006). The potential inhibitory effect of antiparasitic drugs and natural products on P-glycoprotein mediated efflux. Eur J Pharm Sci, 29, 70–81.
- Hsiu SL, Hou YC, Wang YH, Tsao CW, Su SF, Chao PD. (2002). Quercetin significantly decreased cyclosporin oral bioavailability in pigs and rats. Life Sci, 72, 227–235.
- Ingersoll RE. (2005). Herbaceuticals: An overview for counselors. J Counsel Dev, 83, 434–443.
- Jantratid E, Prakongpan S, Dressman JB, Amidon GL, Junginger HE, Midha KK, Barends DM. (2006). Biowaiver monographs for immediate release solid oral dosage forms: cimetidine. J Pharm Sci, 95, 974–984.
- Katragadda S, Budda B, Anand BS, Mitra AK. (2005). Role of efflux pumps and metabolising enzymes in drug delivery. Expert Opin Drug Deliv, 2, 683–705.
- Laffont CM, De Vrieze G, Maas R, Bousquet-Mélou A, Fink-Gremmels J. (2003). Comparative evaluation of p-gp activity in cattle, sheep, goats, pigs, horses, and rats using a limphocyte-based ex vivo model. J Vet Pharm Therap, 26, 82–307.
- Legen I, Salobir M, Kerc J. (2005). Comparison of different intestinal epithelia as models for absorption enhancement studies. Int J Pharm, 291, 183–188.
- Mandava N, Oberol RK, Minocha M, Mitra AK. (2010). Transporter targeted drug delivery. J Drug Deliv Sci Technol, 20, 89–99.
- Neirinckx E, Vervaet C, Michiels J, De Smet S, Van den Broeck W, Remon JP, De Backer P, Croubels S. (2011). Feasibility of the Ussing chamber technique for the determination of in vitro jejunal permeability of passively absorbed compounds in different animal species. J Vet Pharmacol Ther, 34, 290–297.
- Rubas W, Jezyk N, Grass GM. (1993). Comparison of the permeability characteristics of a human colonic epithelial (Caco-2) cell line to colon of rabbit, monkey, and dog intestine and human drug absorption. Pharm Res, 10, 113–118.
- Schwarz UI, Seemann D, Oertel R, Miehlke S, Kuhlisch E, Fromm MF, Kim RB, Bailey DG, Kirch W. (2005). Grapefruit juice ingestion significantly reduces talinolol bioavailability. Clin Pharmacol Ther, 77, 291–301.
- Sica DA. (2006). Interaction of grapefruit juice and calcium channel blockers. Am J Hypertens, 19, 768–773.
- Spahn-Langguth H, Langguth P. (2001). Grapefruit juice enhances intestinal absorption of the P-glycoprotein substrate talinolol. Eur J Pharm Sci, 12, 361–367.
- Sukhai M, Piquette-Miller M. (2000). Regulation of the multidrug resistance genes by stress signals. J Pharm Pharm Sci, 3, 268–280.
- Sun J, He ZG, Cheng G, Wang SJ, Hao XH, Zou MJ. (2004). Multidrug resistance P-glycoprotein: crucial significance in drug disposition and interaction. Med Sci Monit, 10, RA5–R14.
- Takagi T, Ramachandran C, Bermejo M, Yamashita S, Yu LX, Amidon GL. (2006). A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol Pharm, 3, 631–643.
- Tang H, Pak Y, Mayersohn M. (2004). Protein expression pattern of P-glycoprotein along the gastrointestinal tract of the Yucatan micropig. J Biochem Mol Toxicol, 18, 18–22.
- Tarirai C, Viljoen AM, Hamman JH. (2010). Herb-drug pharmacokinetic interactions reviewed. Expert Opin Drug Metab Toxicol, 6, 1515–1538.
- Taur JS, Rodriguez-Proteau R. (2008). Effects of dietary flavonoids on the transport of cimetidine via P-glycoprotein and cationic transporters in Caco-2 and LLC-PK1 cell models. Xenobiotica, 38, 1536–1550.
- Varma MV, Ashokraj Y, Dey CS, Panchagnula R. (2003). P-glycoprotein inhibitors and their screening: A perspective from bioavailability enhancement. Pharmacol Res, 48, 347–359.
- Varma MV, Perumal OP, Panchagnula R. (2006). Functional role of P-glycoprotein in limiting peroral drug absorption: optimizing drug delivery. Curr Opin Chem Biol, 10, 367–373.
- Viljoen AM, Kamatou GPP, Basér KHC. (2008). Head-space volatiles of marula (Sclerocarya birrea subsp. caffra). S Afr J Bot, 74, 325–326.